Abiotic stress-induced changes in antioxidative system and secondary metabolites production in Andrographis paniculata
Автор: Mourya Suraj Kumar, Mohil Praveen, Kumar Anil
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.18, 2022 года.
Бесплатный доступ
Andrographis paniculata is an annual herbaceous plant of the family Acanthaceae. Andrographolide (a bicycle diterpene) is the main component of A. paniculata and responsible for its bitter taste. Abiotic stress considerably affects the life cycle of plants through morphological, biochemical, and physiological changes. These stresses also act as elicitors for the synthesis of secondary metabolites (SMs) in plants. Secondary metabolites help plants to survive in adverse conditions via alleviating osmotic stress and reducing Reactive Oxygen Species (ROS). In this review, we discuss the response of the antioxidative system and biosynthesis of secondary metabolites in A. paniculata under abiotic stress. The mechanism involve in biosynthesis of ent-labdane diterpenoids is also discussed.
Environmental stress, elicitors, andrographolide, reactive oxygen species, antioxidants
Короткий адрес: https://sciup.org/143179059
IDR: 143179059
Текст обзорной статьи Abiotic stress-induced changes in antioxidative system and secondary metabolites production in Andrographis paniculata
Abbreviation: AACA- Acetyl-CoA Acetyltransferase; ABA - Abscisic acid; AG – Andrographolide; APX -Ascorbate peroxidase; As- Arsenate; CAT – Catalase; Co – Cobalt; Cu – Copper; CYT - Cytochrome oxidase; DDAG – deoxy-11,12-didehydroandrographolide; DXR - 1- deoxyxylulose-5-phosphate synthase; DXS - 1- deoxyxylulose-5-phosphate reductoisomerase; FPP- Farnesyl pyrophosphate; GGPP - Geranylgeranyl diphosphate; GGPS - Geranylgeranyl pyrophosphate synthase; GR - Glutathaione reductase; HMGR - 3-hydroxy-3-methyl glutaryl coenzyme A reductase ; HMGS - 3-hydroxy-3-methyl glutaryl coenzyme A synthase; IPP - Isopentyl pyrophosphate; ISPH - 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase; LP - Lipid peroxidase; MeJA - Methyl jasmonate; MEP - Methyl erythritol phosphate; MVAP - Mevalonic acid pathway; NeoAG – Neoandrographolide; PGRs - Plant growth regulators; POD – Peroxidase; PPO - Polyphenol oxidase; ROS - Reactive oxygen species; SA - Salicylic acid; Sms - Secondary metabolites; Sn – Tin; SOD - Superoxide dimutase; Tfs - Transcription factors
Andrographis paniculata (Burm.f.) Wall. ex Nees (Kalmegh in Hindi) is a useful annual herbaceous medicinal plant related to the family Acanthaceae. It is also known as the king of bitter because of its bitter taste. The bitterness of A. paniculata is due to the presence of andrographolide (a bicyclic diterpenoid lactones) (Siripong et al ., 1992). A. paniculata is indigenous in India, Sri Lanka and has a broad range of diversity in some Asian and other countries such as Pakistan, Java, Malaysia (covering Penang, Malacca, & Pangkor Island of Borneo), Indonesia, China, Hong Kong, and Philippines. It is cultivated extensively for traditional herbal medicines in China, Thailand, and some regions of the East and West Indies (Jamaica, Barbados, and Bahamas) (Akbar, 2011; Niranjan et al ., 2011; Joseph, 2014). In India, A. paniculata is found wild through the plains, mostly in Assam, Chhattisgarh, Maharashtra, West Bengal, Uttar Pradesh, Madhya Pradesh, Uttarakhand, Karnataka, and Tamil Nadu (Prajapati et al ., 2003).
A. paniculata generally propagated through seeds and grows at various areas in pine, evergreen, and deciduous forest and, along the roadside in villages (Mishra et al., 2007). The plant is highly branched, erect, up to 30-70 cm in length, stem herbaceous, quadrangular, dark green with varies in diameter from 2 to 6 mm. Leaves are simple, opposite, lanceolate with acute apex and short petiole, varying in length and breadth from 2-10 cm and 1-2.5 cm respectively, along with unicostate reticulate venation (Patil and Jain, 2021). It possesses terminal, axillary, and panicle type of inflorescence. It has white flowers with violet spots that are complete, bisexual, irregular (zygomorphic), hypogynous, pentamerous, pedicellate, and tubular. Flower are consist of linear 5 small sepals with excess hairs, petals are fuse about 6 mm long, white with a maroon stripe on the upper lip of petals. The plant possesses two stamens with hairy filaments, tricolporate pollen grains, and a bi-celled superior ovary. Seeds are very small, numerous and fruit is capsule (Sareer et al ., 2014; Sharma et al ., 2018). The flowering of A. paniculata starts from mid-October and it is in full bloom till December (Sharma and Jain, 2015) (Fig. 1).
The principal components of A. paniculata are ent-labdane diterpenoids, i.e., andrographolide (AG), neoandrographolide (NeoAG), 14-deoxy-11,12-didehydroandrographolide (DDAG) (Rafi et al ., 2020), and flavonoids (Song et al ., 2013). The chemical structure of biochemical components of A. paniculata is shown in Fig. 2. It exhibits several medicinal properties such as anticancer, antiviral, antibacterial, antidiabetic, antimalarial, antiinflammatory, liver protective, and cardioprotective (George and Pandalai, 1949; Yao et al ., 1992; Matsuda et al ., 1994; Zeha and Rawal, 2001; Chowdhury et al ., 2012; Subramanian et al ., 2012; Uttekar et al ., 2012; Sandborn et al ., 2013). The plant also used as antipyretic agents for the treatment of common diseases like fever, cold, cough, diarrhea, and inflammatory disease (Chang and But, 1987).
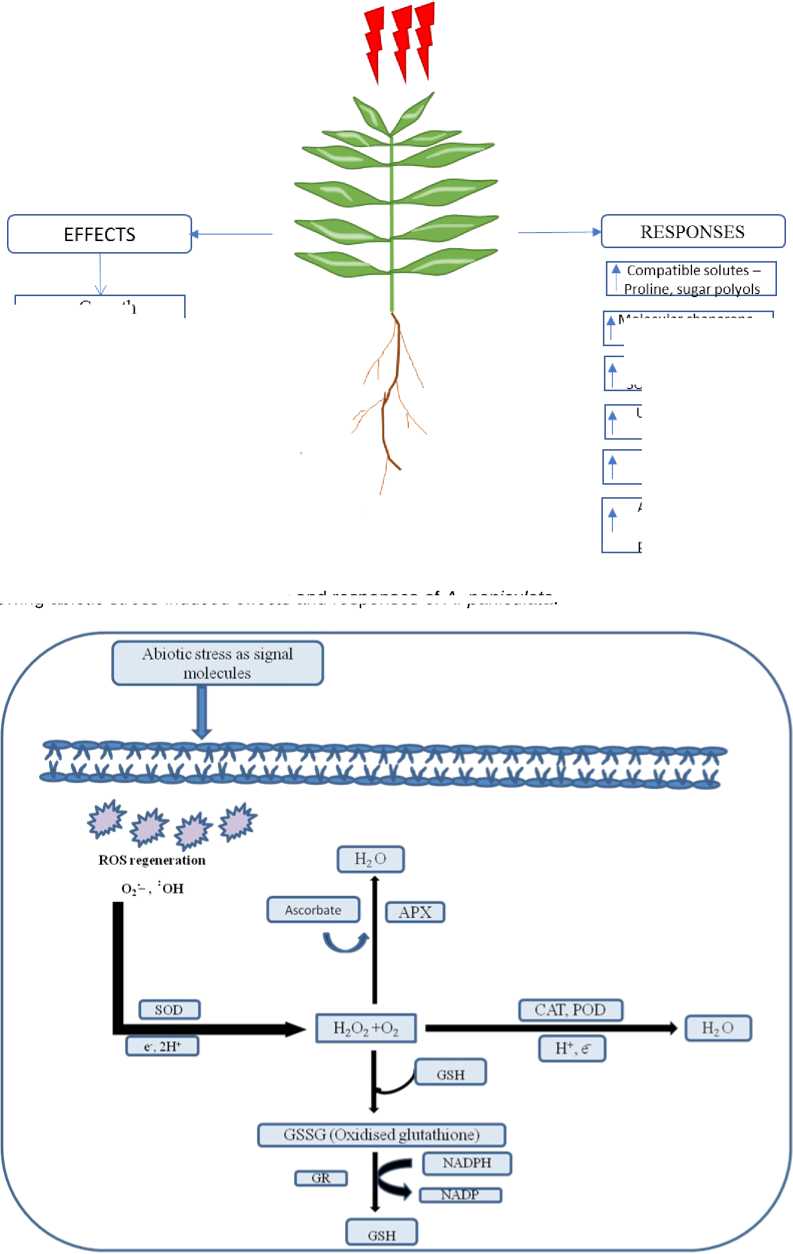
Environmental stresses are adverse or non-ideal conditions for plant that causes changes in morphology, physiology and metabolism of the plant (Kumar, 2020). The effects of stress can lead to reduction in growth, yields, permanent damage and if the environmental stress is more than tolerance limits lead to death (Kumar and Aery, 2011, 2012; Verma et al ., 2013). Adverse environmental conditions may reduce the performance and yield from 50 to 70% of the crop. Under the stress conditions, decreased length and biomass production of plants are the primary responses. At the cellular levels plants faces oxidative stress and generates ROS. The ROS are toxic to plants and are neutralised by antioxidative system. Antioxidative system includes both enzymatic and nonenzymatic antioxidants which play important role in scavenging of excess ROS (Noctor and foyer, 1998). Enzymatic antioxidant include superoxide dismutase (SOD), Peroxidase (POD), catalase (CAT), gluthatione reductase (GR) and Ascorbate peroxidase (APX) (Arora et al ., 2002), whereas nonenzymatic antioxidant include tocopherol, ascorbate, carotenoids and phenolics. In plants maintaining antioxidant capacity to remove excess ROS, is associated to enhance tolerance to environment stress (Zaefyzadeh et al ., 2009). Abiotic stress induced effects and responses of A. paniculata is shown in Fig. 3
Plant SMs are synthesized from primary metabolites (e.g., carbohydrates, lipids, and amino acids) by diverse physiological changes in the plant’s life cycle. These are unique sources for medicine, flavors, fragrances and industrially useful biochemicals and act as multifunctional metabolites that help the plant to interact with its environment for adaptation and defense against herbivores, pathogens, and stress (Jan et al., 2021; Hartmann, 2007). The levels of SMs in plants are also influenced by several abiotic stresses such as nutrient deficiencies, metal ions, wounding, UV radiation, light, salinity, drought, seasonality, and temperature (Gouvea et al., 2012; Verma and Shukla, 2015). SMs are classified the basis of chemical structure. The main group of SMs present in plants is alkaloids (nitrogencontaining compounds-caffeine, vinblastine), phenolics terpenes (alkaloids, cyanogenic glycosides, and glucosinolates), phenolic compounds (tannins xanthones, flavonoids, iso-flavonoid, and coumarines), terpenes (mono, sesqui, di, tetra and poly terpenes) and saponins (Fang et al., 2011; Hussein and El-Anssary, 2019). Here, we review the recent progress in understanding on the production of secondary metabolites and mechanisms of ent-labdane diterpenoids synthesis in A. paniculata under abiotic stress.
ABIOTIC STRESS AND PLANT DEFENSE SYSTEM
Abiotic stresses occur mainly by fluctuations in non-biological factors such as environmental and nutritional factors that adversely impact plant growth, physiology, reproduction, and overall life cycle for a short and mild period (Gull et al., 2019). Abnormalities in water status (drought and waterlogging), nutrient levels, moisture in wind, temperature (cold, frost, and heat), salinity, and mineral toxicity are some of the types of abiotic stresses in the environment and show impact on growth, development, yield and seed quality of crop and other plants (Mittler, 2006; Gull et al. 2019). At the cellular level, due to reduction in turgor pressure the growth of plant decrease as most stress-sensitive process. In higher plants under drought stress, due to disruption of water movement from xylem to the surrounding cells of treachery element, cell elongation is reduced (Nonami, 1998). Under adverse environment, cellular activities such as cell division, cell elongation and expansion are not occurred properly, as per demand of plant that, results in reduction in germination of seeds, height, biomass production, leaf area, and poor establishment of crops and causes reduced yield and quality (Kaya et al., 2006; Hussain et al., 2008). Heavy metal contamination of agricultural lands limits crop productivity and is also dangerous to human health (Kumar and Aery, 2016). Biochemical and physiological functions are reduced in the plant at stress by changing the functionality of gene/protein at the molecular level (Djanaguiraman et al., 2018; Takahashi and Shinozaki, 2019).
Due to various environment stresses such as metal toxicity, high temperature, pollutant, UV-radiation salinity and pathogen infection lead to oxidative stress in plants, and affect many biological processes (Xie et al ., 2019). Oxidative stress is a complex physiological phenomenon of overproduction and storage of ROS, which causes dysfunction and damage of cell components. In plant, oxygen produce by photolysis of water during photosynthesis is disturbed by unbalance metabolism under stress and results in production of ROS (Asada, 1999). Superoxide radicle (O 2 .-), singlet oxygen (1O 2 ), hydrogen peroxide (H 2 O 2 ) and hydroxyl radical (OK ) are oxygenated and possess at least one unpaired electron, that makes them highly reactive and induce oxidative stress through oxidation of cell components when they are found in excess in the cells and go beyond the cellular defence systems (Demidchik, 2014). Major target for ROS in plant cell are protein and lipid (Xie et al ., 2019). Antioxidative systems act to decrease the oxidation of proteins, lipids, DNA, and other biomolecules by blocking the oxidation reaction and antioxidants directly scavenge free radicles and prevent damage (Huang et al ., 2005). Plant defences system sense environmental changes and take action by generating suitable cellular mechanism, after receiving the signal, transferred to the nucleus where transcription machinery activate and respond to stress (Gull et al ., 2019), by the antioxidative system and metabolic product i.e., Secondary metabolites (SMs).
The SMs significantly improve plant survival under different environmental stresses like drought, salinity, metal stress and therefore act as important metabolites in these adverse conditions (Agostini-Costa et al., 2012; Verma and Shukla, 2015; Kurepin et al., 2017; Zandalinas et al., 2018). Many elicitors such as abiotic or biotic stress enhance the production of SMs and make plants stronger (Jan et al., 2021). The mevalonic acid pathway and methyl erythritol phosphate pathway are the main pathway for terpenoid synthesis whereas shikimic acid pathway is the main pathway for phenolic compound under normal as well as stress conditions (Jamwal et al., 2018). Plant of different as well as same species differ in endogenous levels of various SMs (Barton, 2007). Several cellular and biochemical factors have an important role in the storage and transportation of SMs.
Generally, SMs are present in plants in normal conditions but the accumulation of SMs increases by various abiotic stress signals or elicitors molecules in response (Akula and Ravishankar, 2011). Salinity in soil and water causes dehydration, and excess removal of water from the cytoplasm that causes cytosolic and vacuolar volumes reduced, resulting in the accumulation of specific secondary metabolites, which adapt to plant in adverse condition (Mahajan and Tuteja, 2005). The production of SMs is also related to heat/temperature, low-temperature decrease, and higher temperature increase SMs production in plants in responses to stress (Morison and Lawlor, 1999; Verma and Shukla, 2015). Unavailability of water and more transpiration rate cause decline in turgidity of cells thereby negatively influencing various physiological processes and altering SMs productions (Lisar et al ., 2012; Verma and Shukla, 2015).
ANTIOXIDATIVE SYSTEM OF A.PANICULATA UNDER ABIOTIC STRESS
Environmental stress is increasing by anthropogenic as well as natural activity. Under these conditions, plants develop several strategies to defend these conditions. Some studies have been conducted on antioxidative system activity of A. paniculata that minimize stress.
According to Antony and Nagella (2021) in phosphomolybdate assay, maximum antioxidative activity was observed in 100 mM Co dose treated A. paniculata whereas those plants treated with Sn dose also exhibit an increase in antioxidative activity. The highest metal chelating activity was reported in 50 mM Cu treated plant with 57.15% over than 56.55% in control. In DPPH assay antioxidant activity was decline in Cu treatment and raise in Sn treated A. paniculata. Arsenate application enhanced the levels of antioxidative enzymes like superoxide dismutase (SOD) glutathione reductase (GR) and lipid peroxidase (LP) along with proline content in A. paniculata. Both enzymatic and non-enzymatic oxidative stresses were significantly higher in wild plants as compared to cultured plants (Das et al., 2021).
Under high light intensity (0.24x103-0.96x103 ìmole.photons.m-2sec-1) peroxidase (POD) activity increased in leaves of A. paniculata in all three stages (vegetative, flowering, fruiting). Maximum POD activity was reported in the fruiting stage followed by flowering than the vegetative stage. The irradiance at higher light intensity increased the POD activity by 70% (Kumar et al. , 2009).
Under the saline condition, A. paniculata accumulates maximum superoxide in 41.10 mM salt-treated leaf (Ismali et al ., 2015). In A. paniculata salinity-induced enhancement of antioxidative enzymes SOD, CAT, POD, cytochrome oxidase (CYT) and polyphenol oxidase (PPO) in roots and leaves was occur with rising salinity (Shao et al ., 2015). An increase in the catalase (CAT) and ascorbate peroxidase (APX) enzyme activity was observed with increasing NaCl concentration (Kumar et al ., 2021).
The antioxidative response of antioxidative system of A. paniculata to plant growth hormones has also observed. The content of ascorbic acid in A. paniculata in PGRs, abscisic acid (ABA), and gibberellic acid (GA 3 ) treatment. The activity of CAT, APX, and SOD enhance with ABA and GA 3 treated root, stem, and leaves of A. paniculata . In ABA and GA 3 treated A. paniculata, SOD and CAT activity increases (Anuradha et al. , 2010).
Antioxidant enzymes functions in sequentially manner- first of all, SOD catalyses the conversion of superoxide radicles to H2O2, by electron transport, further CAT, POD, and APX act upon H2O2 and convert it into H2O. GR acts as an important enzyme, it maintains reduced/oxidized glutathione inside the cell (Yaghoubian et al., 2014) (Fig. 4)
Table 1: Effect of different abiotic stress on the biosynthesis of secondary metabolites in A. paniculata
|
S. No. |
Abiotic stress |
Secondary Metabolites |
Response |
References |
|
1 |
Heavy metals (Copper, Tin) |
Andrographolide |
Increased |
Antony and Nagella (2021) |
|
2 |
CuSO 4 |
Andrographolide |
Four fold overproduction |
Dawande and Sahay (2020) |
|
3 |
AgNO 3 |
Andrographolide |
Seven-fold overproduction of andrographolide |
Das and Bandyopadhyay (2020) |
|
4 |
As |
Andrographolide, neoandrographolide, didehydroandrographolide |
Increased |
Das et al . (2020) |
|
5 |
Sodium Azide |
Andrographolide, andrographonin |
Increased |
Chandran and Pillai (2018) |
|
6 |
High Sulphur plus low /high nitrogen |
Andrographolide, dehydroanddrographolide |
Increased |
Jian et al. (2021) |
|
7 |
Salinity |
Andrographolide, neoandrographolide |
Increased |
Kumar et al. (2021) |
|
8 |
Salinity |
Andrographolide, deoxyandrographolide and dehydroandrographolide |
All increased |
Shao et al . (2015) |
|
9 |
Salinity |
Andrographolide, neoandrographolide |
Tolerant plants accumulate higher amounts than sensitive plants |
Talei et al . (2013); Talei et al . (2015) |
|
10 |
Salinity |
Andrographolide |
Increased |
Gandi et al . (2012) |
|
11 |
Drought |
Andrographolide, 14-deoxy-11,12 didehydroandrographolide |
Increased |
Chen et al . (2020) |
|
12 |
Drought |
Andrographolide |
Increased in leaves but decreased in stems |
Saravanan et al. (2009) |
|
13 |
Light |
Flavonoid |
Increased |
Kumar et al . (2012) |
|
14 |
Shading and fertiliser |
Andrographolide |
Increased |
Purwanto et al . (2011) |
|
15 |
Salicylic acid and chitosan |
Andrographolide |
18.5 and 59.5 fold overproduction in SA and chitosan treatment |
Vakil and Mendhulkar (2013) |
|
16 |
Methyl jasmonate (MeJA) |
Andrographolide |
5.25 fold overproduction |
Sharma et al . (2014) |
|
17 |
Jasmonic acid and salicyclic acid |
Andrographolide |
3.3 and 3.4 fold overproduction in JA and SA |
Zaheer and Giri (2015) |
|
18 |
Aspartic acid and methyl jasmonate |
Andrographolide |
Three-fold overproduction |
Das and Bandyopadhyay (2020) |
|
19 |
Methyl-jasmonate |
Andrographolide |
Increased |
Sharma et al . (2015) |
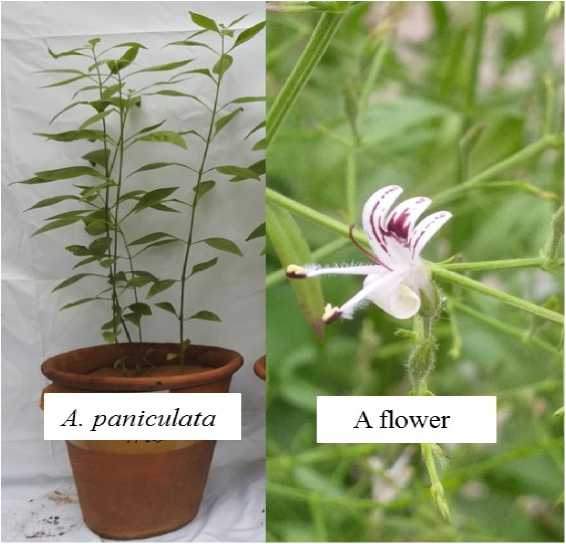
Figure 1 Showing habit of A. paniculata growing in pot and enlarged single flower
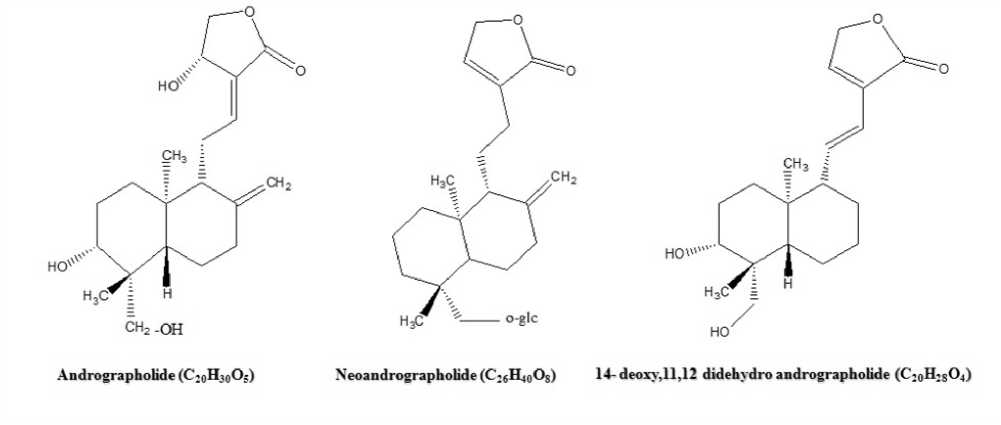
Figure 2 Showing the chemical structure of important biochemical components of A. paniculata
ABIOTIC
STRESS
Membrane injury
Growth reduction
Protein denaturation
Decreases photosynthesis Decreases nitrogen assimilation
Activity of photo enzymes and phytochemicals
Secondary metabolites
ROS Scavengers-S OD, CALAPX, POD
Unsaturated fatty acids _______
Figure 4 Showing mechanism of ROS scavenging by different antioxidant enzymes produced under abiotic stress
Figure 3 Showing abiotic stress induced effects and responses of A. paniculata
Molecular chaperone
_______HSP_______
НМВРР
GGPP
Pyruvic acid
Glvceraldehyde-3-phospha
Chloroplast
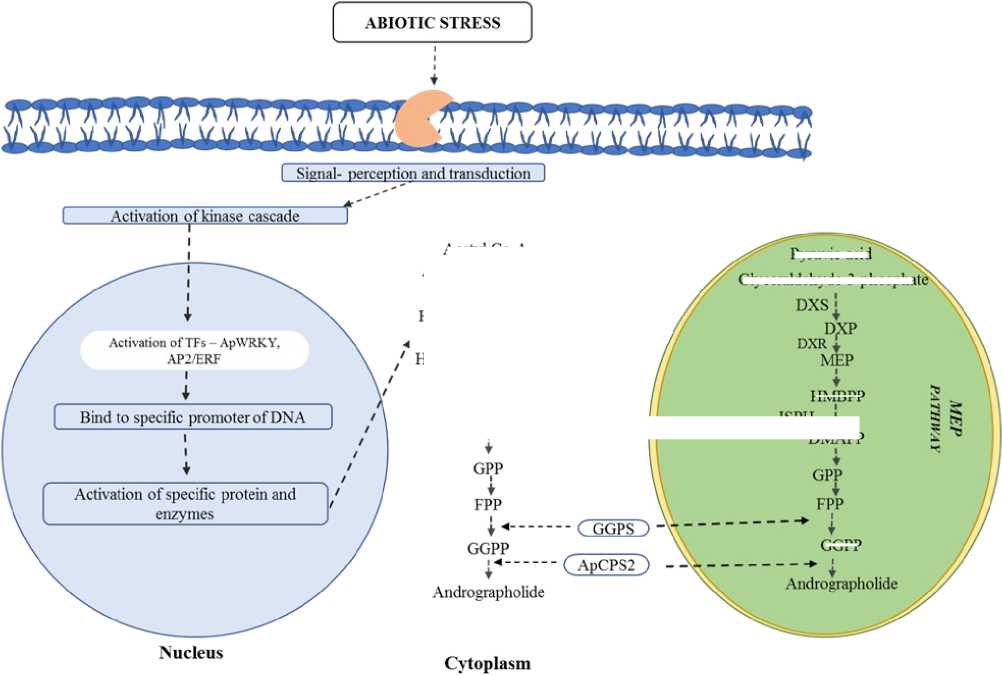
Figure 5 Mechanism of SMs production in response to abiotic stress in A. paniculata
Acetyl Co-A AACA I Acetoacetyl-Co-A
HMGS ;
HMG-Co-A
Mevalonic acid
+
IPP
DMAPP
ABIOTIC STRESS AS AN ELICITOR OFSECONDARY METABOLITES
Abiotic stresses are diverse types of adverse conditions that plants face in their life cycle. Several studies have been undertaken to use these stresses as elicitors of SMs production. Heavy metals have a positive role in the AG content in A. paniculata. The improved level of AG was observed in 50 mM Cu and Sn (Antony and Nagella, 2021). Among different salts of metal, 5 mM Cd has strong effect on AG production and is produced about five times compared to control. The order of AG biosynthesis in different salts was found to be CdCl2 > CuCl2 > AgNO3 > HgCl2 (Gandi et al., 2012). In a study conducted by Dawande and Sahay (2020), fourfold elevation in the AG content was reported after treatment with Cu at 100 to 500 μM concentrations. In A. paniculata, AG production was seven-fold increase in tissues when treated with Ag and three-fold overproduction of AG occur in seedlings treated with L- aspartic acid and methyl jasmonate (Das and Bandyopadhyay, 2020). Main terpenes NeoAG, DDAG AG, and flavonoid 5,7,2,3-tetra-methoxy-flavanone enhanced in leaves of A. paniculata treated with As (Das et al., 2021). Treatment of 0.01% sodium azide resulted in maximum amount of andrographonin (0.004%) and andrographolide (0.06%) (Chandran and Pillai, 2018). The concentration of total diterpene lactones (AG and dehydroandrographolide) was significantly increased with high sulphur at both high and low nitrogen levels. A higher quantitative concentration of total diterpene lactones was reported in leaves of A. paniculata (Jian et al., 2021).
In A. paniculata highest AG content (17,600 μg g-1) was observed in the 100 mM salt dose whereas maximum NeoAG content (12,600 μg g-1) was observed in 50 mM salinity treated plants (Kumar et al ., 2021). The sodium chloride (12 dS m-1) led to a significant increase in AG quantity up to 11.18%. AG content in A. paniculata varied from 1.48% to 2.55% (Talei et al .
2015). An enhancement in the quantity of anti-cancerous phytochemicals AG, NeoAG, and DDAG was reported in A. paniculata under salinity of 12 dSm-1. The tolerant accessions accumulate higher amount of AG and NeoAG than the sensitive accessions (Talei et al . 2013). In A. paniculata AG, deoxy-AG and dehydro-AG were increased significantly with moderate salt concentration 143.7 mM salt. The maximum rise in the quantities of AG, dehydroandrographolide and total lactone were reported at 143.7 mM were 31.5, 39.8, and 30.8% respectively. The quantity of deoxy-AG was maximum (60.7%) at 92.4 mM salinity than other salt concentrations. At 193.4 mM NaCl, there were no significant changes reported in bicycle terpenes (Shao et al., 2015).
AG content in leaves of A. paniculata increased (2.85-3.30%) under water-stressed plants (Saravanan et al. 2009). The effect of drought stress on A. paniculata is dependent on the quantity and time of water. AG content was significantly affected by prolonged treatment of drought. Severe and moderate drought-induced increase of AG, DDAG in A. paniculata has been reported by Chen et al . (2020).
Zaheer and Giri (2015) reported that the treatment of jasmonic acid (JA) and salicylic acid (SA) has enhancive effect on andrographolide content in A. paniculata . JA at 25 µM increases highest (3.3 fold) andrographolide than 1.0 µM (2.6 fold) and 50 µM (3.0 fold). SA at 10, 20, and 50 µM concentrations resulted in 3.0, 3.4, and 3.1 fold AG content, respectively (Zaheer and Giri, 2015). Among the various concentrations 2.5, 5.0, 10.0 μM of Methyl-jasmonate (MeJA) applied to A. paniculata , the accumulation of andrographolide was 1.86, 5.25, 2.1 fold higher after 24 hours of treatment. These observations reveal the stimulatory role of MeJA on the biosynthesis of andrographolide (Sharma et al. , 2015). In A. paniculata SA (0.05 mM) and chitosan influenced elicitation of andrographolide content when expose for different duration has been observed. In SA and chitosan treatment for 24 and 48 hours increases the andrographolide content 18.5 fold (37.0 μg g-1) and 59.5 fold (119.0 μg g-1), respectively (Vakil and Mendhulkar, 2013).
The maximum percentage 2.43% of the andrographolide was recorded in the leaves of sunlight-grown plants during the vegetative stage. It was also reported that the plants grown under sunlight conditions showed the highest concentration of the alkaloid compared to that of shade conditions (Kumar et al. , 2012). According to Purwanto et al . (2012) AG content increased under 50% shading level and straw compost fertilizer application.
In addition to stress, some PGRs are also studied as elicitor of secondary metabolite production. ABA and GA 3 enhances AG content in A. paniculata tissues 30.20 mg g-1 and 29.35 mg g-1, respectively (Anuradha et al. , 2010). According to Gudhate et al (2009) all the plant growth regulator treatments (GA 3 , IAA, NAA, and BAP) resulted in increased andrographolide content. Among the all PGRs, IAA 50 mg l-1 had a pronounced effect on the andrographolide content followed by NAA. GA3 and BAP showed slight increase in andrographolide content in A. paniculata (Gudhate et al ., 2009).
MECHANISM OF ENT-LABDANE DITERPENOIDS ELICITATION UNDER STRESS
Transcription factors (TFs), play important role in the regulation of plant secondary metabolism by regulating the expression of genes that encode biosynthetic enzymes at the transcriptional level (Hao et al ., 2020; Deng et al ., 2020). Genes liable for SMs synthesis in different plant species under stress are regulated by transcription factors such as WRKY, MYB, AP2/ERF, bZIP, and bHLH (Jan et al ., 2021). Among these TFs, WRKY and AP2/ERF have broadly participated in stress responses via regulation of plant SMs (Phukan et al ., 2016; Wasternack and Song, 2017; Jan et al ., 2021). The mechanism of TFs and subsequent gene expression response under abiotic stress is given in Fig. 4. In A. paniculata most WRKY genes are upregulated and WRKY transcripts induce in stem and root while depress in the leaves under stress (Wang et al. , 2021). According to Yao et al . (2020) AP2/ERF genes are expressed along with CPS2, which encodes key enzymes liable for the synthesis of active compounds AG of A. paniculata .
Plant SMs synthesized by the mevalonic acid pathway (MVAP) and methyl-ethritol-phosphate (MEP) pathway, occurs in the cytosol and plastid respectively, are used for the synthesis of central precursors of isoprenoids, isopentenyl diphosphate (IPP), and dimethylallyl diphosphate (DMAPP) (Lange et al ., 2001, Rodríguez-Concepción and Boronat, 2002) (Fig. 4).
The synthesis of AG correlates with the expression of regulatory genes such as HMGS, HMGR, DXS, DXR, GGPS and ISPH in association with MVAP and MEP pathways (Sharma et al . 2015). In A. paniculata , ent-copalyl-diphosphate synthase (ApCPS2) involve in ent-labdane-diterpens (ent-LRDs) synthesis process, it catalysed conversion of GGPP to ent-Copalyl-diphosphate(ent-CPP) (Misra et al ., 2015; Garg et al ., 2015; Das et al ., 2021). These gene expression levels are influenced by abiotic stress or elicitors and enhance SMs (ent-labdane-diterpens) elicitation directly or indirectly. Therefore, high transcript-level expression of these genes significantly coordinates to the elicitation of AG and other ent-LRDs under elicitors or abiotic stress (Ho et al ., 2020).
CONCLUSION AND FUTUREPROSPECTIVE
Abiotic stress is a major worldwide problem and is continuously increasing by anthropogenic activities. A. paniculata is a medicinal plant and has extensive possibilities of various compounds of medicinal purpose. The morphology, biochemistry, and physiology of A. paniculata alter under abiotic stress. Under environmental stress, the growth of A. paniculata reduces but high amount of secondary metabolites are produced. These metabolites play a significant role as a reservoir of key phytochemicals protecting plants against multiple environmental constraints. However, several reports are indicating the increased biosynthesis of SMs in A. paniculata under stress but the exact mechanism is yet to be studied. Using various combinations of stress can synthesize novel terpenoids and flavonoids of importance in medical science. Further, the effect of combined abiotic stresses on the growth performance and efficiency of SMs synthesis is not studied.
ACKNOWLEDGEMENT
Authors are thankful to MHRD, New Delhi for the financial help as RUSA 2.0 project. SKM is thankful to CSIR New Delhi for providing financial assistance in the form of SRF.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Abiotic stress-induced changes in antioxidative system and secondary metabolites production in Andrographis paniculata
- Agostini-Costa T.D.S., Vieira R.F., Bizzo H.R., Silveira D. and Gimenes M.A. (2012) Secondary Metabolites, Chromatography and Its Applications. IntechOpen, London, UK, 131-132.
- Akbar S. (2011) Andrographis paniculata: a review of pharmacological activities and clinical effects. Altern. Med. Rev., 16(1), 66-77.
- Akula R. and Ravishankar G.A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav., 6(11), 1720-1731.
- Antony A. and Nagella P. (2021) Heavy metal stress influence the andrographolide content, phytochemicals and antioxidant activity of Andrographis paniculata. Plant Sci. Today, 8(2), 324-330.
- Anuradha V.E., Jaleel C.A., Salem M.A., Gomathinayagam M. and Panneerselvam R. (2010) Plant growth regulators induced changes in antioxidant potential and andrographolide content in Andrographis paniculata Wall. ex Nees. Pestic Biochem. Physiol., 98(2), 312-316.
- Arora A., Sairam R.K. and Srivastava G.C. (2002) Oxidative stress and antioxidative system in plants. Curr. Sci., 82(10), 1227-1238. Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. plant boil., 50(1), 601639.
- Barton K.E. (2007) Early ontogenetic patterns in chemical defense in Plantago (Plantaginaceae): genetic variation and trade-offs. Am. J. Bot., 94(1), 56-66.
- Chandran N.A. and Pillai P.U. (2018) In vitro studies on the effect of sodium azide treatment on secondary metabolites production in Andrographis paniculata (Burm. F.) Nees. J. Pharmacogn. Phytochem., 7, 2411-2415.
- Chang H.M. and But P.P.H. (1987) Pharmacology and application of Chinese material medica. World Scientific, Singapore, 1, 918-928.
- Chen X., Xie Y., Wei K., Lan Z., Li C., Li Y. and Guo X. (2020) Drought stress enhanced andrographolides contents in Andrographis paniculata. Acta Ecol. Sin., 40(2), 113- 121.
- Paul S. (2012) Pharmacological potentials of Andrographis paniculata: an overview. Int. J. Pharmacol., 8(1), 6-9.
- Das D. and Bandyopadhay M. (2020) Novel approaches towards overproduction of andrographolide in in-vitro seedling cultures of Andrographis paniculata. S. Afr. J. Bot., 128, 77-86.
- Das P., Khare P., Singh R.P., Yadav V., Tripathi P., Kumar A., Pandey V., Gaur P., Singh A., Das R. and Hiremath C. (2021) Arsenic-induced differential expression of oxidative stress and secondary metabolite content in two genotypes of Andrographis paniculata. J. Hazard. Mater., 406, 124302.
- Dawande A.A. and Sahay S. (2020) Copper sulphate elicitation of optimized suspension culture of Andrographis paniculata Nees yields unprecedented level of andrographolide. J. Microbiol. Biotechnol. Food Sci., 9(4), 688-694.
- Demidchik V. (2014) Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot., 109, 212-228.
- Kai G. (2020) SmMYB2 promotes salvianolic acid biosynthesis in the medicinal herb Salvia miltiorrhiza. J. Integr. Plant Biol., 62(11), 1688-1702.
- Djanaguiraman M., Perumal R., Jagadish S.V.K., Ciampitti I.A., Welti R. and Prasad P.V.V. (2018) Sensitivity of sorghum pollen and pistil to high-temperature stress. Plant Cell Environ., 41(5), 1065-1082.
- Fang X., Yang C.Q., Wei Y.K., Ma Q.X., Yang L. and Chen X.Y. (2011) Genomics grand for diversified plant secondary metabolites. Plant Divers., 33(1), 53-64.
- Gandi S., Rao K., Chodisetti B. and Giri A. (2012) Elicitation of andrographolide in the suspension cultures of Andrographis paniculata. Appl. Biochem. Biotechnol., 168, 1729-1738.
- Garg A., Agrawal L., Misra R.C., Sharma S. and Ghosh S. (2015) Andrographis paniculata transcriptome provides molecular insights into tissue-specific accumulation of medicinal diterpenes. BMC Genom., 16(1), 1-16.
- George M. and Pandalai K.M. (1949) Investigations on plant antibiotics, Part IV. Further search for antibiotics substances in Indian medicinal plants. Indian J. Med. Res., 37, 169-181.
- Gouvea D.R., Gobbo-Neto L., Sakamoto H.T., Lopes N.P., Lopes J.L.C., Meloni F. and Amaral J.G. (2012) Seasonal variation of the major secondary metabolites present in the extract of Eremanthus mattogrossensis Less (Asteraceae: Vernonieae) leaves. Quim. Nova, 35(11), 2139-2145.
- Gudhate P.P., Lokhande D.P. and Dhumal K.N. (2009) Role of plant growth regulators for improving andrographolide in Andrographis paniculata. Pharmacogn. Mag., 5(19), 249.
- Gull A., Lone A.A. and Wani, N.U.I. (2019) Biotic and abiotic stresses in plants. Abiotic and biotic stress in plants, Intechopen, London, UK, 1-19.
- Hao X., Pu Z., Cao G., You D., Zhou Y., Deng C., Shi M., Nile S.H., Wang Y., Zhou W. and Kai G. (2020) Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res., 23, 1-12.
- Hartmann T. (2007) From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochem., 68, 28312846.
- Ho T.T., Murthy H.N. and Park S.Y. (2020) Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell Chowdhury A., Biswas S.K., Raihan S.Z., Das J., and Deng C., Wang Y., Huang F., Lu S., Zhao L., Ma X. and and organ cultures. Int. J. Mol. Sci., 21(3), 716.
- Huang D., Ou B. and Prior R.L. (2005) The chemistry behind antioxidant capacity assays. J. Agric. Food Chem., 53(6), 1841-1856.
- Hussain M., Farooq M. and Ashraf M.Y. (2008) Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci., 194(3), 193-199.
- Hussein R.A. and El-Anssary A.A. (2019) Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. J. Herb. Med., 1, 13.
- Ismail N.A., Hossain M.S., Mustafa N.H.M. and Phang C. (2015) Morpho-physiological characteristics, selected macronutrient uptake, and oxidative stress level of Andrographis paniculata under saline condition. J. Teknol., 77(24), 135-140.
- Jamwal K., Bhattacharya S. and Puri S. (2018) Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants, 9, 26-38.
- Jan R., Asaf S., Numan M. and Kim K.M. (2021) Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agron., 11(5), 968.
- Jian S.F., Huang X.J., Yang X.N., Zhong C. and Miao J.H. (2021) Sulfur regulates the trade-off between growth and andrographolide accumulation via nitrogen metabolism in Andrographis paniculata. Front. Plant Sci., 12.
- Joseph S.M. (2014) Scientific aspects of the therapeutic use of A. paniculata (kalmegh): A review. Int. J. Pharm. Sci. Rev. Res., 27(1), 10-13.
- Kaya M.D., Okcu G., Atak M., Cikili Y. and Kolsarici O. (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron., 24(4), 291295.
- Kranner I., Minibayeva F.V., Beckett R.P. and Seal C.E. (2010) What is stress? Concepts, definitions and applications in seed science. New Phytol., 188(3), 655-673.
- Kumar A. (2020) Inorganic Soil Contaminants and Their Biological Remediation. In Plant Responses to Soil Pollution. Springer, Singapore, 133-153.
- Kumar A. and Aery N.C. (2011) Effect of tungsten on growth, biochemical constituents, molybdenum and tungsten contents in wheat. Plant Soil Environ., 57(11), 519-525.
- Kumar A. and Aery N.C. (2012) Effect of tungsten on the growth, dry-matter production, and biochemical constituents of cowpea. Commun. Soil Sci. Plant Anal., 43(7),1098-1107.
- Kumar A. and Aery N.C. (2016) Impact, metabolism, and toxicity of heavy metals in plants. In Plant responses to xenobiotics. Springer, Singapore. 141-176.
- Kumar A., Rodrigues V., Verma S., Singh M., Hiremath C., Shanker K., Shukla A. and Sundaresan V. (2021) Effect of salt stress on seed germination, morphology, biochemical parameters, genomic template stability, and bioactive constituents of Andrographis paniculata Nees. Acta Physiol. Plant., 43(4), 1-14.
- Kumar R.N., Chakraborty S. and Kumar N. (2012) Influence of light and developmental stages on active principles of Andrographis paniculata (Burm.f.) Wall. ex Nees. Indian J. Sci., 3(1), 91-95.
- Kumar R.N., Chakraborty S. and Kumar N. (2009) Effect of light stress on peroxidase, succinate dehydrogenease and total chlorophyll content in Andrographis paniculata. Asian J. Environ. Sci., 4(1), 34-38.
- Kurepin L.V., Ivanov A.G., Zaman M., Pharis R.P., Hurry V. and Huner N.P. (2017) Interaction of glycine betaine and plant hormones: protection of the photosynthetic apparatus during abiotic stress. In Photosynthesis: Structures, mechanisms, and applications. Springer, Cham. New York, USA, 185202.
- Lange B.M., Ketchum R.E. and Croteau R.B. (2001) Isoprenoid biosynthesis. Metabolite profiling of peppermint oil gland secretory cells and application to herbicide target analysis. J. Plant Physiol., 127(1), 305-314.
- Lisar S.Y., Rahman I.M., Hossain M.M. and Motafakkerazad R. (2012) Water Stress in Plants: Causes, Effects and Responses. IntechOpen, London, UK.
- Mahajan S. and Tuteja N. (2005) Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys., 444(2), 139-158.
- Matsuda T., Kuroyanagi M., Sugiyama S., Umehara K., Ueno A. and Nishi K. (1994) Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull., 42, 1216-1225.
- Plant review Andrographis paniculata (Kalmegh): A review. Pharmacogn. Rev., 1(2), 283-298.
- Mittler R. (2006) Abiotic stress, the field environment and stress combination. Trends Plants Sci., 11(1), 15-19.
- Morison J.I.L. and Lawlor D.W. (1999) Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ., 22(6), 659-682.
- Niranjan A., Tewari S. and Lehri A. (2010) Biological activity of kalmegh (Andrographis paniculata Nees) and its active principles- A review. Indian J. Nat. Prod. Resour., 1(2), 125-135.
- Noctor G. and Foyer C.H. (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Boil., 49(1), 249-279.
- Nonami H. (1998) Plant water relations and control of cell elongation at low water potentials. J. Plant Res., 111(3), 373-382.
- Patil R. and Jain V. (2021) Andrographolide: A review of analytical methods. J. Chromatogr. Sci., 59(2), 191203.
- Phukan U.J., Jeena G.S. and Shukla R.K. (2016) WRKY transcription factors: molecular regulation and stress responses in plants. Front. Plant Sci., 7, 760.
- Prajapati N.D., Purohit S.S., Sharma A.K. and Kumar T. (2003) A handbook of medicinal plants. Agrobios, Jodhpur (India).
- Purwanto E., Samanhudi S. and Sudarmi S. (2012) Studies of shading levels and nutrition sources on growth, yield and andrographolide content of Sambiloto (Andrographis paniculata Ness). AGRIVITA, J. Agric. Sci., 33(3), 300-306.
- Rafi M., Devi A.F., Syafitri U.D., Heryanto R., Supart I.H., Amran M.B., Rohman A., Prajogo B. and Lim L.W. (2020) Classification of Andrographis paniculata extracts by solvent extraction using HPLC fingerprint and chemometric analysis. BMC Res. Notes, 13(1), 1-6.
- Sandborn W.J., Targan S.R., Byers V.S., Rutty D.A., Mu H., Zhang X. and Tang T. (2013) Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol., 108(1), 90.
- Saravanan R., Khristi S., Gajbhiye N.A. and Maiti S. (2009) Effect of plant population and soil moisture stress on herbage yield and andrographolide content in Andrographis paniculata. Indian J. Hortic., 66(1), 120-125.
- Sareer O., Ahmad S. and Umar S. (2014) Andrographis paniculata: a critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat. Prod. Res., 28, 2081-2101.
- Shao Y.H., Gao J.L., Wu X.W., Li Q., Wang J.G., Ding P. and Lai X.P. (2015) Effect of salt treatment on growth, isoenzymes and metabolites of Andrographis paniculata (Burm. f.) Nees. Acta Physiol. Plant., 37(2), 1-12.
- Sharma B.K. and Jain A.K. (2015) Studies on Some Aspects of Reproductive biology of Andrographis paniculata (Acanthaceae). Int. J. Plant Reprod. Biol., 7(2), 153-158.
- Sharma S., Sharma Y.P. and Bhardwaj C. (2018) HPLC quantification of andro-grapholide in different parts of A. paniculata (Burm.F.) wall. Ex Nees. J. Pharmacogn. Phytochem., 7(3), 168-171.
- Siripong P., Kongkathip B., Preechanukool K., Picha P., Tunsuwan K. and Taylor W.C. (1992). Cytotoxic diterpenoid constituents from Andrographis paniculata, Nees leaves. J. Sci.Soc. Thail., 18(4), 187-194.
- Song Y.X., Liu S.P., Jin Z., Qin J.F. and Jiang Z.Y. (2013) Qualitative and quantitative analysis of Andrographis paniculata by rapid resolution liquid chromatography/time-of-flight mass spectrometry. Molecules, 18(10), 12192-12207.
- Subramanian R., Asmawi M.Z. and Sadikun A. (2012) A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem. Rev., 11(1), 39-75.
- Takahashi F. and Shinozaki K. (2019) Long-distance signaling in plant stress response. Curr. Opin. Plant Biol., 47, 106-111.
- Talei D., Valdiani A., Maziah M., Sagineedu S.R. and Abiri R. (2015) Salt stress-induced protein pattern associated with photosynthetic parameters and andrographolide content in Andrographis paniculata Nees. Biosci. Biotechnol. Biochem., 79(1), 51-58.
- Talei D., Valdiani A., Maziah M., Sagineedu S.R. and Saad M.S. (2013) Analysis of the Anticancer Phytochemicals in Andrographis paniculata Nees. under Salinity Stress. Biomed Res. Int., 2013. Article ID 319047.
- Trivedi N.P. and Rawal U.M. (2001) Hepatoprotective and antioxidant property of Andrographis paniculata (Nees) in BHC induced liver damage in mice. Indian J. Exp. Biol., 39(1), 41-46.
- V., Gupta S.K. and Bhat S.V. (2012) Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem., 56, 368374.
- Vakil M.M.A. and Mendhulkar V.D. (2013) Salicylic acid and chitosan mediated abiotic stress in cell suspension culture of Andrographis paniculata (Burm. f.) Nees. for andrographolide synthesis. Int. J. Pharm. Sci. Res., 4(9), 3453.
- Verma N. and Shukla S. (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants, 2(4), 105-113.
- Verma S., Nizam S. and Verma P.K. (2013) Biotic and abiotic stress signalling in plants. Stress Signaling in Plants: Genomics and Proteomics Perspective, Springer Science, New York, USA, 1, 25-49.
- Wang Q., Zeng W., Ali B., Zhang X., Xu L. and Liang Z. (2021) Genome-wide identification of WRKY gene family and expression analysis under abiotic stresses in Andrographis paniculata. Biocell, 45(4), 1107.
- Wasternack C. and Song S. (2017) Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot., 68(6), 1303-1321.
- Xie X., He Z., Chen N., Tang Z., Wang Q. and Cai Y. (2019) The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res. Int., 2019, Article ID 9732325.
- Yaghoubian Y., Goltapeh E.M., Pirdashti H., Esfandiari E., Feiziasl V., Dolatabadi H.K., Verma A. and Hassim M.H. (2014) Effect of Glomus mosseae and Piriformospora indica on growth and antioxidant defense responses of wheat plants under drought stress. J. Agric. Res., 3(3), 239-245.
- Yao W., An T., Xu Z., Zhang L., Gao H., Sun W., Liao B., Jiang C., Liu Z., Duan L. and Ji A. (2020) Genomic-wide identification and expression analysis of AP2/ERF transcription factors related to andrographolide biosynthesis in Andrographis paniculata. Ind. Crops Prod., 157, 112878.
- Yao X.J., Wainberg M.A. and Parniak M.A. (1992) Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virol., 187(1), 56-62.
- Zaefyzadeh M., Quliyev R.A., Babayeva S.M. and Abbasov M A. (2009) The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces.Turk. J. Boil., 33(1), 1-7.
- Zaheer M. and Giri C.C. (2015) Multiple shoot induction Uttekar M.M., Das T., Pawar R.S., Bhandari B., Menon and jasmonic versus salicylic acid driven elicitation for enhanced andrographolide production in Andrographis paniculata. Plant Cell, Tissue Organ Cult., 122(3), 553-563.
- Zandalinas S.I., Mittler R., Balfagón D., Arbona V. and Gómez-Cadenas A. (2018) Plant adaptations to the combination of drought and high temperatures. Physiol. Plant., 162(1), 2-12.


