Achillea aleppica and Achillea arabica (Asteraceae) phytochemical FT-IR analysis
Автор: Saleh B.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.20, 2024 года.
Бесплатный доступ
Achillea genus is a flowering, aromatic and perennial herbs, and widespread all over the world. Plants phytochemical analysis has a capital role in pharmaceutical and medicine preliminary studies. Two medicinal Achillea species of A. aleppica DC and A. arabica Kotschy grown wild in the Middle-Southern regions of Syria were investigated for their phytochemical analysis using fourier-transform infrared (FT-IR) spectroscopy. FT-IR spectra highlighted 11 and 12 functional groups characteristics for A. aleppica and A. arabica species, respectively; of which 9 were common between the two studied species [Aromatics (1), Ethers (3), Carboxylic acids (2), Olefinic (alkene) (1), Alkanes (1) and Aliphatic primary amine (1) groups]. These constituents have been previously investigated for different biological activities. The current study could consider as the first report regarding FT-IR Achillea analysis.
Achillea aleppica, achillea arabica, phytochemical analysis, ftir
Короткий адрес: https://sciup.org/143182395
IDR: 143182395
Текст научной статьи Achillea aleppica and Achillea arabica (Asteraceae) phytochemical FT-IR analysis
Achillea genus belongs to Asteraceae family the largest angiosperms’ family that involves approximately 1500 genera and 23000 species, distributed in three subfamilies and seventeen tribes. This genus included 115 species of perennial herbs; all of them are native to temperate regions of the northern hemisphere (Heywood, 1998; Moradkhani et al. , 2012).
Plants phytochemical analysis using different analytical methods has a capital role in pharmaceutical and medicine studies for plants essential oils in particularly and crude extracts analysis. Among them, ultraviolet (UV), infrared spectroscopy (I ) and mass spectrometry (MS) (Stojanović et al. , 2005; Moradkhani et al. , 2014); nuclear magnetic resonance (NM ) (Moradkhani et al. , 2012; 2014); fourier transform infrared spectroscopy (FTI ) (Dev et al. , 2017); gas chromatography-mass spectrometry (GC-MS) (Dev et al. , 2017; Albayrak and Silahtarlıoğlu, 2020; Saleh, 2019); high-performance liquid chromatography (HPLC) (Albayrak and Silahtarlıoğlu, 2020); high-performance liquid chromatography with diode array detector (HPLC-DAD)( Şabanoğlu et al. , 2019) and recently, fourier transform infrared photoacoustic spectroscopy (FTI -PAS) and diffuse reflectance infrared spectroscopy (FTI -D IFT) (Brangule et al. , 2020) and others.
Achillea species exhibited wide range in medicine and pharmaceutical applications; e.g . as antimicrobial (Stojanović et al. , 2005; Toncer et al. 2010; Tabanca et al. , 2011; Albayrak and Silahtarlıoğlu, 2020); antioxidant (Toncer et al. , 2010; Manayi et al. , 2012; Polatoglu et al. , 2013; Albayrak and Silahtarlıoğlu, 2020); insecticidal (Toncer et al. , 2010; Tabanca et al. , 2011; Polatoglu et al. , 2013); herbicidal (Toncer et al. , 2010; Polatoglu et al. , 2013); cytotoxic (Albayrak and Silahtarlıoğlu 2020); antinociceptive and anti-inflammatory (Toncer et al. , 2010) properties. Moreover, they used in traditional remedies against rheumatic pain and digestive complaints, fever, common cold, pneumonia and hemorrhage (Manayi et al. , 2012). Başer (2016) reviewed 31 Achillea species for their essential oil yield and composition.
The genus Achillea is represented in Syrian Flora with about 9 species (Mouterde, 1983), of which A. aleppica DC and A. arabica Kotschy (Synonyms. Achillea biebersteinii Afanasiev) were wild grown in Syria.
No information available regarding the two previous Achillea species in respect to their phytochemical analysis in Syria. Thereby, the current study aimed to study their phytochemical analysis using FT-I spectra for the first time.
MATERIALS AND METHODS
Plant materials and samples preparation
Aerial parts of A. aleppica (A.A) and A. arabica (A. ) (10 plants for each species) were harvested and bulked as representative for each Achillea sp. Sampling has been performed during blooming stage from two wild Achillea species grown in their natural habitat from Middle-Southern regions in Syria. Achillea aleppica DC was collected from rural Damascus regions; whereas, Achillea arabica Kotschy was collected from rural Homs regions (Table 1).
Samples were shade dried for two weeks, and were milled to fine powder by special electric mill and stored separately in glass bowls until FT-I analysis.
FT-IR assay
The fine powder was used as template for FT-I analysis in the wavenumber range of 4000-500 cm-1. I measurement has been performed using NX FTI (Thermo, USA) instrument for FT-I analysis.
RESULTS AND DISCUSSION
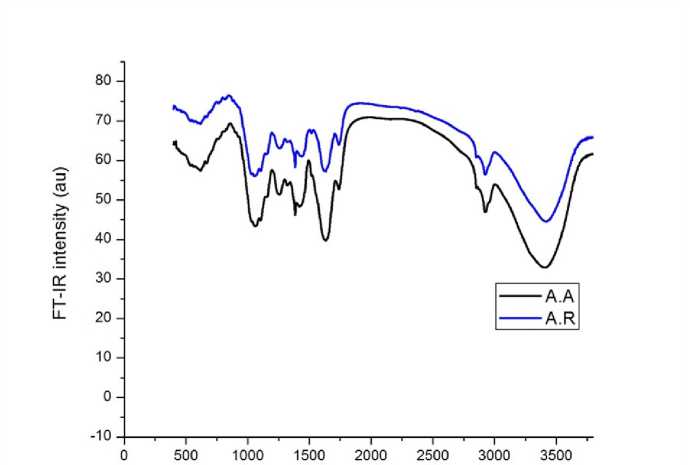
Phytochemical analysis of wild A. aleppica and A. arabica medicinal species grown in the Middle-Southern regions of Syria, has been assessed using FT-I spectroscopy. FT-I spectra of the A. aleppica and A. arabica aerial parts was presented in Figure 1. FT-I analysis showed 11 and 12 functional groups characteristics for A. aleppica and A. arabica species, respectively; of which 9 were commonly sharing between the two studied species (Table 2). These common peaks were: 800 cm-1 (assigned to =C-H oop bend-Aromatics group); 1080, 1100 and 1700 cm-1 (assigned to C–O secondary alcohol stretch C–O stretch-Ethers group), 1200 and 1300 cm-1 (assigned to C–O stretch-Carboxylic acids group), 1630 cm-1
[(assigned to Alkenyl C=C stretch-Olefinic (alkene) group], 2924 cm-1 (assigned to C–H stretch –Alkanes group) and 3400 cm-1 (assigned to NH stretch-Aliphatic primary amine group).
Saeidnia et al. (2011) reviewed the Achillea phytochemistry and reported that terpenoids (monoterpenes, sesquiterpenes, diterpenes, triterpenes), phenolic acids, flavonoids, coumarins and sterols have been frequently reported as secondary metabolites in Achillea species.
Başer (2016) reported the presence of camphor, 1,8-cineole, p-cymene, α-pinene, α-terpineol, α-bisabolol oxide, T-cadinol, caryophyllene oxide and spathulenol as a main components in A. aleppica essential oils.
Different researches focused on A. arabica Kotschy (Synonyms. Achillea biebersteinii Afanasiev) essential oils (EOs) composition; e.g . Tabanca et al. (2011) reported the presence of camphor, 1,8-cineole, borneol, p-cymene and piperitone in its EOs. Whereas, these components were camphor, 1,8-cineole, piperitone, p-cymene and ascaridol (Toncer et al. , 2010). Moreover, Polatoglu et al. (2013) reported that camphor, 1,8-cineole, α-thujone, p-cymene, ß-thujone, borneol and piperitone were presented in its EOs. Whereas, Şabanoğlu et al. (2019) reported the analyzed of some phenolic components, e.g. gallic acid, chlorogenic acid, caffeic acid, ferulic acid, p -coumaric acid, rutin, quercetin, luteolin, apigenin and kaempferol in three methanolic Achillea species ( A. biebersteinii, A. setacea and A. wilhelmsii leaf, flower and root) extracts using HPLC-DAD analysis.
Other researches focused on phytochemical analysis of other Achillea species; e.g. Stojanović et al. (2005) reported the presence of alkanes, fatty acids, monoterpenes, guaiane sesquiterpenes (rupicolin A and B, 1-deoxy-1alpha-peroxy-rupicolin A and B), and flavonoids (apigenin and centaureidin) in A. clavennae (hexane:ether:methanol=1:1:1) extract using different spectral analyses (1D and 2D NM , UV, I and MS). Whereas, Manayi et al. (2012) reported the presence of sterols, tannins and terpenoids in methanolic A. tenuifolia root extract; whereas, sterols and terpenoids were presented in its Ethyl acetate one in preliminary test. Whereas, Moradkhani et al. (2012) reported for the first time the presence of β-sitosterol (compound1), 5-hydroxy, 4',6,7– trimethoxy flavone (salvigenin compound 2), and methyl-gallate (compound 3) in dichloromethane fraction A. tenuifolia using NM and Mass spectral analyses. Moreover, Moradkhani et al. (2014) reported for the first time the presence of two known flavones 3', 5- dihydroxy- 4', 6, 7- trimethoxy flavone (eupatorine, compound 3), 5- hydroxy- 3',4', 6, 7- tetramethoxyflavone (compound 4), besides stearic acid (compound 1), lupeol (compound 2), daucosterol (β- sitosterol 3-O- β- D- glucopyranoside, compound 5), 2, 4- dihydroxy methyl benzoate (compound 6) in the methanolic A. tenuifolia extract using UV, I , Mass and 1H- NM (1D and 2D) and 13C-NM .
More attention has been given to A. millefolium phytochemical analysis; e.g. Dias et al. (2013) reported higher content of fat and saturated fatty acids, proteins, ash, energy value, sugars and flavonoids in commercial methanolic A. millefolium (yarrow) compared to wild one. Whereas, the wild type showed higher carbohydrates, organic acids, unsaturated fatty acids, tocopherols and phenolic acids content compared to cultivated one, using HPLC analysis. Whereas, Georgieva et al. (2015) investigated phytochemicaly (phenolic acids, flavonoid aglycones and flavonoid glycosides) the aqueous A. millefolium inflorescences and upper leaves extracts using HPLC analysis.
Table 1 : Collection sites of A. aleppica and A.arabica species
|
Species |
Collection site |
Code |
Altitude (m) |
Annual rainfall (mm) |
|
A. aleppica |
Damascus |
A.A |
950 |
260 |
|
A. arabica |
Homs |
A. |
265 |
400 |
FT-IR shift (cm1)
Figure 1. :FT-I spectra of A. aleppica (A.A) and A.arabica (A.R) species.
Table 2: Observed functional groups identified in A. aleppica and A.arabica species usingFT-I analysis
|
Species |
Peak N° |
I frequency (cm-1) |
Observed I (cm-1) |
Bond |
Functional groups |
|
A. aleppica |
1 |
700-600 |
617 |
Aliphatic bromo compounds, C–Br stretch |
Aliphatic organohalogen |
|
2 |
900-690 |
800 |
=C-H oop bend |
Aromatics |
|
|
3 |
2000-1000 |
1080 |
C–O secondary alcohol stretch C–O stretch |
Ethers |
|
|
4 |
2000-1000 |
1100 |
C–O secondary alcohol stretch C–O stretch |
Ethers |
|
|
5 |
1300-1200 |
1200 |
C–O stretch |
Carboxylic acids |
|
|
6 |
1300-1200 |
1300 |
C–O stretch |
Carboxylic acids |
|
|
7 |
1600-1400 |
1417 |
C=C stretch aromatic |
Aromatics |
|
|
8 |
1680-1620 |
1630 |
Alkenyl C=C stretch |
Olefinic (alkene) |
|
|
9 |
2000-1000 |
1700 |
C–O secondary alcohol stretch C–O stretch |
Ethers |
|
|
10 |
2970-2850 |
2924 |
C–H stretch |
Alkanes |
|
|
11 |
3400-3380 |
3400 |
NH strech |
Aliphatic primary amine |
|
|
A. arabica |
1 |
900-690 |
810 |
=C-H oop bend |
Aromatics |
|
2 |
2000-1000 |
1080 |
C–O secondary alcohol stretch C–O stretch |
Ethers |
|
|
3 |
2000-1000 |
1100 |
C–O secondary alcohol stretch C–O stretch |
Ethers |
|
|
4 |
1300-1200 |
1200 |
C–O stretch |
Carboxylic acids |
|
|
5 |
1300-1200 |
1300 |
C–O stretch |
Carboxylic acids |
|
|
6 |
1600-1400 |
1400 |
C=C stretch aromatic |
Aromatics |
|
|
7 |
1600-1400 |
1515 |
C=C stretch aromatic |
Aromatics |
|
|
8 |
1680-1620 |
1630 |
Alkenyl C=C stretch |
Olefinic (alkene) |
|
|
9 |
2000-1000 |
1700 |
C–O secondary alcohol stretch C–O stretch |
Ethers |
|
|
10 |
2970-2850 |
2924 |
C–H stretch |
Alkanes |
|
|
11 |
3400-3380 |
3400 |
NH strech |
Aliphatic primary amine |
|
|
12 |
3645-3630 |
3645 |
primary alcohol, OH stretch |
Alcohol and hydroxyy compound |
CONCLUSION
Phytochemical analysis of A. aleppica and A. arabica species grown wild in Syria was investigated using FTI assay. FT-I spectra highlighted 11 and 12 functional groups characteristics for A. aleppica and A. arabica species, respectively; of which 9 were common between the two studied species. Whereas, Aliphatic bromo compounds, C–Br stretch-Aliphatic organohalogen functional group was observed in A. aleppica and not in A. arabica species. While, primary alcohol, OH stretch-Alcohol and hydroxy compound functional group was observed in A. arabica and not in A. aleppica species. These constituents have been previously investigated for different biological activities. The current study could consider as the first report regarding FT-I Achillea analysis.
ACKNOWLEDGMENTS
The author would like to thank Prof. Othman I, Director General of AECS and Prof. MirAli N, Head of Molecular Biology and Biotechnology Department for their support, and also to the Plant Biotechnology group for technical assistance.
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
Список литературы Achillea aleppica and Achillea arabica (Asteraceae) phytochemical FT-IR analysis
- Albayrak S, Silahtarlioglu N. (2020). Determination of biological activities of essential oil and extract obtained from Achillea coarctata Poir. Advances in Traditional Medicine. 20: 77-88. Baçer KHC. (2016). Essential oils of Achillea species of
- Turkey. Nat. Volatiles & Essent. Oils, 3(1): 1-14. Brangule A, Sukele R, Bandere D. (2020). Herbal medicine characterization perspectives using advanced FTIR sample techniques - diffuse reflectance (DRIFT) and photoacoustic spectroscopy (PAS). Front. Plant Sci, 11: 356.
- Dev P, Ramappa VK, Gopal R, Sangeeta. (2017). Analysis of chemical composition of Mulberry Silkworm pupal oil with fourier transform infrared spectroscopy (FTIR), gas chromatography/mass Spectrometry (GC/MS) and its antimicrobial property. Asian Jl Agri Res. 11: 108-115.
- Dias MI, Barros L, Dueñas M, Pereira E, Carvalho AM, Alves RC, Oliveira MBPP, Santos-Buelga C, Ferreira ICFR. (2013). Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Comparative Study Food Chem. 141(4): 4152-4160.
- Georgieva L, Gadjalova A, Mihaylova D, Pavlov A. (2015). Achillea millefolium L. - phytochemical profile and in vitro antioxidant activity. Int Food Res J. 22(4): 1347-1352.
- Heywood VH. (1998) Flowering Plants of the World. BT Batsford Ltd, England, 263-268.
- Manayi A, Mirnezami T, Saeidnia S, Ajani Y. (2012). Pharmacognostical evaluation, phytochemical analysis and antioxidant activity of the roots of Achillea tenuifolia LAM. Pharm J. 4 (30): 14-19.
- Moradkhani S, Ayatollahi AM, Ghanadian M, Moin MR, Razavizadeh M, Shahlaei M. (2012). Phytochemical analysis and metal-chelation activity of Achillea tenuifolia Lam. Iranian J Pharm Res, 11 (1): 177-183.
- Moradkhani S, Kobarfard F, Ayatollahi SA. (2014). Phytochemical investigations on chemical constituents of Achillea tenuifolia Lam. Iranian J Pharma Res. 13(3): 1049-1054.
- Mouterde P. (1983). Nouvelle Flore du Liban et de la Syrie. Dar El- Machreck, Beyrouth. Vol 3, pp. 398402.
- Polatoglu K, Karakoc OC, Goren N. (2013). Phytotoxic, DPPH scavenging, insecticidal activities and essential oil composition of Achillea vermicularis, A. teretifolia and proposed chemotypes of A. biebersteinii (Asteraceae). Ind Crops Prod. 51: 3545.
- Çabanoglu S, Gökbulut A, Altun ML. (2019). Characterization of phenolic compounds, total phenolic content and antioxidant activity of three Achillea species. J Res Pharm. 23(3): 567-576.
- Saeidnia S, Gohari AR, Mokhber-Dezfuli N, Kiuchi F. (2011). A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 19(3): 173186.
- Saleh B. (2019). Volatile constituents of three Hypericum (Hypericaceae) species using GC-MS analysis. Int J Pharm Life Sci. 10(11-12): 63496354.
- Stojanovic G, Radulovic N, Hashimoto T, Palic R. (2005). In vitro antimicrobial activity of extracts of four Achillea species: the composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol. 101(1-3): 185-190.
- Tabanca N, Demirci B, Gurbuz I, Demirci F, Becnel JJ, Wedge DE, Baser KHC. (2011). Essential oil composition of five collections of Achillea biebersteinii from central Turkey and their antifungal and insecticidal activity. Nat Prod Comm. 6(5): 701-706.
- Toncer O, Basbag S, Karaman S, Diraz E, Basbag M. (2010). Chemical composition of the essential oils of some Achillea species growing wild in Turkey. Int J Agri Biol. 12(4): 527-530.


