Acrylamide toxicity in processed foods: a growing public health concern
Автор: Lende P. W., Harinkhede P. S., Marbate V. D., Dhurvey V. T., Nagwanshi A. M., Shahare S. S., Sharma A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.21, 2025 года.
Бесплатный доступ
The rising demand for processed foods, driven by population growth and urbanization, has heightened concerns about food safety, particularly regarding acrylamide (ACR), a compound formed during high-temperature processing of carbohydrate-rich foods. Originally identified as an industrial neurotoxicant, ACR is now recognized for its carcinogenic, neurotoxic, genotoxic properties and reproductive toxicity, making its presence in fried, baked, and roasted foods, is a significant public health concern. This review explores acrylamide formation, dietary sources, and harmful effects on organs like liver, intestine and gonads, including its effects on oxidative stress, inflammation, and DNA damage, as revealed by experimental studies. Emphasizing its health risks, this work highlights the urgent need for innovative effective mitigation strategies to reduce ACR levels in processed foods, ensuring consumer safety without compromising the sensory and nutritional quality of processed foods.
Processed food, food safety, acrylamide, high temperature, carcinogenic, neurotoxic, genotoxic, reproductive toxicity, health risk, mitigation, nutritional quality
Короткий адрес: https://sciup.org/143184735
IDR: 143184735
Текст обзорной статьи Acrylamide toxicity in processed foods: a growing public health concern
Food is the component required for physiological process such as proper growth, metabolic processes and survival. With increase in population and urbanization, the demand for variety of foods, including both nutritious as well as indulgent food is also increasing. The children’s and modern-day people are more exposed to indulgent foods, which are often rich, flavourful, and appealing, commonly contain various types of additives to enhance their taste, texture, appearance, and shelf life
Processing toxicants are substances formed during food processing or preparation that can have adverse physiological effects on human health. Ingredients used in food formulations can undergo chemical reactions under processing conditions such as heat, irradiation, or fermentation. The reaction products formed can be beneficial or harmful, depending on the conditions and processes used (Lineback and Stadler, 2009).
ACR was first discovered in 1997 due to a tunnel leakage in Sweden, where tunnel workers developed signs of impaired nerve function (Spencer and Schaumburg, 1974; Exon, 2006). The presence of ACR haemoglobin adducts suggested the possibility that ACR might also be consumed through the diet (Reynold, 2002; Timilsena et al., 2010 ). In 2002, Swedish researchers reported the presence of ACR in several heat-treated carbohydrate-rich foods at significantly higher levels than other well-known food carcinogens (Tareke et al., 2002; Koszucka et al., 2019).
It has been placed in Group 2A carcinogen according to International Agency for Research on Cancer (IARC) specifying ACR as probably carcinogenic to humans based on previous experiment, studies and limited evidence in humans (Stadler et al., 2002; Tareke et al., 2002; Larguinho et al., 2014, Capuano and Fogliano, 2011; Parzefall, 2008; Tan et al., 2013) .
ACR (2 - propenamide) is a colorless, odourless, non-volatile, unsaturated amide with a molecular weight of approximately 71.08 kDa and it is found to be soluble in water and other polar solvents (acetone, methanol, ethanol) but is nearly insoluble in nonpolar solvents like carbon tetrachloride. It has high boiling point of about
125 °C (25 mm Hg), and melting point is at 84.5 °C, (Erkekoğlu and Baydar, 2010; Timilsena et al., 2009; Krishnakumar and Visvanathan, 2014; Zamani et al., 2017).
Effect on Liver
Histology enables the observation of tissue and any distinctive alterations the tissue might have experienced. This helps to determine adverse effects in multiple organs of the model organism (Larguinho et al., 2014). To evaluate how ACR influences biochemical responses and diagnose any alteration, serum concentrations of various analytes are measured. (Wolf, 2018; Larguinho et al., 2014). Liver plays a key role in many metabolic processes, and liver injury disrupts these functions.
Effect of ACR on Histology of Liver
Acrylamide (ACR) exposure has been shown to induce dose-dependent hepatotoxicity across various aquatic and terrestrial species. Ibrahim et al., (2019) reported progressive liver damage in Clarias gariepinus exposed to 20–80% of the 96-hour LC50 (133 mg/L), ranging from mild necrosis and vessel congestion to extensive necrosis and cytoplasmic destruction at higher doses. Similarly, Kilicle et al., (2020) observed sinusoid vasodilatation, nuclear anomalies, and severe fibrosis in Capoeta capoeta following intraperitoneal ACR administration (10–30 mg/L). In Carassius auratus , Larguinho et al., (2013) found eosinophilic alterations, necrosis, and inflammatory responses at concentrations up to 150 mg/L. Supporting these findings in mammals, Faridi et al., (2024) showed that rats treated with 2 mg/kg ACR over four weeks developed significant hepatocellular damage, including necrosis, vacuolar degeneration, and pyknotic nuclei
Biochemical Effects of ACR on Liver
Acrylamide (ACR) exposure has been linked to oxidative stress and metabolic disruption in various models. Ibrahim et al. (2019) reported increased malondialdehyde (MDA) levels and reduced GSH, SOD, and TAC in Clarias gariepinus, indicating lipid peroxidation and impaired antioxidant defenses. Kim et al., (2014) found that zebrafish exposed to 150–300 ppm ACR showed elevated GOT and GPT levels, along with increased serum glucose and triglycerides, reflecting liver damage and metabolic disturbances. In rats, Yalçin et al. (2020) observed reduced TAS, increased TOS, and heightened caspase-3 activity, suggesting oxidative stress and hepatocyte apoptosis. Similarly, Mallepogu et al. (2023) reported that ACR-treated chick embryos exhibited elevated ALT, AST, and ALP levels with reduced glutathione, further confirming oxidative liver damage.
Effects of ACR On Gene Expression in Liver
In a study conducted by Ibrahim et al., (2019) where fish were exposed to ACR at various concentrations of the 96-hour LC50 value (133 mg/L) for 2 weeks. According to this study genotoxicity was evident through DNA fragmentation assays, which showed dosedependent increases in DNA fragmentation in the liver, indicating significant DNA damage. Accordingly, a study by Sheikh et al. (2010) exposed fertilized chick embryos to single doses of acrylamide (0.1 mg, 0.2 mg, and 0.3 mg in 50 µL saline) showed a dose-dependent induction of GST isoforms, including theta (CL1), mu (CL2), and alpha (CL3) classes, demonstrating an adaptive upregulation of detoxification pathways in response to acrylamide exposure. In another work by Chen et al., (2021), acrylamide was administered orally to mice at doses of 50–75 mg/kg/day for 30 days, this showed upregulation of cytochrome P450-related genes, cancer-related genes (e.g., EGFR and Rad51), and inflammatory markers, while anti-oncogenes such as Bcl2 and P21 were downregulated. Kandemir et al., (2020) carried his research by administering acrylamide at doses of 50 and 100 mg/kg body weight for a duration of 14 days in rats. Acrylamide affected gene expression by upregulating pro-apoptotic markers, including P53, which suggests enhanced apoptosis in liver cells.
Effects On Intestine
Histological, Biochemical and Neurochemical biomarkers provide valuable data on specific organ toxicity, particularly in organs like the intestine, which are directly involved in the processing of food and toxins. Studies using these biomarkers help quantify the exposure to pollutants and elucidate the mechanisms of toxicity, providing insights into how toxicants like acrylamide affect the organism's physiological functions (Samanta et al., 2016). The intestines play a key role in the metabolism of orally ingested toxic substances, making them a critical organ for evaluating the impact of acrylamide in organisms (Van Dyk et al., (2009).
Effect of ACR on Histology of Intestine
Acrylamide (ACR) exposure has been shown to cause significant gastrointestinal toxicity in both aquatic and mammalian models. Modi et al. (2022) reported that zebrafish exposed to 1.22 mM ACR for 21 days experienced early mortality and intestinal damage, including goblet cell hyperplasia and enterocyte exfoliation. In rats, Ige et al. (2022) observed increased intestinal transit time, reduced mucosal cell counts, and severe duodenal lesions such as villi sloughing, crypt disruption, and inflammation following 28-day ACR exposure (7.5 and 15 mg/kg). Similarly, Koledin et al., (2016) found that subchronic ACR treatment (25–50 mg/kg/day) in juvenile rats disrupted mucin production, notably reducing neutral, acidic, and MUC2 mucins, leading to compromised mucosal integrity. Sayyad et al., (2017) also reported damage to the duodenal lining, villi, and lingual papillae in rats exposed to ACR and fried potato chips, along with evidence of DNA damage in intestinal and lingual tissues.
Biochemical Effects of ACR on Intestine
Acrylamide (ACR) exposure has been shown to induce significant neurochemical and inflammatory changes in the gastrointestinal tract. Palus et al., (2019) demonstrated that juvenile pigs exposed to ACR at 0.5 and 5 µg/kg/day for 28 days exhibited increased immunoreactivity to SP, CGRP, GAL, and VAChT in ileal and duodenal enteric neurons, with reduced nNOS in the ileum, suggesting altered ENS function. A follow-up study by Palus et al., (2020) further showed that ACR exposure increased VIP-like immunoreactive neurons and altered their co-localization with neuroactive substances such as nNOS, SP, and CART, highlighting disruptions in intestinal neuronal signaling. Complementing these findings, Amirshahrokhi (2021) reported that rats treated with higher ACR doses (20–30 mg/kg/day) showed elevated colonic inflammation, oxidative stress markers, pro-inflammatory cytokines, and NF-κB expression, along with reduced antioxidant defenses, indicating severe gastrointestinal and immunological disturbances.
Effects On Gonad –
Effect of ACR on Histology of Gonad
Male Gonad
Acrylamide exposure induces significant structural damage to testicular tissues. Studies in rodents show reduced testicular weight, degeneration of seminiferous tubules, and vacuolation in Sertoli cells (Mazen and Elnegris, 2013; Yilmaz et al., 2017). Leydig cell hyperplasia and interstitial edema are frequently observed, accompanied by disrupted spermatogenesis and germ cell apoptosis (Wang et al., 2010; Kucukler et al., 2020). Histopathological findings reveal the severity of acrylamide’s effects on the structural and functional integrity of testicular tissues.
Female Gonad
Acrylamide exposure reduces follicular reserves and induces atresia in ovarian follicles. Pregnant guinea pigs exposed to acrylamide showed heightened follicular apoptosis, affecting ovarian function in offspring (Hułas-Stasiak et al., 2013). Studies highlight the vulnerability of the ovarian reserve to acrylamide, particularly during critical developmental windows. Acrylamide crosses the placental barrier, disrupting fetal ovarian and testicular development. This includes decreased follicle numbers, impaired Leydig cell function, and epigenetic alterations that may affect future generations (Aldawood et al., 2022). Acrylamide’s transgenerational impact underscores the need for protective measures, particularly for pregnant populations.
Biochemical Effects of ACR on Gonad
Acrylamide induces oxidative stress by increasing reactive oxygen species (ROS) and lipid peroxidation while depleting antioxidant defenses like superoxide dismutase (SOD) and glutathione peroxidase (GPx) (Khalil et al., 2014; Zhang et al., 2010). Elevated malondialdehyde (MDA) levels in testes and ovaries indicate heightened lipid peroxidation, a marker of cellular damage (Lebda et al., 2014). This imbalance between ROS production and antioxidant defenses plays a central role in acrylamide-induced gonadotoxicity. Acrylamide exposure alters key reproductive hormones, including testosterone, estradiol, luteinizing hormone (LH), and folliclestimulating hormone (FSH). Decreased testosterone and increased LH levels in males disrupt spermatogenesis, while altered estradiol levels in females impair follicular development (Ma et al., 2011; Wei et al., 2014).
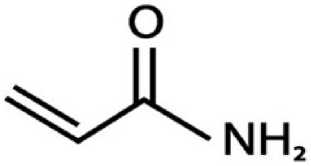
Acrylamide | C,H5NO
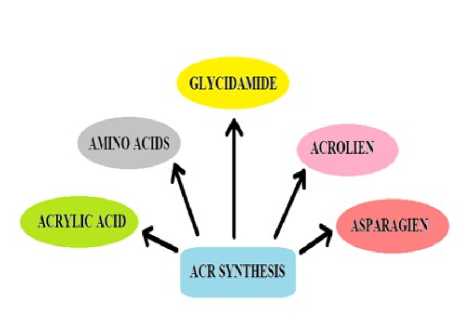
Figure 1. Structure of Acrylamide. Figure 2. Probable ways of ACR formation
Table 1. - Effect of Acrylamide on histology of liver
|
Model Organism |
Dosage |
Duration |
Inference |
References |
|
Clarias gariepinus |
20%, 40%, 60%, and 80% |
2 weeks |
vessel congestion, necrobiotic changes, diffuse necrosis, cytoplasmic destruction, nuclear damage, severe liver damage |
Ibrahim et al., (2020) |
|
Capoeta capoeta |
10mg/L, 20mg/L, 30mg/L |
4 days |
sinusoid and central vein vasodilatation, melanomacrophages, necrosis, nuclear anomalies, pyknotic, karyorrhectic, and karyolytic nuclei, fibrosis, blood leakage into the liver parenchyma |
Kilicle et al., (2020) |
|
Carassium auratus |
50 - 200 mg l–1 |
96h |
eosinophilic alterations, focal necrosis, nuclear pleomorphisms (pyknosis), diffuse eosinophilic hepatocellular alterations, lipidic and eosinophilic degeneration, inflammatory responses, reduced pancreatic intrusions into the hepatic parenchyma |
Larguinho et al., (2013), |
|
Rattus norvegicus |
2mg/kg |
4 weeks |
necrosis, vacuolar degeneration, granular cytoplasmic changes, pyknotic nuclei in liver tissue |
Faridi et al., (2024) |
Table 2. Biochemical effect of Acrylamide on liver
|
Model Organism |
Dosage |
Duration |
Inference |
References |
|
Clarias gariepinus |
20%, 40%, 60%, 80% |
2 weeks |
increased malondialdehyde (MDA) levels, decreased levels of reduced glutathione (GSH), superoxide dismutase (SOD), and total antioxidant capacity (TAC) |
Ibrahim et al., (2019) |
|
Danio rerio |
300 ppm |
26 hours |
increases in serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels, acute elevations in serum glucose and triglyceride levels |
Kim et al., (2015) |
|
Rattus norvegicus |
20mg/kg |
30 days. |
reduced the total antioxidant status (TAS) and increased the total oxidant status (TOS) in serum, immunohistochemically, ACR significantly increased caspase-3 immunoreactivity. |
Yalçin et al., (2020) |
|
fertilized eggs from the Babcock strain |
0.5 mg and 1.0 mg |
11 days |
elevated levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), alongside decreased glutathione levels, which denote oxidative stress. |
Mallepogu et al., (2023) |
Table 3. Effect of Acrylamide on Gene Expression in Liver
|
Model Organism |
Dosage |
Duration |
Inference |
References |
|
Clarias gariepinus |
20%, 40%, 60%, 80% |
2 weeks |
increases in DNA fragmentation in the liver, indicating significant DNA damage |
Ibrahim et al., (2019) |
|
Chick embryo |
0.1, 0.2, and 0.3 mg |
72 hours |
dose-dependent induction of GST isoforms (theta (CL1), mu (CL2), and alpha (CL3) classes), |
Sheikh et al., (2010) |
|
Rattus norvegicus |
25- 75 mg/kg |
30 days |
upregulation of cytochrome P450-related genes, cancer-related genes (e.g., EGFR and Rad51), and inflammatory markers, while anti-oncogenes such as Bcl2 and P21 were downregulated |
Chen et al., (2021) |
|
Rattus norvegicus |
50 and 100 mg/kg body weight |
14 days |
upregulating pro-apoptotic markers, including P53, which suggests enhanced apoptosis in liver cells |
Kandemir et al., (2020) |
Table 4. Effect of ACR on histology of Intestine.
|
Model Organism |
Doses |
Duration |
Inference |
Reference |
|
Zebrafish |
0.152, 0.305, 0.610 and 1.22 mM |
21 days |
widening of intracellular gaps, protein fluid accumulation, ovarian depletion of yolk granules in mature oocytes, disruption of intestinal prismatic structure, leading to goblet cell hyperplasia and enterocyte exfoliation. |
Modi et al., (2022) |
|
Wistar Rat |
0.2ml/ animal/day, 7.5mg/kg BD, 15mg/kg BD |
28 days |
Acrylamide reduced mucosal and intestinal cell counts in the duodenum, jejunum, and ileum. Duodenal samples showed severe coagulative necrosis, villus sloughing, necrotic debris, crypt disruption, moderate cell infiltration, and vascular congestion. |
Ige et al., (2022) |
|
Rat |
0,25, 50 mg/kg/day |
five days per week for 21 days |
Acrylamide reduced mucin production and secretion, with a notable decrease in goblet cells in the upper crypt. Neutral mucins and MUC2 mucins declined significantly, while acidic mucins showed a dosedependent decrease. Sulfomucins were absent in the lower crypt and significantly reduced alongside sialomucins in the upper crypt. |
Koledin et al., (2016) |
|
Neonatal rats |
30,50 mg/kg body weight/day |
21 days |
Acrylamide caused mucosal injury, cell sloughing, glandular disarrangement, reduced mucosal thickness, and decreased PAS-positive reactions. Microscopic analysis revealed damage to parietal, chief, mucous, and enteroendocrine cells. Quercetin co-treatment mitigated most of these effects. |
Sayyad et al., (2017) |
Table 5. Biochemical effect of ACR on Intestine.
|
Model Organism |
Doses |
Duration |
Inference |
|
female Danish Landrace pigs |
0.5 ug/kg bw, 5 ug/kg bw |
28 days |
increase in substance P (SP), calcitonin generelated peptide (CGRP), galanin (GAL) and vesicular acetylcholine transporter (VAChT)-like immunoreactive (LI) neurons as well as a decrease in neuronal nitric oxide synthase (nNOS) -like immunoreactivity in all types of ileum intramural plexuses. |
|
0.5 -5 µg/kg body weight/day |
4 weeks |
Acrylamide caused increased immunoreactivity to neuroactive substances (e.g., substance P, CGRP, galanin, nNOS, and VACHT) within the duodenal ENS, indicating neurochemical changes. Increased oxidative stress and neurotoxic effects were suggested, impacting the enteric nervous system, as well as potential systemic effects such as neurotoxicity and carcinogenicity |
|
|
Mouse (NMRI strain) |
20 and 30 mg/kg/day. |
21 days |
Acrylamide exposure worsened colitis in mice, increasing disease activity index (DAI), body weight loss, mortality, and oxidative stress markers (MDA, carbonyl proteins). It also elevated proinflammatory cytokines (TNF-α, IL-1β, IL-6) and caused greater macroscopic and histological damage. |
|
Pig (Danish landrace gilts) |
Low dose (0.5 µg/kg body weight/day) and high dose (5 µg/kg body weight/day) |
28 days |
Acrylamide exposure led to significant changes in the population of vasoactive intestinal peptide (VIP)-like immunoreactive neurons in the small intestine’s enteric nervous system. Increased VIP immunoreactivity was associated with signs of local inflammation, oxidative stress, and potential neuronal plasticity as an adaptation to acrylamide exposure. |
Table 6. Histological Effects of Acrylamide on Gonads
|
Model Organism |
Dosage |
Duration |
Inference |
References |
|
Albino rat |
15 mg/kg |
8 Weeks |
Shrunken tubules, vacuolations, sloughed spermatogenic cells into the lumen |
Mazen and Elnegris (2013) |
|
Sprague Dawley |
5 mg/kg/day and 10 mg/kg/day |
8 Weeks |
germ cell depletion, leydig cell hyperplasia, vacuolation in seminiferous tubules |
Wang et al., (2010) |
|
Mice |
1mM and 10mM |
24 Hours |
Oxidative stress, apoptosis in Leydig and Sertoli cell. |
Yilmaz et al., (2017) |
|
Guinea pigs |
3 mg/kg/day |
32 Days |
Reduction in the number of healthy ovarian follicles, increase in atretic follicles. |
Hulas-Stasiak et al., (2013) |
|
Wistar Albino |
2.5, 10 or 50 mg/kg |
14 Days |
Reduction in ovaries weight, apoptosis increased in granulosa cells. |
Aldawood et al., (2020) |
Table 7. Biochemical effects of Acrylamide on Gonads
|
Model Organism |
Dosage |
Duration |
Inference |
References |
|
Albino rat |
5, 10 and 15 mg/kg bw |
4 or 12 Weeks |
Imbalance in the oxidant /antioxidant status, impairment in the testicular function and perturbations in the immune response genes in rats. |
Khalil et al., (2014) |
|
Mice |
0.05% (w/v) |
21 Days |
Elevation in malondialdehyde level (MDA), reduction in the level of reduced glutathione (GSH) and the glutathione-S-transferase (GST), glutathione peroxidase (GPX) and glutathione reductase (GR), reduced serum total testosterone and progesterone but increased estradiol (E2) levels. |
Lebda et al., (2014) |
|
Sprague Dawley |
5, 15, and 30 mg/kg per day |
4 Weeks |
Reduced testis and seminal vesicle weights, altered hormone levels (increased FSH and testosterone, decreased LH), and led to histological damage in the reproductive organs, such as epithelial cell degeneration in the epididymis. |
Ma et al., (2011) |
|
Mice |
20 mg/kg and 40 mg/kg per day |
30 Days |
Potential disruption of ovarian function and decrease in the number of corpora lutea |
Wei et al., (2014) |
Table 8. Molecular Effects of Acrylamide on Gonads
|
Model Organism |
Dosage |
Duration |
Inference |
References |
|
Mice |
1mM and 10mM |
24 Hours |
upregulated pro-apoptotic genes like Bax and Caspase-3, downregulated anti-apoptotic genes such as Bcl-2 in leydig and sertoli cells |
Yilmaz et al., (2017) |
|
Mice |
ACR- 0, 100, 500 and 1000uM GLY 25, or 250 μM |
7 Days |
Elevated expression of p53, a tumor suppressor gene, suggesting DNA damage. |
Aras et al., (2017) |
|
F344 |
0.0, 0.5, 1.5, 3.0, 6.0 or 12 mg/kg |
31 Days |
Reduced expression of the StAR, altered expression of FSHR and LHR affects germ cell function |
Recio et al., (2017) |
|
Mice |
0.001, 0.01, 0.1, 1 and 10 µg/ml |
1 Year |
Downregulation of DNA repair genes increases susceptibility to mutations and chromosomal aberrations |
Nixon et al., (2012) |
Effects of ACR on Gene Expression in Gonad
Acrylamide upregulates pro-apoptotic genes like Bax and Caspase-3 while downregulating anti-apoptotic genes such as Bcl-2 in Leydig and Sertoli cells (Yilmaz et al., 2017). Elevated expression of p53, a tumor suppressor gene, suggests a cellular response to DNA damage (Aras et al. 2017). These genetic alterations initiate apoptosis and exacerbate gonadal dysfunction. Acrylamide disrupts genes involved in steroid hormone biosynthesis and spermatogenesis. Reduced expression of the StAR (steroidogenic acute regulatory) protein impacts testosterone production, while altered expression of FSHR and LHR affects germ cell function (Recio et al., 2017; Ma et al. 2011). Such disruptions in gene expression interfere with the production of critical reproductive hormones and gametes. Glycidamide, acrylamide’s active metabolite, forms DNA adducts that interfere with replication and repair. Downregulation of DNA repair genes increases susceptibility to mutations and chromosomal aberrations (Nixon et al., 2012; Katen and Roman, 2015).
SUMMARY AND CONCLUSION
Acrylamide (ACR), a chemical formed during high-temperature cooking of carbohydrate-rich foods, is increasingly recognized as a public health concern due to its carcinogenic, neurotoxic, genotoxic, and reproductive effects. Experimental studies have shown that ACR exposure causes oxidative stress, inflammation, DNA damage, and hormonal imbalances, affecting key organs like the liver, intestine, and gonads in both animals and humans.
Given its widespread presence in processed foods and its toxic effects on multiple organ systems, reducing acrylamide exposure is essential. This can be achieved through improved food processing techniques, regulatory policies, and public education, ensuring food safety while maintaining nutritional and sensory quality.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.


