Aiming COVID-19 SARS-cov-2 proteins by natural antiviral flavonoids through in-silico drug repurposing
Автор: Karumalaiyan Palanisamy, Pandiyarajan Sabarison, Velayutham Gurunathan, Govindasamy Hariharan, Ho-Chiao Chuang, Sheng-Tung Huang
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.21, 2025 года.
Бесплатный доступ
The World Health Organisation (WHO) has proclaimed the quickly spreading, extremely infectious, and pathogenic SARS-CoV-2 (SARS-Coronavirus 2) linked COVID-19 (Coronavirus disease 2019) a pandemic. SARS-CoV-2 conquers host cell by connecting glycoprotein (S-protein) spike viral surface with ACE2 (cellular angiotensin converting enzyme 2). That necessary virus molecular association through host cell provides clear beneficial goal on behalf of discovering SARS-CoV-2 antiviral medications. These medications recycling will offer fast and possible therapy to extend COVID-19 exponentially. The present study is to estimate and classify natural antiviral analogues as repurposing medicines like 4',5-Dihydroxy,3,3',7-trimethoxyflavone, 3,3'-Dimethoxyquercetin, Fisetin, O-Glucosyl-7-methyl-5-genistein, Glycosil-7-O-luteolin, Hesperetin, Isoquercitrin, Justicidin B, Luteolin–7-O-glucoside and Morin for COVID-19 main protease and compared with antiviral medication Remdesivir. Molecular docking studies have shown that Luteolin–7-O-glucoside and Justicidin B were natural flavonoid derivative of exceptional inhibition ability through binding energy of -9.5,-9.4 kcal/mol of 5N5O and 6LU7 enzyme, relative to the other compounds and Remdesivir antiviral medication (Binding energy -7.4 and -7.7 Kcal/mol). The need for the most time is the prompt discovery and commitment of appropriate medication to tackle and convince the global COVID-19 crisis. Besides, timely in vivo experiments were needed to approve the inhibition efficacy of the anti-SARS-CoV-2 compounds.
Anti-viral, ADME, COVID-19, Justicidin B, Luteolin–7-O-glucoside, Molecular docking
Короткий адрес: https://sciup.org/143185123
IDR: 143185123
Текст научной статьи Aiming COVID-19 SARS-cov-2 proteins by natural antiviral flavonoids through in-silico drug repurposing
There is a horrific worldwide public health epidemic as a consequence of a febrile respiratory pandemic such as air syndrome triggered via latest coronavirus, dubbed SA S-CoV-2, which triggers COVID-19. The representative of the Coronaviridae group was SA S-CoV-2, that is an optimistic strategy-intellect, enclosed beached NA virus that causes contagions of marine, mammalian, and avian organisms throughout the globe (Wan et al ., 2020; Malik et al ., 2020). Medical initiation of COVID-19 disease is described through fatigue, dry cough, multi-organ failure, fever, and sometimes mortality in extreme cases (Huang et al ., 2020). As of April 13, 2020, over 1,800,000 people have been adversely affected worldwide, and over 100,000 deaths from Interior China and other 213 pretentious countries have already been reported (WHO, 2020). Alphacoronavirus infections (NL63-CoV and HCoV-229E) are mostly asymptomatic and trivial, while betacoronaviruses, together with ME S-CoV (Middle East Coronavirus espiratory Syndrome) and SA S-CoV, have triggered severe diseases (Liu et al ., 2020). In 2002, the SA S-CoV epidemic formed in China and contributed to 8,000 cases testified (WHO, 2015). Later in Saudi Arabia, recurrence in the form of ME SCoV was recorded at 35% of the fatality rate (WHO, 2016; Huang et al ., 2020).
HCoV-HKU1, HCoV-OC43, and NL63-CoV have limited other human-infected coronaviruses (Gaunt et al ., 2010). The coronaviral reappearance, by way of SA S-CoV-2 did by the finale of 2019, devours frightened the globe and developed a troubling state of affairs needing immediate care to avoid the prospective passing of contaminated patients (Malik et al ., 2020; Smith & Freedman, 2020). Despite substantial clinical work globally, no appropriate antiviral medications or treatments to cure people or avoid the virus's spread are still used. Present measures to deter human-to-human infection are geared to quarantine and containment of diseased patients (Smith & Freedman, 2020; Wu & McGoogan, 2020). Therefore, reports are available on the repurpose of antiviral medicines such as antimalarial, ritonavir, lopinavir, and remdesivir in contradiction of SA S-CoV-2 (Touret & Lamballerie,
2020). Also, monoclonal antibody neutralization therapies remain now produced to tackle the COVID-19 epidemic (Elshabrawy et al ., 2012; Dhama et al ., 2020). In human beings, coronavirus infection is guided primarily by associations amongst host cell receptor (ACE2) and the SProtein (envelope-anchored spike glycoprotein) of the coronavirus (Hoffmann et al ., 2020; Wong et al ., 2004). Environmental and economic characteristics can significantly promote the efflux of secondary metabolites such as tropical plant bioactive compounds. Additionally, secondary plant-concealed metabolites are deemed prodigiously in tropical regions and are progressed in remedies (Guerriero et al ., 2018; Yang et al ., 2018). Innumerable medicinal plant natural products were already evaluated for antiviral action (Zakaryan et al ., 2017; Seema & Thyagarajan, 2016; Jo et al ., 2020) .
MATERIALS AND METHODS
In-silico docking
The AutoDock Software 1.5.6 application bundle was used to build Autodock Vina input data. Discovery studio 2019 program package was utilized for binding pocket prediction of main protease (PDB ID: 5N5O and 6LU7) via co-crystallized ligands. The 5N5O Protein Quest Grid has been recognized as centre x,y,z: -23.002, -3.023, 4.681 with measurements x,y,z: 24 through 1.0 Å interval. The 6LU7 protein quest grid was defined as centre x,y,z:-10.656, 17.223, 67.024 in dimension x,y,z: 20 in 1.0 Å positioning and meaning of completeness remained fixed to 8. The other restrictions have been fixed and not specified by default for Autodock Vina. The compound which devotes the smallest inhibitory value is the main inhibitor, and the consequences were visually examined by Discovery studio 2019.
Molecular property and ADME prediction
Herein, Lipinski's law of five' being utilized for the theoretical prediction of ADMEs and the toxicity of 4',5-Dihydroxy,3,3',7-trimethoxyflavone , 3,3'-Dimethoxyquercetin , Fisetin , O -Glucosyl-7-methyl-5-genistein , Glycosil-7- O -luteolin , Hesperetin , Isoquercitrin , Justicidin B , Luteolin–7- O -glucoside , Morin and antiviral Remdesivir compounds (Lipinski et al ., 2001). A Swiss ADME online tool was also used to estimate the Lipinski parameters (Swiss ADME, 2020). To predict the transportation and biocompatibility of an active compound over blood-brain obstacle, the tPSA (topological polar surface) has been utilized (Ertl et al ., 2000). Bioavailability is too multidimensional but mainly concerned with the absorption of the digestive system (Daina & Zoete, 2016). The percentage of absorption remained determined as of formulas: percent ABS = 109 (TPSA x 0.345). Further predictions involved CYP2D6, PLD (phospholipidosis), P-glycoprotein inhibition, water solubility, and CYP2D9.
RESULTS AND DISCUSSION
In-silico assessment
In-silico docking replications remained carried out to progress appreciative of the conceivable progression of biotic activity. Phytoconstituents 4',5-Dihydroxy,3,3',7-trimethoxyflavone , 3,3'-Dimethoxyquercetin , Fisetin , O -Glucosyl-7-methyl-5-genistein , Glycosil-7- O -luteolin , Hesperetin , Isoquercitrin , Justicidin B ,
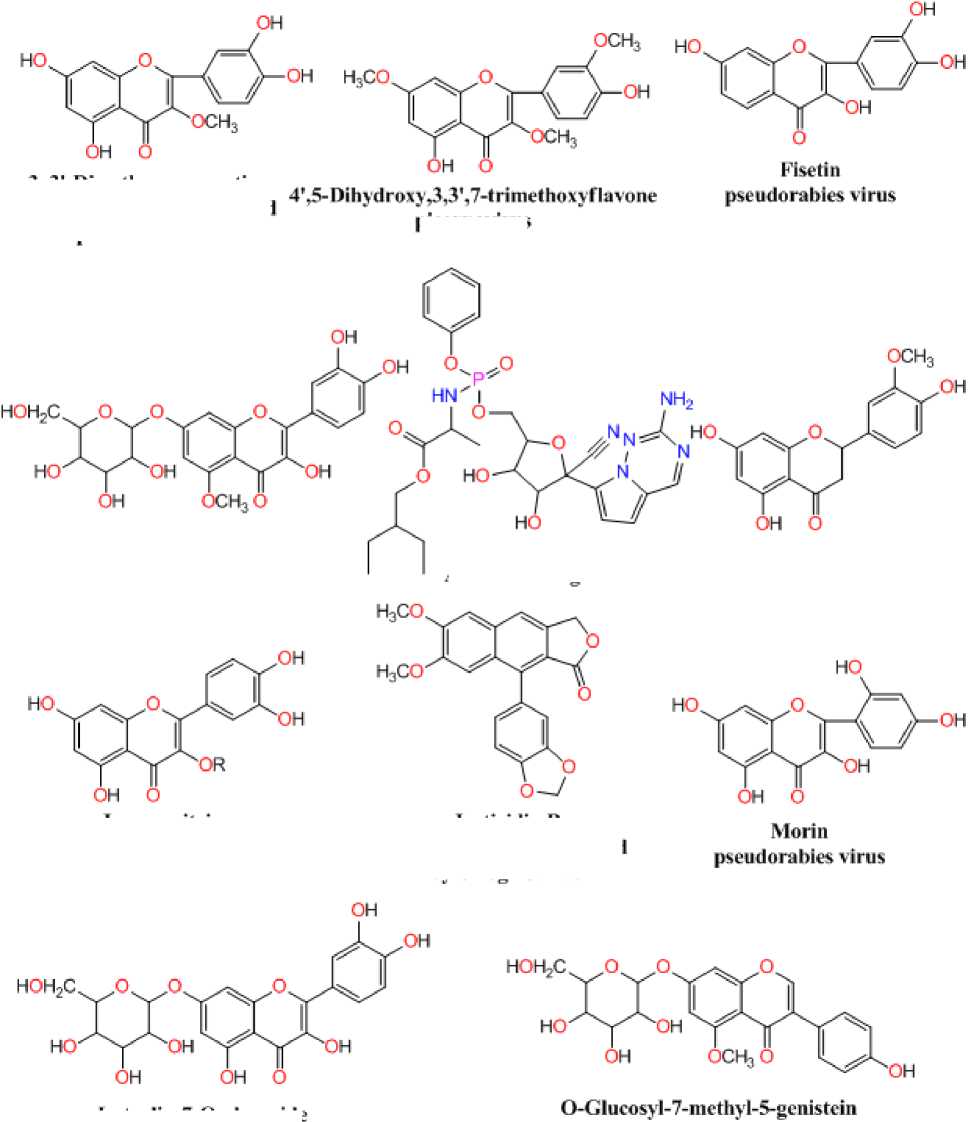
Luteolin–7- O -glucoside and Morin as well as antiviral Remdesivir compounds were evaluated for their inhibition capability concerning SA S coronavirus proteins 5N5O and 6LU7 through the software Autodock Vina. All these checked inhibitors demonstrate negative binding energy. The natural derivative Luteolin–7- O -glucoside demonstrates astonishing inhibition capability through binding ability of -9.5 kcal/mol over former derivatives 4’,5-Dihydroxy,3,3’,7-trimethoxyflavone (-7.4kcal/mol), 3,3’-Dimethoxyquercetin (-7.3 kcal/mol), Fisetin (-7.3 kcal/mol), O -Glucosyl-7-methyl-5-genistein (-7.7 kcal/mol), Glycosil-7- O -luteolin (-8.1 kcal/mol), Hesperetin (-7.4 kcal/mol), Isoquercitrin (-7.4 kcal/mol), Justicidin B (-7.8 kcal/mol), Morin (-7.5 kcal/mol) and antiviral drug Remdesivir (-7.4 kcal/mol) in 5N5O receptor individually. The essential aspect of bonding equilibrium between ligand and proteinis hydrogen bonding, and the supporting bonding gap between atoms H-acceptor and H-donor is less than 3.5 Å (Taha et al ., 2015). The related hydrogen bonding distances for the specific object receptor inhibitors remained fewer than 3.5 Å, demonstrating the strong hydrogen link amongst ligands and receptors. Luteolin– 7- O -glucoside demonstrates two associations between hydrogen bonding and the 5N5O receptor. Asn142 and Gly143 amino acid residues were associated with bond lengths of 3.09 and 2.12 Å in contact with hydrogen. The residues of Met49, Cys145, and Gln189 amino acids came in contact with hydrophobics. Figure 2 indicates the hydrophobic and hydrogen bonding interaction of compound Luteolin–7- O -glucoside with amino acid residues in 5N5O protein. The antiviral Remdesivir treatment demonstrates two associations with hydrogen bonding with the target 5N5O. Cys44 and Glu166 amino acid residues are entangled through associations between hydrogen and the bond lengths 2.20 and 2.49 Å. Thr25, Met165, Leu167, Pro168, and Gln189 amino acid residues were mixed within hydrophobic encounters. Figure 3 indicates the hydrophobic and hydrogen bonding interaction of Remdesivir antiviral medication with amino acid residues in 5N5O receptor.
Molecular property and ADME prediction
The growth of bioactive components as healers is driven by high oral bioavailability (Newby et al ., 2015). This analysis's key forecasters were demonstrated, for example, the intestinal absorption, low polar surface region, decreased molecular versatility, and hydrogen bonding ability (Azam et al ., 2012). The natural antiviral analogues 4',5-Dihydroxy,3,3',7-trimethoxyflavone , 3,3'-Dimethoxyquercetin , Fisetin , O -Glucosyl-7-methyl-5-genistein , Hesperetin , Isoquercitrin ,
Fails " ule of 5" with two infringement HBA, MW > 500
HBD and oB (Table 3).
Justicidin B , and Morin satisfies Lipinski's "law of 5" without any infringement and compounds Glycosil-7- O -luteolin , Luteolin–7- O -glucoside and Remdesivir
Isoquercitrin Herpes simplex virus
Glycosil-7-O-luteolin poliomelytis and herpes viruses
3,3'-Dimethoxyquercetin vesicular stomatitis vims and picornaviruscs
Justicidin В
Sindbis virus, murine, and cytomegalovirus
Rcmdcsivir
Antiviral Drug
Hesperetin vesicular stomatitis virus
Herpes virus
Figure 1: Natural antiviral derivatives.
Luteolin-7-()-glucoside poliovirus and herpes virus
picomavirus
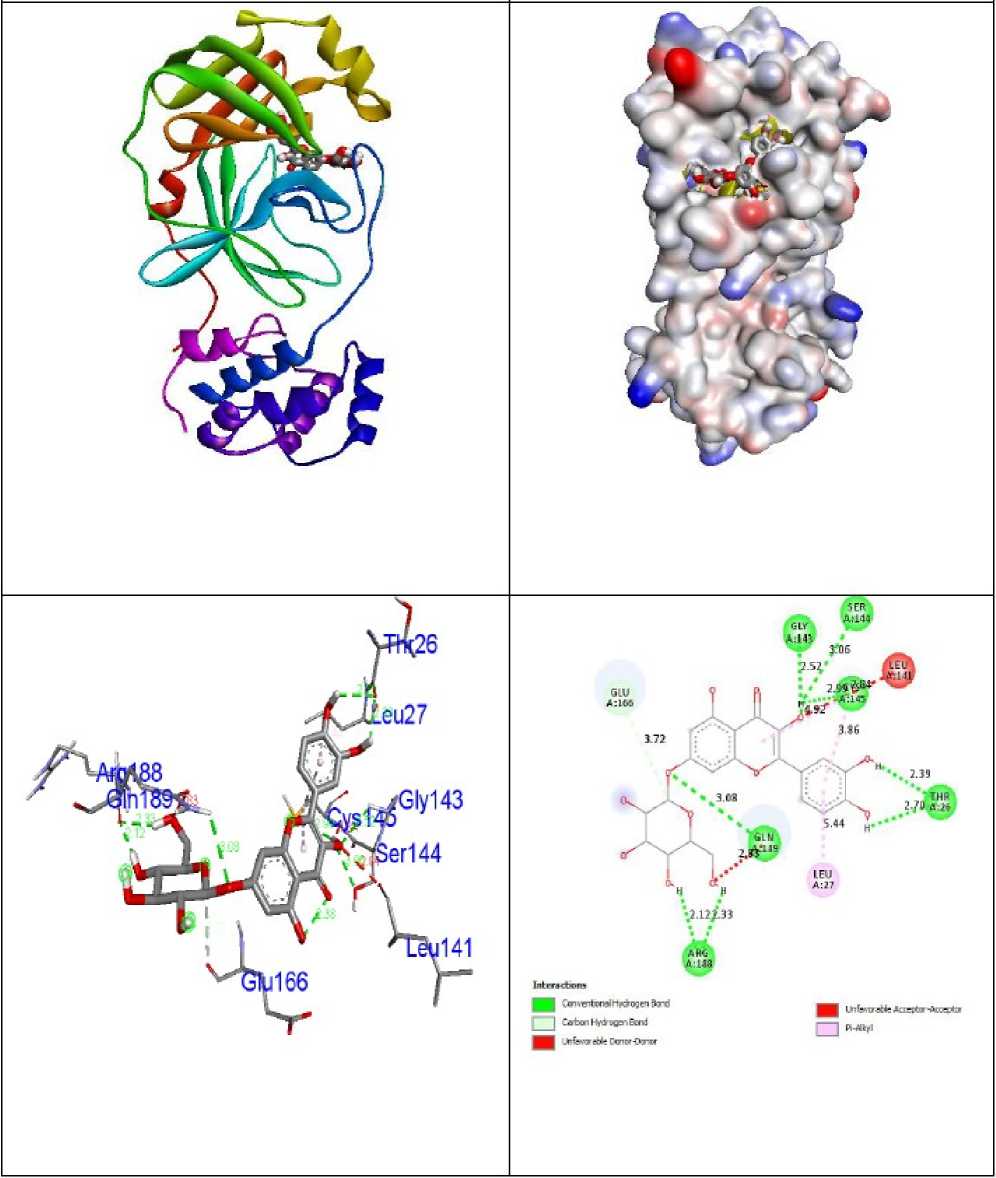
Figure 2: Interactions of Luteolin–7- O -glucoside inside the binding pocket of receptor 5N5O .
Figure 3: Interactions of antiviral drug Remdesivir inside the binding pocket of receptor 5N5O .
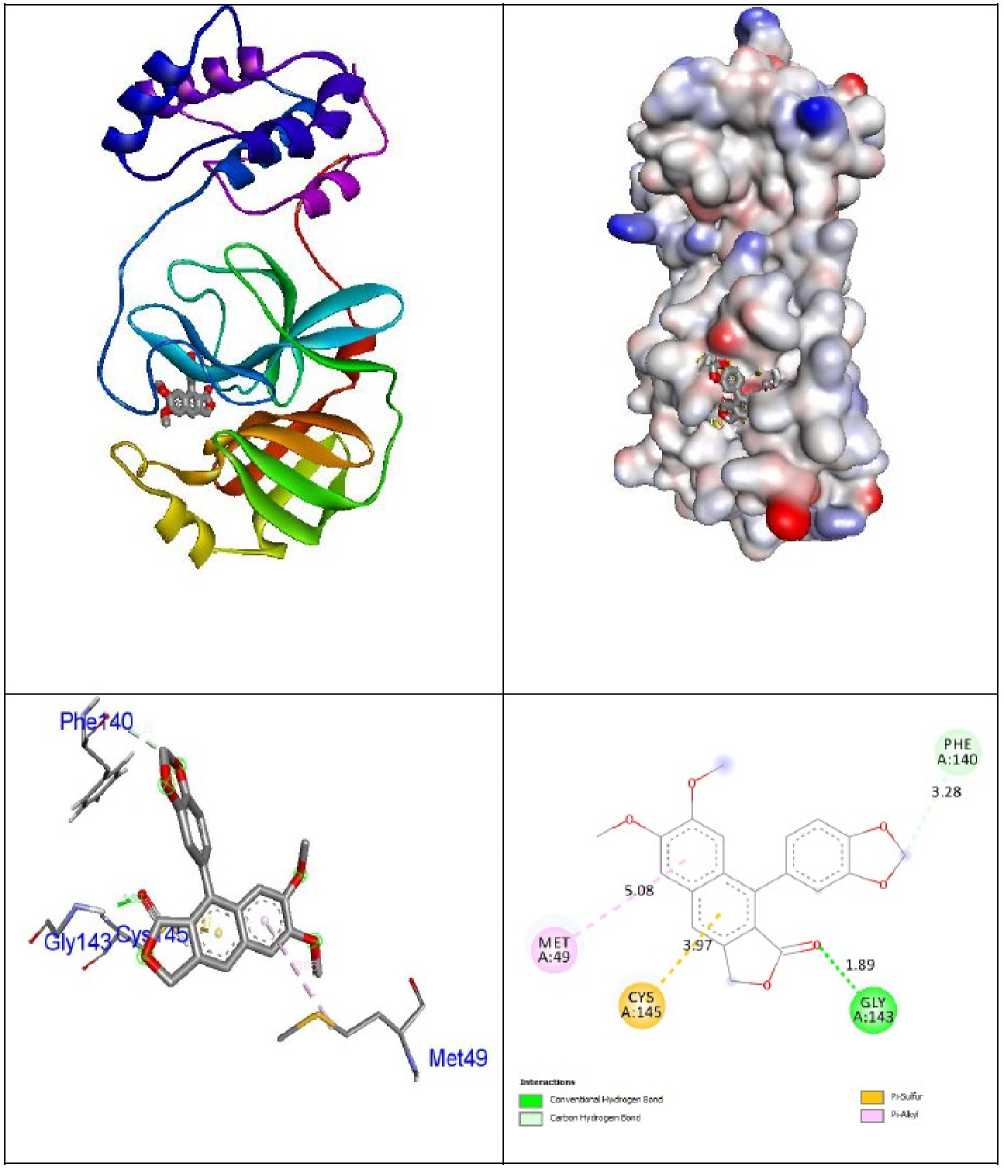
Figure 4: Interactions of Justicidin B within the binding pocket of receptor 6LU7 .
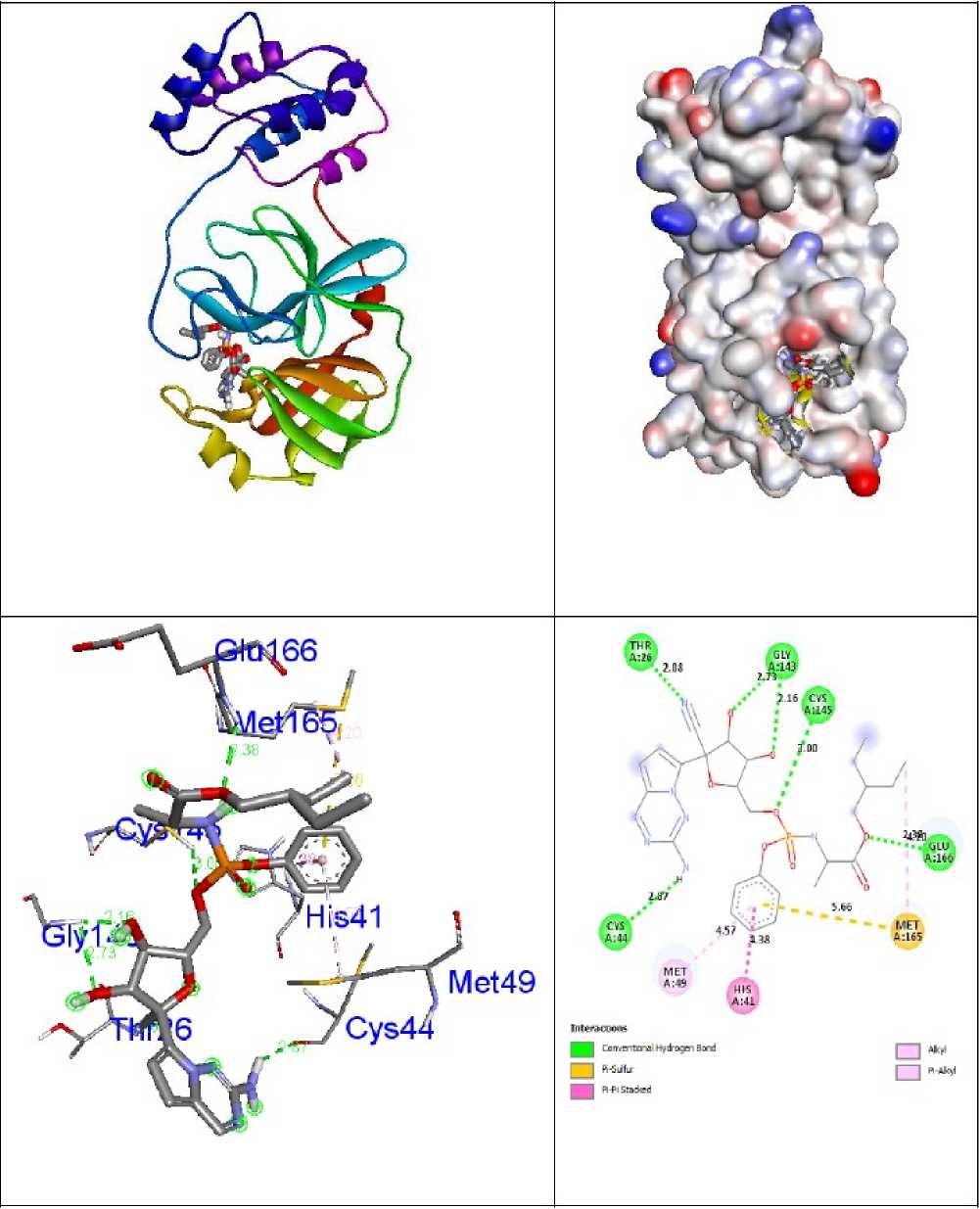
Figure 5: Interactions of antiviral drug Remdesivir inside the binding pocket of receptor 6LU7 .
Table 1. Interactions of compounds ( 1a-1k ) against SA Scoronavirus key protease (PDB ID: 5N5O).
|
Compounds |
Binding affinity (kcal/mol) |
No. of H-bonds |
H-bonding residues |
|
4’,5-Dihydroxy,3,3’,7-trimethoxyflavone (1a) |
-7.4 |
2 |
Gly143, Cys145 |
|
3,3’-Dimethoxyquercetin (1b) |
-7.3 |
1 |
Gly143 |
|
Fisetin (1c) |
-7.3 |
1 |
Glu166 |
|
O -Glucosyl-7-methyl-5-genistein (1d) |
-7.7 |
6 |
Thr26, Gly143, Glu166, Arg188. Gln189 |
|
Glycosil-7- O -luteolin (1e) |
-8.1 |
3 |
Thr26, Gln189, Thr190 |
|
Hesperetin (1f) |
-7.4 |
3 |
Asn142, Glu166, Arg188 |
|
Isoquercitrin (1g) |
-7.4 |
3 |
Gly143, Ser144 |
|
Justicidin B (1h) |
-7.8 |
2 |
Gly143, Cys145 |
|
Luteolin–7- O -glucoside(1i) |
-9.5 |
8 |
Thr26, Gly143, Ser144, Cys145, Arg188, Gln189 |
|
Morin (1j) |
-7.5 |
1 |
Arg188 |
|
Remdesivir (1k) |
-7.4 |
2 |
Cys44, Glu166 |
Table 2. Interaction of compounds ( 1a-1k ) against SARS coronavirus key protease (PDB ID: 6LU7).
|
Compounds |
Binding affinity (kcal/mol) |
No. of H-bonds |
H-bonding residues |
|
4’,5-Dihydroxy,3,3’,7-trimethoxyflavone (1a) |
-7.2 |
1 |
Gly143 |
|
3,3’-Dimethoxyquercetin (1b) |
-7.2 |
0 |
- |
|
Fisetin (1c) |
-7.3 |
0 |
- |
|
O -Glucosyl-7-methyl-5-genistein (1d) |
-7.9 |
6 |
Phe140, Asn142, Cys145, Glu166, Asp187 |
|
Glycosil-7- O -luteolin (1e) |
-7.8 |
5 |
Thr24, Ser144, Cys145, Glu166, Arg188 |
|
Hesperetin (1f) |
-7.1 |
3 |
Leu141, Arg188, Thr190 |
|
Isoquercitrin (1g) |
-7.8 |
2 |
Leu141, Ser144 |
|
Justicidin B (1h) |
-9.4 |
1 |
Gly143 |
|
Luteolin–7- O -glucoside(1i) |
-7.9 |
5 |
Thr26, Cys44, Ser144, Cys145 |
|
Morin (1j) |
-8.4 |
3 |
Gly143, Ser166, Asp187 |
|
Remdesivir (1k) |
-7.7 |
6 |
Thr26, Cys44, Gly143, Cys145, Glu166 |
Table 3 Molecular property and computer-generated ADME (absorption, distribution, metabolism, excretion) forecast of
|
potent compounds ( 1a-1k ). |
||||||||||
|
Compound |
tPSAa |
%Absb |
MWc |
oB d |
HBDe |
HBAf |
M g |
IlogPh (MlogP) |
LoigS |
CYP2D6 Inhibitor |
|
Rule |
≤140 ´Å2 |
>50 |
≤500 |
≤10 |
≤5 |
≤10 |
40– 130 |
<5 |
>-4 |
- |
|
4’,5- Dihydroxy,3,3’,7- |
98.36 |
75.06 |
344.32 |
4 |
2 |
7 |
91.44 |
3.26 (0.17) |
-4.46 |
Yes |
|
trimethoxyflavon e (1a) |
||||||||||
|
3,3’-Dimethoxy quercetin (1b) |
120.3 6 |
67.47 |
316.26 |
2 |
4 |
7 |
82.50 |
2.00 (-0.31) |
-3.89 |
Yes |
|
Fisetin (1c) |
111.1 3 |
70.66 |
286.24 |
1 |
4 |
6 |
76.01 |
1.50 (-0.03) |
-3.35 |
Yes |
|
O -Glucosyl-7-methyl-5- |
159.0 5 |
54.12 |
446.40 |
5 |
5 |
10 |
110.58 |
1.93 (-1.39) |
-3.05 |
No |
|
genistein (1d) |
||||||||||
|
Glycosil-7- O -luteolin (1e) |
199.5 1 |
40.16 |
478.40 |
5 |
7 |
12 |
114.63 |
1.50 (-2.37) |
-2.91 |
No |
|
Hesperetin (1f) |
96.22 |
75.80 |
302.28 |
2 |
3 |
6 |
78.06 |
2.25 (0.41) |
-3.62 |
No |
|
Isoquercitrin (1g) |
131.3 6 |
63.68 |
302.24 |
1 |
5 |
7 |
78.03 |
1.63 (-0.56) |
-3.16 |
Yes |
|
Justicidin B (1h) |
63.22 |
87.18 |
364.35 |
3 |
0 |
6 |
97.76 |
3.15 (2.60) |
-4.85 |
Yes |
|
Luteolin–7- O -glucoside(1i) |
210.5 1 |
36.37 |
464.38 |
4 |
8 |
12 |
110.16 |
1.54 (-2.59) |
-3.04 |
No |
|
Morin (1j) |
131.3 6 |
63.68 |
302.24 |
1 |
5 |
7 |
78.04 |
1.47 (-0.56) |
-3.16 |
Yes |
|
Remdesivir (1k) |
213.3 6 |
35.39 |
602.58 |
14 |
4 |
12 |
150.43 |
2.74 (0.18) |
-4.12 |
No |
Abbreviations: a Topological polar surface area; b Absorption; c Molecular weight; d Number of rotatable bonds; e Number of hydrogen bond donors; f Number of hydrogen bonds acceptors; g Molar refractivity; h Logarithm of compound partition coefficient between n-octanol and water; i Logarithm of water solubility.
The molecular conformational changes defined the number of revolving ties and the potential for the receptor binding. Phytoconstituents 4',5-Dihydroxy,3,3',7-trimethoxyflavone , 3,3'-Dimethoxyquercetin , Fisetin , O -Glucosyl-7-methyl-5-genistein , Hesperetin , Isoquercitrin , Justicidin B , Morin , Glycosil-7- O -luteolin , Luteolin–7- O -glucoside were under ten rotatable bonds except for Remdesivir (14 rotatable bonds), which are formed without the chirality core and have poor oral bioavailability conditions. The belongings tPSA (topological Polar
Surface Area) reveals passive molecular transport across membranes and blood-brain barrier penetration (Ertl et al ., 2000).
Checked substances except for compounds O -Glucosyl-7-methyl-5-genistein , Glycosil-7- O -luteolin , Luteolin–7- O -glucoside , and Remdesivir with tPSA values < 140Å2 fulfill the requirements for subsequent oral administration for gastrointestinal absorption. In comparison, all of the compounds studied except for O -Glucosyl-7-methyl-5-genistein (tPSA = 159.05Å2 ), Glycosil-7- O -luteolin (tPSA = 199.51 Å2), Luteolin–7- O -glucoside (tPSA = 210.51 Å2), Morin (tPSA = 131.36
Å2), Isoquercitrin (tPSA = 131.36 Å2), Fisetin (tPSA = 111.13 Å2), Hesperetin (tPSA = 96.22 Å2), 4’,5-Dihydroxy,3,3’,7-trimethoxyflavone (tPSA = 98.36 Å2), 3,3’-Dimethoxyquercetin (tPSA = 120.36 Å2), and Remdesivir (tPSA = 213.36 Å2) devour lower bloodbrain barrier (tPSA > 90 Å2), which reveals detrimental belongings of CNS (Central Nervous System).
The tested compounds except Glycosil-7- O -luteolin (% Abs = 40.16) and Luteolin–7- O -glucoside (% Abs = 36.37) demonstrated absorption percentage (percentage Abs = > 50), suggesting strong bioavailability.
Bioavailability by oral route was appropriate (> 50 percent). The compounds 3,3'-Dimethoxyquercetin , Fisetin , O -Glucosyl-7-methyl-5-genistein , Glycosil-7- O -luteolin , Hesperetin , Isoquercitrin , Luteolin–7- O -glucoside and Morin were very water-soluble (-logS > -4) excluding than other derivatives 4',5-Dihydroxy,3,3',7-trimethoxyflavone (-logS-4.46) Justicidin B (-logS -4.85) and Remdesivir (-logS -4.12) have modest water solubility. Liver impairment side effects remained not suspected in the case of derivatives O -Glucosyl-7-methyl-5-genistein
Glycosil-7- O -luteolin , Hesperetin , Luteolin–7- O -glucoside , and Remdesivir since they were predicted to be CYP2D6 non-inhibitors. A part of the P-gp (P-glycoprotein) family transporter ABC (ATP-binding cassette) comprises the pharmaceutical metabolism, intestinal absorption, and brain penetration; its caginess may expressively modify the bioavailability and defense of the drug (Fromm, 2000). Phospholipidosis convinced medication is a condition known for further developing phospholipids in soft tissue and medication-associated poisonousness (Nonoyama & Fukuda, 2008).
The findings indicate that the studied natural derivatives 4',5-Dihydroxy,3,3',7-trimethoxyflavone 3,3'-Dimethoxyquercetin, Fisetin, O-Glucosyl-7- methyl-5-genistein,Glycosil-7-O-luteolin, Isoquercitrin, Luteolin–7-O-glucoside, and Morin were not a part of the P-gp substrate, and phospholipidosis was not promoted. The checks for P-gp-phospholipidosis were anticipated in Hesperetin Justicidin B, and Remdesivir. The overall findings of ADME and toxicity indicate respectable pharmacological profile and rapid gastrointestinal ingestion through blood-brain blood barrier penetration in the isolated compound was Justicidin B. All assessed compounds remained acknowledged by way of drug-like and passed " ule of 5" of Lipinski except Glycosil-7-O-luteolin, Luteolin–7-O-glucoside, and Remdesivir. The restrictions predicted are all within the context of accepted principles.
CONCLUSIONS
COVID-19 devours arisen in the anthropological community in China and is a possible danger to wellbeing internationally. However, here is no precisely approved medication to overcome the situation. The already offered COVID-19 medications coping with essential protease. The present work aimed to inspect some natural analogues extracted from medicinal plants that might be tossed off to combat COVID-19. The utmost frequently proposed phytoconstituents in healing plants that may function by way of substantial inhibitors of COVID-19 essential proteases (PDB ID: 5N5O, 6LU7) were 4',5-Dihydroxy,3,3',7-trimethoxyflavone , 3 ,3'-Dimethoxyquercetin , Fisetin , O -Glucosyl-7-methyl-5-genistein , Glycosil-7- O -luteolin , Hesperetin , Isoquercitrin , Justicidin B , Luteolin–7- O -glucoside and Morin with negative binding energies. Studies in molecular docking have shown that compounds Luteolin–7- O -glucoside and Justicidin B were natural flavonoid derivative of exceptional inhibition ability through binding energy of -9.5, -9.4 kcal/mol of 5N5O and 6LU7 enzyme, relative to the other compounds and Remdesivir antiviral medication (Binding energy -7.4 and -7.7 Kcal/mol). However, an advanced study is necessary to inspect the possible application of these compounds in medicinal plants.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.


