Alterations in biochemical and genomic content of selected tissues of snail Pila globosa under copper and mercury sublethal toxicity
Автор: Patel R., Kurhe A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Human destructive influence on the aquatic environment is in the form of subethal pollution, which results in chronic stress conditions that have a negative effect on aquatic life. Alterations in biochemical and genomic content of selected tissues of snail Pila globosa under copper and mercury sublethal toxicity was evaluated. In acute treatment, snails were exposed to sublethal doses of 0.88 ppm and 0.475 ppm of copper and mercury, respectively, for up to 96 hours. During chronic treatment, test animals were exposed to sublethal doses of 0.176 ppm and 0.095 ppm of copper and mercury respectively, for up to 28 days. Significant depletion in the total carbohydrate, total protein, total lipid, total DNA and total RNA content were found compared to controls with an increasing exposure period in all studied tissues of Pila globosa after acute and chronic treatment with copper and mercury. Heavy metals can cause oxidative stress in molluscs, which causes depletion of the nutritional reserves by breaking down biomolecules to meet their elevated metabolic demand, which exceeds normal levels. The results demonstrate that heavy metals Cu and Hg have adverse effects on the biochemical composition and genomic content of Pila globosa , The measurement of the health of snails can be used to assess the health of an aquatic ecosystem. This study will be of some importance in the fight against pollution hazards by supporting environmental laws, policies, standards and control methods.
Biochemical, copper, genomic, mercury, pila globosa, toxicity
Короткий адрес: https://sciup.org/143184720
IDR: 143184720
Текст научной статьи Alterations in biochemical and genomic content of selected tissues of snail Pila globosa under copper and mercury sublethal toxicity
Human destructive influence on the aquatic environment is in the form of subethal pollution, which results in chronic stress conditions that have a negative effect on aquatic life (Mason, 1991). Heavy metals are important environmental pollutants due to their persistence in nature, toxicity, bioaccumulative, and bioamplifying nature. Copper sulfate pentahydrate is a fungicide used to prevent fungal and bacterial infections in vegetables, fruits, nuts, and field crops. It is also used as an algaecide, nematicide, herbicide, insecticide, molluscicide, and in irrigation and municipal water treatment systems. Human-related sources of mercury include the combustion of fossil fuels, mining, the operation of chloralkali plants., the disposal of batteries and fluorescent lamps, and agricultural applications. The uses of mercury compounds as pesticides include seed dressers, algaecide, molluscicides, insecticides, and slimicides. Mercury is known to be the most toxic of all heavy metals.
Biochemical parameters are a sensitive index to monitor changes due to xenobiotics and can constitute an important diagnostic tool in toxicological studies (Radwan et al. , 2008). The mode of action of toxicants and causes for the death of poisoned aquatic animals is better understood from biochemical investigations beside mortality studies. Different stress conditions exert control over the activity, synthesis, and breakdown of nucleic acids. The nucleic acid contents can cause alterations in genetic information and genome functioning, so it is important to investigate the levels of DNA and RNA periodically in different tissues of animals undergoing stress situations.
The snail Pila globosa is chosen for study because of its ecological, economical, nutritional, and ethnomedical importance, abundance in freshwater ecosystems, relatively longer life span, ease of handling and maintenance in laboratories, resistance to disease, tolerance to persistent toxic chemicals, high academic interest, and suitable size.
It is very clear from a literature review that very meager work has been done on this topic. Johnson (1990) studied the level of protein in the digestive gland and foot of Pila globosa exposed to a lethal concentration of copper and Shivramakrishna (1992) investigated the effect of lethal and sublethal concentrations of mercury on the content of protein in the foot and digestive gland of Pila globosa. Therefore, an investigation was conducted to study the alterations in biochemical and genomic content of selected tissues of snail Pila globosa under copper and mercury sublethal toxicity.
MATERIALS AND METHODS
For the present study, live specimens of freshwater Indian apple snails ( Pila globosa ) were hand-picked from the River Godavari (Lat. 20°00′33.2′′ N and Long. 73°46′44.7′′ E), Nashik district of Maharashtra state, India, and were immediately brought to the laboratory for acclimation. During the acclimation period of two weeks, snails were fed a daily with Hydrilla, Nymphaea, and green algae (Haniffa, 1980), and the water in the glass aquarium tank was renewed once a day. Feeding was stopped one day prior to the toxicity test. Tests were rejected when mortality during acclimation period exceed 5% (USEPA, 1975).
Sublethal Toxicity Study:
Acclimatized healthy, active, and mature snails of average body weight (21 ± 2 g) and approximately uniform size (shell length: 43.00 ± 14.10 mm, shell width: 37.9 ± 13.61 mm), irrespective of sex, were selected for experiments.
Analytical reagent-grade heavy metals copper as copper sulfate (CuSO 4 .5H 2 O) and mercury as mercuric chloride (HgCl 2 ), E. Merck, Mumbai, were used in the experiment. The acclimatized snails were divided into three groups for acute and three groups for chronic sublethal exposures, each of copper and mercury, along with a control with three replicates for each group. In acute treatment, snails were exposed to sublethal doses of 0.88 ppm and 0.475 ppm (LC 50/2 ppm of 96 hrs.) of copper and mercury, respectively, for up to 96 hours. During chronic treatment, test animals were exposed to sublethal doses of 0.176 ppm and 0.095 ppm (LC 50/10 ppm of 96 hrs.) of copper and mercury respectively, for up to 28 days. The above experimental concentrations were selected based on the acute toxicity (96 hrs. LC 50
for copper, 1.76 mercury, 0.95) levels (Patel, 2009). Water was renewed every 24 hours. Tests were rejected when the control mortality exceeded 10% (OECD, 2009 USEPA, 2002).
Different water parameters like temperature, pH, dissolved oxygen, total hardness, total alkalinity, and salinity of water were checked periodically at the beginning and end of the test and before and after water renewal during the course of the investigation and were maintained at 25 ± 1.47ºC, 7.6 ± 0.45, 6.8 ± 2 mg/l, 107 ± 3.56 mg/l as CaCO 3 , 112 ± 1.94 mg/l, and 63. 27 ± 1.71 mg/l, respectively. The snails were maintained in a natural daylight photoperiod in the laboratory (APHA, 2017)
Tissue sample preparation:
At the end of 24 hrs., 48 hrs., 72 hrs. and 96 hrs. of acute sublethal test and 7 days, 14 days, 21 days and 28 days of chronic sublethal test, the control and experimental snails (alive) were dissected to isolate the desired organs like the foot, mantle, digestive gland and whole soft body. These tissues were dried in a hot air oven maintained at 600 C for 3 to 4 days until a constant weight was obtained and ground into a fine powder. These powders were used for the estimation of various parameters like total carbohydrate, total protein, total lipid, total DNA and total RNA.
Biochemical and Genomic Analysis:
Total protein, total carbohydrate, and total lipid content of the tissues were estimated biochemically by the Folin-phenol reagent method of Lowry et al. (1951), the Anthrone reagent method of Roe (1955), and the sulfo-phospho-vanillin method of Folch et al. (1957), Barnes and Blackstock (1973), respectively. The total DNA content was determined by a diphenylamine reagent, and the total RNA content was determined by an orcinol reagent following the method of Schneider (1957).
Statistical Analysis:
Differences in control and experimental animal groups were tested for significance (at p ≤0.05, p ≤0.01, and p ≤0.001) by using the student's ‘t’ test (Bailey, 1965).
RESULTS AND DISCUSSION
Acute sublethal toxicity study:
Chronic sublethal toxicity study:
respectively (Fig.7).
nllidhdhdh
F M DG WSB F M DG WSB F M DG WSB F M DG WSB
24 hrs 48 hrs 72 hrs 96 hrs
H Control ■ Си-content H Hg-content
Figure 1: Impact of acute exposure of Cu & Hg on carbohydrate content of Pila globosa . (F-Foot, M-Mantle, DG-Digestive gland, WSB- Whole Soft Body)
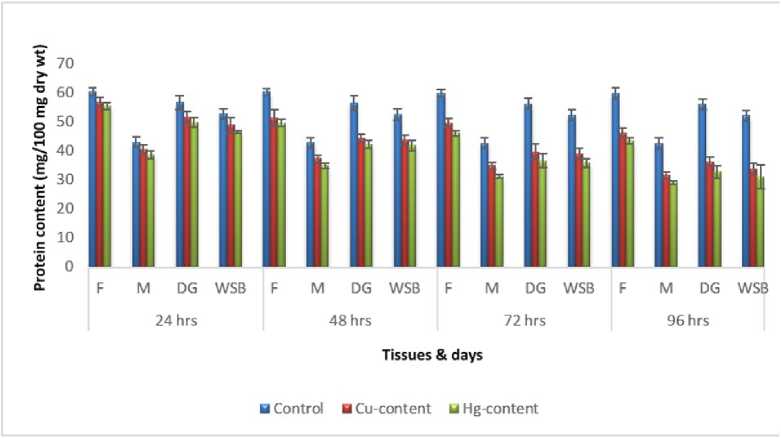
Figure 2. Impact of acute exposure of Cu and Hg on protein content of Pila globosa
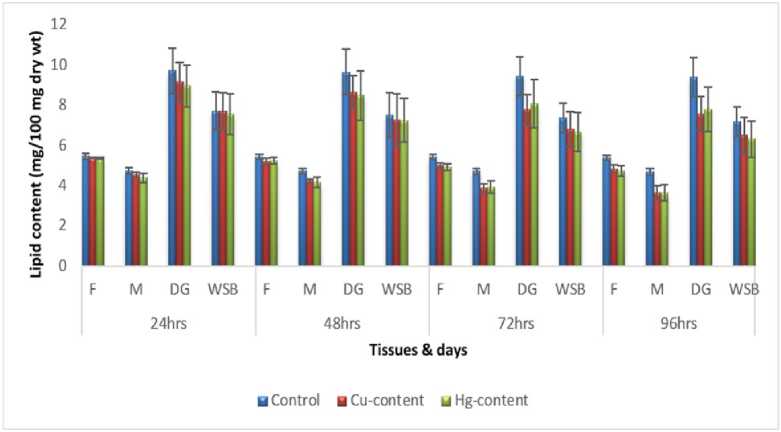
Figure 3: Impact of acute exposure of Cu and Hg on lipid content of Pila globosa
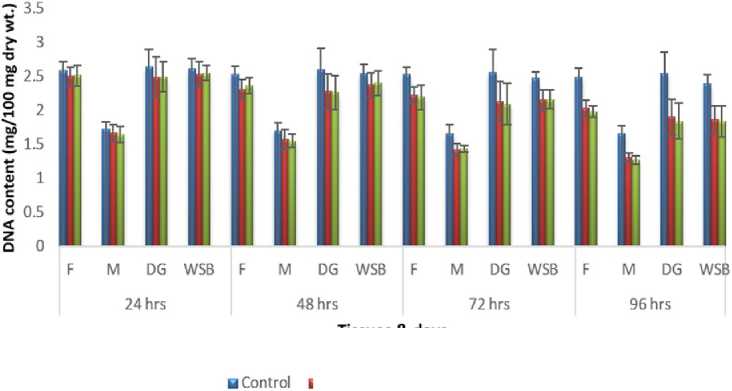
Figure 4: Impact of acute exposure of Cu and Hg on DNA content of Pila globosa
F M DG WSB F M DG WSB F M DG WSB F M DG WSB
24 hrs 48 hrs 72 hrs 96 hrs
W Control иСи-content MHg-content
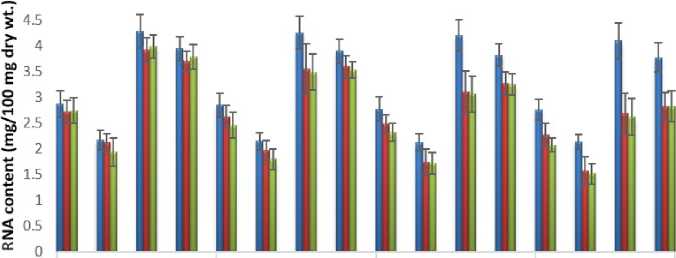
Figure 5: Impact of acute exposure of Cu and Hg on RNA content of Pila globosa
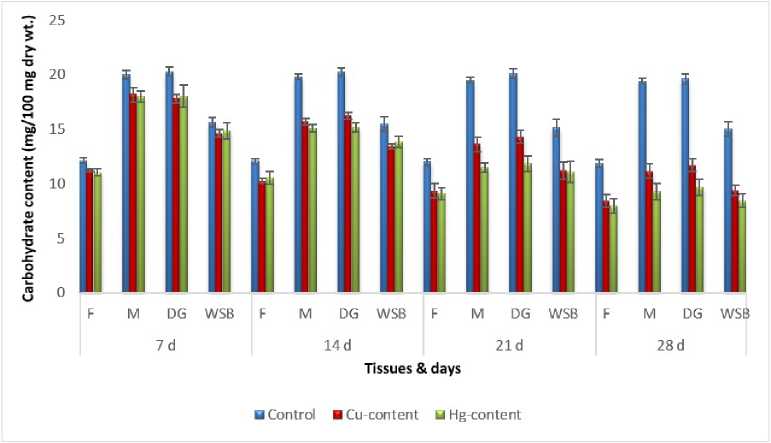
Figure 6: Impact of chronic exposure of Cu & Hg on carbohydrate content of Pila globosa
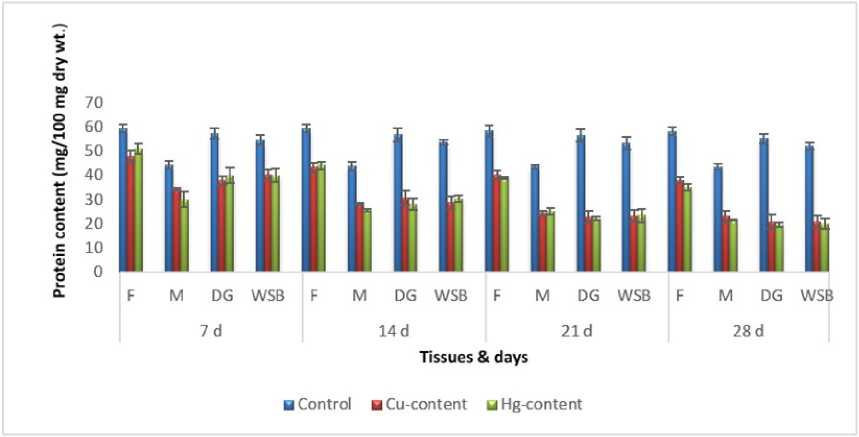
Figure 7: Impact of chronic exposure of Cu and Hg on protein content of Pila globosa
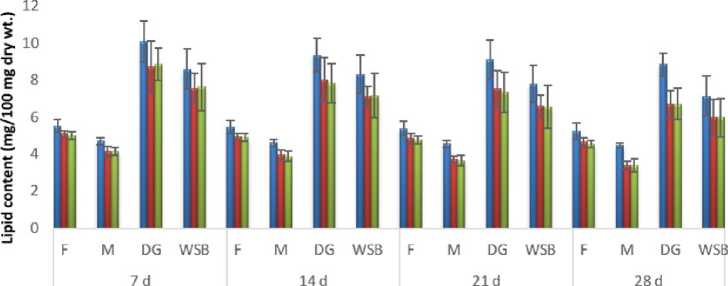
И Control иСи-content uHgcontent
Figure 8: Impact of chronic exposure of Cu and Hg on lipid content of Pila globosa
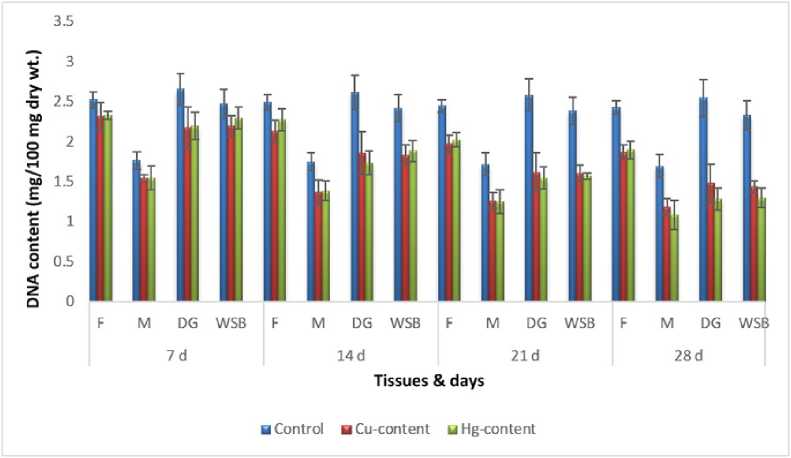
Figure 9: Impact of chronic exposure of Cu and Hg on DNA content of Pila globosa
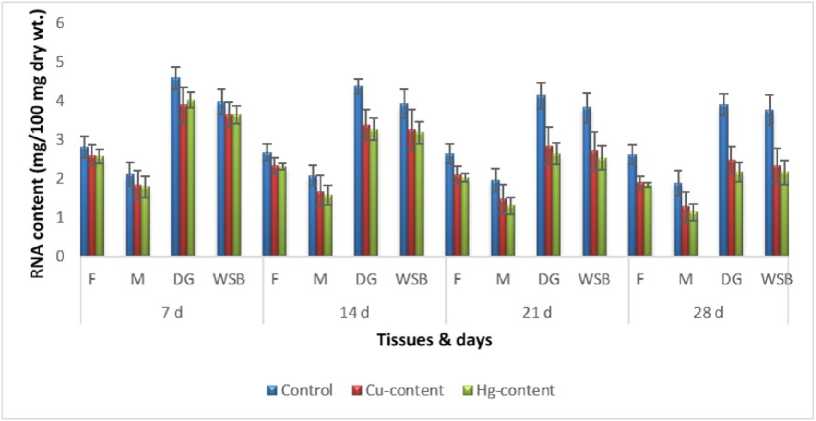
Figure 10: Impact of chronic exposure of Cu and Hg on RNA content of Pila globosa
In the present investigation, significant depletion in the total carbohydrate, total protein, total lipid, total DNA and total RNA content were found compared to controls with an increasing exposure period in all studied tissues of Pila globosa after acute and chronic treatment with copper and mercury.
Heavy metals can cause oxidative stress (Semedo et al. , 2012) in molluscs, which causes depletion of the nutritional reserves by breaking down biomolecules like carbohydrate, protein, and lipid to meet their elevated metabolic demand, which exceeds normal levels. The heavy metals react directly with DNA or generate reactive oxygen species (Geter et al. , 2008), which cause damage to DNA by binding to histone proteins (Bal et al. , 2000) or through inhibition of DNA repair enzymes (Hartwig et al. , 2002). The low level of RNA observed might be attributed to damage to DNA or a poor rate of synthesis of enzymes necessary for transcription or increased catabolism of RNA or degradation of cells and tissues, or a decreased rate of protein synthesis (Andhale & Zambare, 2011).
The heavy metals Cu and Hg have adverse effects on the biochemical composition and genomic content of Pila globosa , and their toxicity is metal-, organ-, or exposure time-specific. This study will be of some importance in the fight against pollution hazards by supporting environmental laws, policies, standards, and control methods.
CONCLUSIONS
Various concentrations of copper and mercury at varying lengths of exposure alter the levels of protein, carbohydrate, lipid, DNA and RNA significantly in the different soft tissues of Pila globosa. The measurement of the health of snails can be used to assess the health of an aquatic ecosystem. This study will be of some importance in the fight against pollution hazards by supporting environmental laws, policies, standards and control methods.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.


