Altitude-induced biochemical variations in Picrorhiza kurrooa from Uttarakhand
Автор: Pokhriyal Anshika, Vijay Suhani, Patni Babita
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.21, 2025 года.
Бесплатный доступ
Picrorhiza kurrooa Royle ex Benth, commonly known as Kutki, is a high-altitude medicinal herb valued for its hepatoprotective and antioxidant properties. This study investigates the effect of altitude on the biochemical composition of P. kurrooa populations from three sites in Uttarakhand: Tungnath (3200 m asl), Baniyakund (2460 m asl), and Pothibasa (2200 m asl). Fresh rhizomes were collected, authenticated, and analyzed for primary and secondary metabolites, including proteins, soluble sugars, starch, proline, phenolics, tannins, and flavonoids. Results revealed a significant increase in soluble protein (67.39±5.9 mg/g fr.wt.), proline (18.50±0.52 µmol/g), phenolics (86.45±1.61 µg/ml GAE), and flavonoid content (29.8±0.52 µg QE/ml) in the Tungnath population compared to lower altitudes. Soluble sugars were also highest in Tungnath, while starch content decreased with altitude. The higher metabolite concentrations at elevated altitudes are attributed to environmental stress factors such as UV-B radiation and cold temperatures, promoting adaptive responses in the plant. These findings underscore the superior adaptability and pharmaceutical potential of high-altitude populations, particularly the Tungnath genotype. Conservation and sustainable cultivation practices are recommended to preserve this endangered species and reduce pressure on wild populations.
Biochemical analysis, high altitude, kutki, picrorrhiza kurrooa, secondary metabolites
Короткий адрес: https://sciup.org/143184739
IDR: 143184739
Текст научной статьи Altitude-induced biochemical variations in Picrorhiza kurrooa from Uttarakhand
Picrorhiza kurrooa Royle ex Benth, commonly known as Kutki, is a small perennial herb native to the Himalayan region, thriving at altitudes between 2,500 and 4,500 meters above sea level. The name "Picrorhiza" originates from the Greek words ‘picros’ (bitter) and ‘rhiza’ (root), while its common name, Kutki, is derived from the Punjabi term "Karu," also meaning bitter (Coventry, 1927). This herb ischaracterized by its perennial creeping nature, spreading via stolons. The rhizomes give rise to a whorl of radical leaves, with adventitious roots emerging from the rhizome base. Medicinally important compounds are primarily concentrated in the roots and rhizomes (Sharma et al. , 2012).
-
P. kurrooa has been used to treat respiratory diseases, fever, allergies, diarrhea, inflammatory conditions, chronic scorpion stings, and liver-related diseases (Bhandari et al. , 2010; Rajani et al. , 2014).
Overharvesting from its natural habitat, coupled with a lack of structured cultivation practices, has led to a significant decline in P. kurrooa populations, resulting in its classification as an endangered species by the International Union for Conservation of Nature (IUCN) (Nayar & Sastry, 2006).
MATERIALS AND METHODS
Collection of plant material
Plant material (fresh roots and rhizome) were obtained from three altitudes in Uttarakhand: Tungnath (3300 m asl), Baniyakund (2460 m asl) and Pothibasa (2200 m asl). Since material collected in September is rated high because of low moisture content (Uniyal et al. , 2011), the collection for our study was done during the month of September. The plant materials were authenticated at HAPPRC, HNBGU, Srinagar Garhwal. A voucher specimen has been deposited in HAPPRC, Srinagar Garhwal, Uttarakhand, against voucher no. HAPPRC2310.
Preparation of Plant extract
Fresh rhizomes of P. kurrooa were washed under running tap water to remove soil adhering and then rootlets were removed from the rhizomes. Fresh rhizomes were dried in hot air oven at 65°C until constant dry weight was attained (Fig. 2). An electric grinder was used to powderize the dried materials. Using the maceration method, 15 g of powdered root was extracted using acetone and aqueous solvents for 72 hours each. Whatman filter paper No. 1 was used to filter all the extracts, which were then concentrated at room temperature until dry. The extracts were then placed in airtight screw cap vials and stored for later use in refrigerator at 4-5°C.
Total protein estimation
The Bradford (1976) method was used to estimate total soluble proteins. Leaf material was homogenized in
5 ml Tris-HCl buffer (pH 7.5) using a mortar and pestle, followed by centrifugation at 10,000 rpm for 10 minutes at 4°C. The supernatant was used for analysis. The Bradford reagent was prepared by dissolving 10 mg Coomassie Brilliant Blue (CBB) in 5 ml of 95% ethanol, adding 10 ml of 85% ortho-phosphoric acid, and diluting to 100 ml with distilled water. Sample dilutions (10–100 µg) were mixed with 4.9 ml Bradford reagent, incubated for 2–3 minutes, and absorbance was measured at 595 nm using a spectrophotometer. A standard curve was generated using Bovine Serum Albumin (BSA).
Total Carbohydrate estimation
Estimation of Total Soluble Sugars
Fresh leaves (200 mg) were homogenized in 5 ml of 80% boiling ethanol, and the extract was centrifuged at 3000 rpm for 10 minutes. The supernatant was used for sugar estimation. Various volumes of the supernatant were diluted to 1 ml with distilled water, and 4 ml of anthrone reagent (200 mg anthrone dissolved in 100 ml of 95% sulfuric acid) was added. The mixture was cyclomixed for 2–3 minutes, and absorbance was recorded at 620 nm using a UV-Visible spectrophotometer. Standard sucrose solutions were used for calibration, and extractions were performed in triplicate.
Estimation of Total Starch Content
The pellet obtained after centrifugation was used for starch estimation. It was suspended in 5 ml of 52% perchloric acid and centrifuged at 3000 rpm for 12 minutes. The supernatant was then diluted to 1 ml with distilled water, and 4 ml of anthrone reagent was added. The mixture was cyclo-mixed for 2–3 minutes, and absorbance was measured at 620 nm. Standard sucrose solutions were used to construct a calibration curve, ensuring accurate starch quantification.
Free Proline content estimation
Proline content was estimated following Bates et al. (1973). Fresh leaf tissue (250 mg) was homogenized in 5 ml of 3% sulfosalicylic acid, and the extract was centrifuged at 5000 g for 10 minutes. During the beginning, pre-monsoon, and post-monsoon phases of the third growing season, 2 ml of the supernatant was mixed with 2 ml acid ninhydrin and 2 ml glacial acetic acid. The mixture was incubated at 100°C, cooled in an ice bath for 10 minutes, and 4 ml of toluene was added.
After cyclo-mixing for 30 seconds, the upper colored layer was collected, and absorbance was measured at 520 nm using a spectrophotometer. Proline concentration was determined from a standard curve prepared with L-proline.
Estimation of Total Phenolic Content (TPC)
The Folin-Ciocalteau method (Singleton and Rossi, 1965) was used to determine TPC in rhizome extracts. A stock solution was prepared by dissolving 10 mg of extract in 10 ml of distilled water and centrifuging at 5000 rpm for 15 minutes. From the supernatant, solutions of 100, 200, and 300 µg/ml were prepared. Each solution (0.5 ml) was mixed with 5 ml of diluted Folin-Ciocalteau reagent (1:10 dilution with distilled water) and incubated at room temperature for 7 minutes. The reaction was stopped by adding 4 ml of 7.5% sodium carbonate, resulting in a blue color. After 1 hour of incubation, absorbance was measured at 765 nm using a UV-Vis spectrophotometer against a blank prepared with distilled water. The TPC was calculated as µg/ml gallic acid equivalent using a standard curve generated with gallic acid.
Estimation of Total Tannin Content (TTC)
The Folin-Ciocalteu method (Tambe and Bhambar, 2014) was used to estimate tannin content. In a 10 ml volumetric flask, 0.1 ml of the sample extract was mixed with 7.5 ml distilled water, 0.5 ml Folin-Ciocalteu reagent, and 1 ml of 35% sodium carbonate solution. The mixture was thoroughly shaken and incubated at room temperature for 30 minutes. Reference standard solutions of gallic acid (20, 40, 60, 80, and 100 µg/ml) were prepared similarly. Absorbance for both test and standard solutions was measured at 725 nm using a UV-Visible spectrophotometer against a blank. Tannin content was expressed as gallic acid equivalents.
Estimation of Total Flavonoid Content (TFC)
The TFC was estimated following the method of Zhishen et al. (1999). A stock solution was prepared by dissolving 3 mg of extract in 10 ml of methanol. From this stock, 300 µl was mixed with 3.4 ml of 30% aqueous methanol, 150 µl of 0.3M aluminum chloride solution, and 150 µl of 0.5M sodium nitrite solution. After a 5-minute incubation at room temperature, 1 ml of 1M sodium hydroxide solution was added. Absorbance was measured at 506 nm against a blank (prepared with distilled water) using a UV-Vis spectrophotometer. The experiment was conducted in triplicate, and TFC was calculated as µg quercetin/ml using a standard curve prepared with quercetin.
RESULTS
Total soluble protein estimation:
Carbohydrate EstimationSoluble Sugar
Total Soluble sugar content was found maximum in Tungnath i.e., 509.61±6.05 mg/ml, followed by Baniyakund i.e., 404.45±9.09 mg/ml. While minimum was observed in Pothibasa i.e., 237.79±5.25 mg/ml as illustrated in Fig. 4.
Starch
Total Starch content was found maximum in Baniyakud i.e., 378.70±69.66 mg/g, followed by Pothibasa i.e., 334.45±40.36 mg/g. While lowest was observed in Tungnath i.e., 152.64±45.30 mg/g as illustrated in Fig. 5.
Total free Proline content
Total Proline content wad found highest in Tungnath i.e., 18.50±0.52 umol/g, followed by Baniyakund i.e., 11.22±1.94 umol/g. While lowest was observed in Pothibasa i.e., 8.91±0.06 umol/g as illustrated in Fig. 6.
Total Phenolic Content:
Total Phenolic Content was found maximum in acetone extract of Picrorhiza kurrooa of Tungnath i.e., 86.45±1.61 µg/ml GAE, followed by aqueous extract of Picrorhiza kurrooa of Tungnath i.e., 68.76±1.45 µg/ml GAE. While minimum was observed in aqueous extract of Picrorhiza kurrooa of Pothibasa i.e., 43.02±1.00 µg/ml GAE as illustrated in Fig.7

Figure 1. Picrorhiza kurrooa plant in field (Baniyakund, 2460 m asl)


Figure 2. a. P. kurrooa rhizomes obtained from Tungnath, b. P. kurrooa rhizomes obtained from Baniyakund, c. P kurrooa rhizomes obtained from Pothibasa
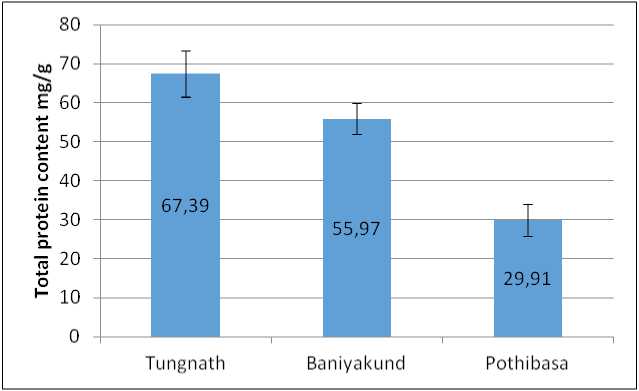
Figure 3. Graph of Total soluble protein content in Picrorhiza kurrooa from different places.
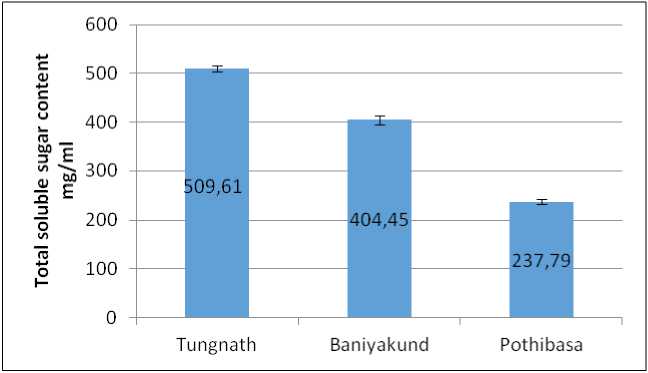
Figure 4. Graph of Soluble sugar content in Picrorhiza kurrooa from different places.
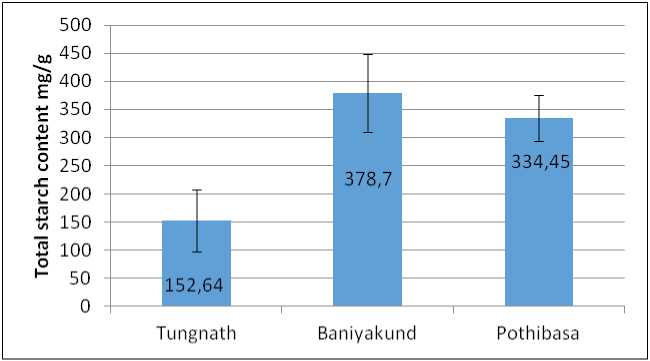
Figure 5. Graph of Starch content in Picrorhiza kurrooa from different places.
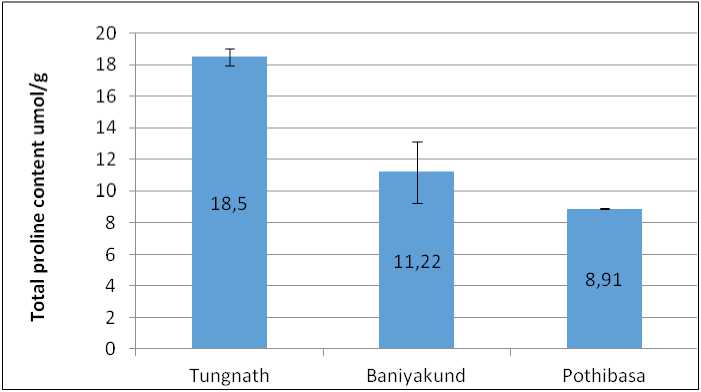
Figure 6. Graph of Proline content in Picrorhiza kurrooa from different places.
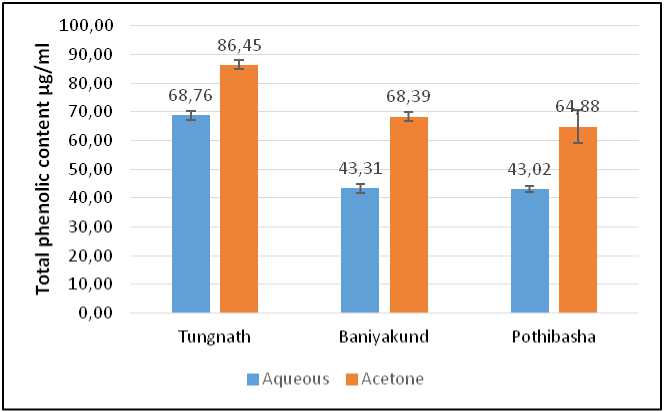
Figure 7. Graph of Phenolic content in Picrorhiza kurrooa from different places.
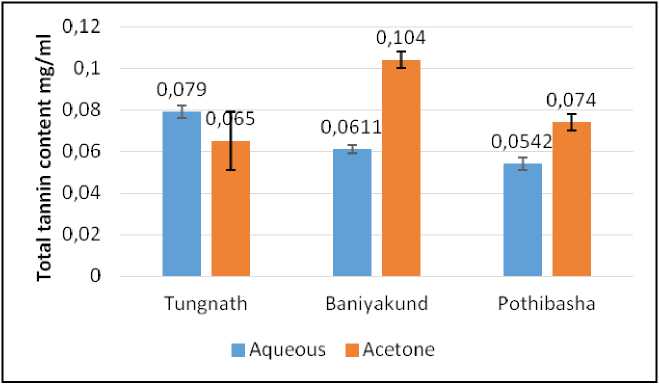
Figure 8. Graph of Tannin content in Picrorhiza kurrooa from different places.
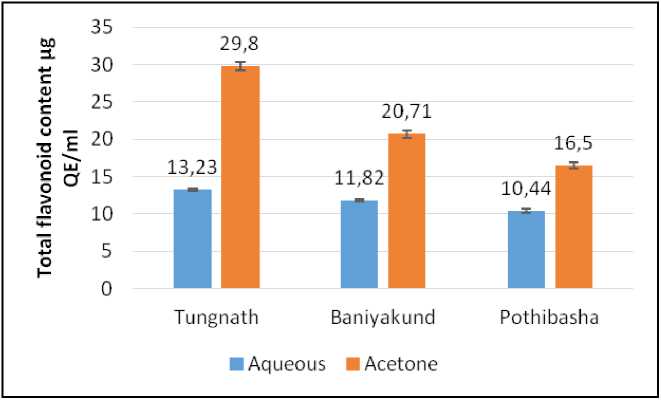
Figure 9. Graph of flavonoid content in Picrorhiza kurrooa from different places.
Total Tannin Content:
Fig. 8 shows that Total Tannin content was highest in acetone extract of Picrorhiza kurrooa obtained from Baniyakund (1.104±0.004 mg/ml TAE) followed by aqueous extract of Picrorhiza kurrooa obtained from Tungnath (0.079±0.003 mg/ml TAE). While minimum was observed in aqueous extract of Picrorhiza kurrooa obtained from Pothibasa, Uttarakhand (0.0542±0.003 mg/ml TAE). Illustrated in Fig. 8
Total Flavonoid Content:
Acetone extract of Picrorhiza kurrooa of Tungnath, showed highest Flavonoid content i.e., 29.8±0.52 µg E/ml, followed by acetone extract of Picrorhiza kurrooa of Baniyakund i.e., 20.71±0.51 µg E/ml. Least Flavonoid content was found to be 10.44±0.25 µg E/ml of aqueous extract of Pothibasa as illustrated in Fig. 9.
DISCUSSION
Altitude serves as a comprehensive indicator of ecological factors such as temperature, humidity, and solar radiation, which significantly influence plant growth and metabolism. Our study revealed a clear trend in Picrorhiza kurrooa , where the levels of primary metabolites (soluble sugars, starch, proteins, and proline) and secondary metabolites (phenolics, flavonoids, and tannins) increased with altitude.
The protein content was significantly higher in high altitude. This increase in protein content at higher altitudes may be linked to the synthesis of anti-freeze proteins, which help plants endure low temperatures (Slaughter et al. , 2022). Proteins can directly participate in tolerance to cold stress through the control and activation of genes and/or metabolic pathways linked to the resistance of plants to different environmental pressures, such as cold stress (Kosová et al. , 2011, 2018). Rahman et al. (2020), also reported similar trends in Plantago major and P. punctata .
Soluble sugars, which act as osmoregulators, were highest in Tungnath (3200 m asl), reflecting their role in protecting plants against freezing stress, as noted by Bano and Fatima, 2009. The accumulation of carbohydrates protects the membrane against freezedamage and control the stability of these membranes
(Mahajan and Tuteja, 2005).Conversely, starch content decreased at higher altitudes, likely due to its hydrolysis into sugars under cold stress, a phenomenon supported by Theron and Jacobs (1996).
Proline levels also showed a significant increase with altitude, peaking at Tungnath. Proline functions as an osmo-protectant and mitigates abiotic stress, including UV-B radiation, which is more intense at higher elevations (Ashraf and Harris, 2013; Hayat et al. , 2012; Saradhi et al. , 1995). Many researchers have reported that proline content positive correlation with altitude (Bano and Fatima, 2009; Mahajan and Tuteja, 2005) .
The total tannin, phenolic, and flavonoid contents exhibited a direct correlation with altitude. Earlier many reviewers have reported that the synthesis of the phenol and flavonoid contents in the plant increases with the increase in UV radiation at higher altitude (Watson, 2014). Phenolics stabilize membranes by decreasing membrane fluidity, hinder the diffusion of free radicals, and restrict peroxidative reactions These results align with studies on other Himalayan plants, including Primula denticulata (Aslam et al. , 2015) and Coleus forskohlii (Rana et al. , 2020).
The harsh alpine conditions, characterized by high UV radiation, extreme temperature fluctuations, and permafrost, drive plants to develop morphological and physiological adaptations. These include the enhanced synthesis of secondary metabolites, which support survival under stress. Thus, the higher metabolite concentrations observed at higher altitudes may play a crucial role in the adaptability and resilience of alpine plants.
CONCLUSION
This study demonstrated that altitude significantly influences the biochemical composition of rhizomes, with higher altitudes promoting greater synthesis of secondary metabolites such as phenolics, flavonoids, tannins, and proline. The Tungnath population (3,200 m asl) exhibited the highest concentrations of these compounds, indicating superior adaptability to alpine conditions. Higher levels of total soluble sugars and proline at greater elevations suggest their role in stress tolerance, while lower starch content reflects starch-to- sugar conversion under cold stress. These findings highlight the potential of the Tungnath population as elite germplasm for commercial cultivation and pharmaceutical applications. It is recommended to prioritize conservation efforts and explore cultivation strategies for this superior genotype to ensure sustainable utilization and reduce pressure on wild populations. Additionally, traditional remedies warrant greater attention, as they often have fewer side effects compared to conventional drugs.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
CONTRIBUTIONS
AP: Drafted the manuscript, performed data calculations, and formulated results. SV: Data collection and conducted testing. BP: Contributed to manuscript writing and corrections.


