Ameliorative effect of aqueous-acetone extract of Raphia sudanica mesocarp on streptozotocin-induced oxidative stress and testicular damage in male Wistar rats
Автор: Upev V.A., Akande T., Ogbe R.A., Ayuba D.T., Abu A.H.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Background; Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia due to defect in insulin secretion, insulin action or both. The present study aims to investigate the effects of 80% aqueous- acetone-extract of Raphia sudanica on streptozotocin induced diabetic male rats. A total of 25 adult male Wistar rats weighing between 200g and 250g were used in the study, The rats were randomly divided into 5 groups: normal control (nc), diabetic control, and diabetic rats treated orally with 100mg and 200mg kg/mg body weight. And 5mg/kg glibenclamide for 28 days. A single dose of 40mg/kg STZ was administered intraperitonially to induce diabetes. Oxidative makers, relative reproductive organ weights and sperm parameters were assessed. Results; The result revealed the presence of phytochemicals (alkaloids, phenols flavonoids, tannins, cardiac glycoside, reducing sugars, terpenoids, saponins and volatile oils while steroids were not detected). The result showed a significant p function show_abstract() { $('#abstract1').hide(); $('#abstract2').show(); $('#abstract_expand').hide(); }
Diabetes mellitus, raphia sudanica, male reproduction, streptozotocin
Короткий адрес: https://sciup.org/143184719
IDR: 143184719
Текст научной статьи Ameliorative effect of aqueous-acetone extract of Raphia sudanica mesocarp on streptozotocin-induced oxidative stress and testicular damage in male Wistar rats
Diabetes mellitus (DM) is a chronic metabolic disorder of the endocrine system characterize hyperglycemia due to defect in insulin secretion, insulin action or both (Yameny, 2024).
The disorder is a public health concern due to its several complications, including reproductive disfunctions and failure of various organs. (Guo-Lian et al ., 2015). The increase in the number of young persons of reproductive age with either type 1 or type 2 diabetes is a great concern as diabetes is associated with declining fertility rate (Agbaje el al., 2007). Several animal and human studies indicate that diabetes causes male infertility due to alterations in spermatogenesis (Kumar, et al ., 2021), degenerative and apoptotic changes in testes, altered glucose metabolism in Sertoli/blood testes barrier (Yang et al ., 2023), reduced testosterone synthesis and secretion (Grossmann, 2010), reduced sex drive (libido) and ejaculatory dysfunction (Schoeller et al ., 2012) Although diabetic complications are related to chronic sustain hyperglycemia. oxidative stress has been reported as a major pathway on the pathogenesis of diabetic complications (Li et al ., 2020)
Oxidative stress may play important role in the pathophysiology of diabetes induced male reproductive dysfunction. Studies in rodent models suggest mechanisms including oxidative stress, DNA damage to sperm, altered hormonal profiles, and abnormal progression through spermatogenesis (Schoeller et al., 2012). More recently, investigations into human sperm samples from diabetic males show an increase in nuclear and mitochondrial damage suggesting that hyperglycemia may cause oxidative stress and free radical damage to sperm DNA (Schoeller et al., 2012). In conventional medical practice, the present therapies of diabetes mellitus are reported to have side effects. The medicinal plants provide a useful source of oral hypoglycemic compounds for the development of new pharmaceutical leads as well as a dietary supplement to existing therapies (Xiang et al., 2017). Some of the plants which are being used for the treatment of diabetes have received scientific or medicinal scrutiny and even the WHO expert committee on diabetes recommends that this area warrant further attention (WHO, 2016). Previous studies revealed that plants with phenolic compounds such as flavonoids and anthocyanin have positive effects on diabetes, by inhibiting the two keys’ enzymes hydrolyzing carbohydrates in the digestive tract (Laoufi et al., 2017). The genus Raphia (Palmae / Arecaceae) contains 22 species and represents a major multiuse resource across tropical Africa (Susanne et al., 2020). Raphia species provide goods that range from food to construction material and medicine (Mogue Kamga et al., 2020). Raphia species are used for medicinal purposes including inflammatory, digestive system, muscular-skeletal system, circulatory system, blood system, mental pain, subcutaneous tissue, pregnancy and birth disorders bruises, and poisoning (Gruca et al., 2015). Many parts of Raphia sudanica plant have been used traditionally for medicinal purposes. There is dearth of scientific information on possible effects of Raphia sudanica extract on sperm parameters and testicular damage in diabetic rats. This study aimed to assess the ameliorative effect of Raphia sudanica on diabetes-induced reproductive dysfunction in male Wistar rats.
MATERIALS AND METHODS
Chemicals and Reagents
Streptozotocin (STZ) (Sigma Aldrich), Aluminum Chloride (AlCl) (Fisher Scientific), Acetic acid (Fisher Scientific), Ferrous Chloride (Qualiken’s), Trichloroacetic Acid, Gallic Acid, Folin-Ciocalteu Reagent, Sodium Carbonate, Potassium Ferricyanide, Phosphate Buffers, Sulfanilic Acid. Epinephrin, Reduced Glutathione, Eliman’s Reagent 5,5’-Dithiobis-(2-Nitrobenzoic Acid) DTNB (Sigma Aldrich)
Collection and Extraction of Plant Material
The whole seeds of Raphia sudanica were obtained from a Farm in Makurdi Local Government area of Benue State and then identified by a taxonomist. The fresh whole seeds of Raphia sudanica were sun dried for one week to enable softening and easy removal of the mesocarp the mesocarp was subjected to size reduction to a coarse powder with electric grinder (JX Japan).
The air-dried power was extracted with ratio 80 20 % aqueous-acetone in three cycles using Soxhlet extractor and the residue evaporated at 450C using electric water bath. (Genlab Limited England) The residue was further stored in an airtight bottle kept in a refrigerator at 4oC till required for use.
Phytochemical Screening Assay
Phytochemical evaluation was performed on the aqueous-acetone extracts of Raphia sudanica to determine the presence of alkaloids, flavonoids, phenols, tannins, cardiac glycoside, saponin, terpenoids, steroids and volatile oils using the method described by (Trease and Evans, 1989 Harbone, 1998)
Determination of Total Phenol, Flavonoids and Tannins
The total phenolic content of aqueous-acetone extract of Raphia sudanica mesocarp was determined using the Folin-Ciocalteu colorimetric method, as previously described by (Duda-Chodak et al ., 2011), Aluminum chloride method was used for flavonoid determination using the method described by (Duda-Chodak et al ., 2011).
Experimental Animals
Twenty-five Wister albino rats weighing 200 to 250g were obtained from a stock bred at College of Health Sciences, Benue State University, Makurdi, Nigeria. The animals were kept in cages and housed at Department of Veterinary Physiology and Biochemistry Joseph Sarwuan Tarka University, Makurdi for a period of two weeks for acclimatization to laboratory setting. The rats were fed with standard commercial rat pellets (UAC Grand Cereal Ltd. Jos, Nigeria) and water were given adlibitum . The animals were used in accordance with the guideline and recommendation of the Animal Ethics and Welfare Committee on the use of animals for research Joseph Sarwuan Tarka University Makurdi with the reference number JOSTUM/CVM/ETHICS/2024/24.
Experimental Design and induction of Diabetes
Diabetes was induced by a single intraperitoneal injection of freshly prepared streptozotocin (STZ) at a dose of 40 mg/kg (dissolved in 0.1 M of cold citrate buffer pH=4.5) (Mythili et al., 2004). All the animals were provided with free access to water and pellet diet after 30 min of administration of STZ. The animals were kept under strict observation, and 48 hours after STZ injection, the fasting blood glucose level was determined using ACCU CHEK Active Glucometer. Animals with fasting (BGL) of greater than 140 mg/dL were selected and included in the study.
The animals used in study were randomly divided into five Groups (N=5) Including;
Group1 Normal Control Not induced with diabetes, received distilled water ad libitium for 28days
Group2 Diabetic non treated control rats, injected STZ 40mgkg-1
Group3 Diabetic rats treated with 5mg/kg glibenclamide
Group4 Diabetes rats treated with 100mg/kg of plant extract
Group5 Diabetic rats treated with 200mg/kg of plant extract
Collection of Samples for Measurement of relative organ weight and estimation of Antioxidants Parameters
After 28 days of treatment, rats were anesthetized on the 29th day, laparotomy was done for scrotal incision to harvest the testes, epididymis, seminal versicle, vas deferens and prostate. The organs were accurately weighed to determine the relative organ weights, and sperm analysis to be determine from the epididymis.
Relative Organ weight = (Organ Weight/ Body Weight of Rat) × 100%
Portions of the testicular samples were flushed and fixed in 10% neutral buffered formalin 72 hours for histological evaluation and the remaining portions of the testes were homogenize in 0.1M phosphate buffered saline for estimation of superoxide dismutase, catalase, glutathione peroxidase, and malondialdehyde assays.
Determination of Superoxide Dismutase (SOD) Activity
The activity of SOD was determined according method of Misra and Fridovich (1972) as adopted by (Marko et al ., 2015) Briefly 0.05 g of epinephrine was dissolved in 200 ml of distilled water containing 0.5 ml of concentrated HCl (37%).
50 µl of sample was added to 2.5 ml of 0.05M carbonate buffer (pH 10.2) and 0.3 ml of epinephrine in a cuvette, mixed by inversion and change in absorbance monitored every 30 sec for 2.5 min at 480 nm. The reference cuvette was the same as for the samples with water replacing the samples.
Calculation 1 unit of SOD activity was given as the amount of SOD necessary to cause 50% inhibition of epinephrin auto-oxidation (IEA)
1 ( A Increase in absorbance per min for sample)
% Inhibition = -— --------------------- —-—-^ x 10OT A Increase in absorbance per min for blank
SOD Activity (U/mg protein) = %IAE/50% * V 1 /V 2 *df /Protein Concentration
SOD specific activity was calculated per mg of protein and expressed in U/mg/Protein
Where Δ is change in absorbance
I - Inhibition Ratio of SOD
-
V 1 - Total Volume of Reaction
-
V 2 - Total Volume Sample Added to The Reaction
0.0436 * sample volume x mg protein/ml
df- Dilution Factor
Determination of catalase activity
Catalase activity was determined according to the method of Claiborne (1985) as cited by (Nilantika and Mahuya, 2013). Hydrogen peroxide (2.95 ml of 19 mM solution) was pipetted into a 1 cm quartz cuvette and 50 µl of sample added. The mixture was rapidly inverted to mix and placed in a spectrophotometer. Change in absorbance was read at 240 nm every minute for 5 min.
Calculation
△ Aiefrnin x reaction volume * dilution factor
Catalase, activity = -----------------------------------------Ь pmole HjOymm/mg protein
Where ΔA 240 is change in absorbance of sample per minute.
Assay for Glutathione Peroxidase Activity
Glutathione peroxidase (GPX) activity was measured according to the method by (Wallin, et. al. , 1993) Briefly, to 0.5 ml of phosphate buffer in a test tube was added to 0.1 ml of NaN 3 , 0.2 ml of GSH, 0.1 ml of H 2 O 2 and 0.5 ml of sample (added last). The reaction mixture was incubated for 3 min at 37˚C after which 0.5 ml of TCA was added and the final mixture centrifuged at 3000 rpm for 5 min. To 1 ml of the supernatants, 2 ml of K 2 HPO 4 and 1 ml of DTNB were added and the absorbance read against a reagent blank at 412nm.
GSH consumed = Change in Abs initial test/min – Change Abs blank/ ε412 × sample volume × mg protein/ml
GPX activity = GSH consumed/mg protein= µg GSH/mg protein.
Assessment of Lipid Peroxidation
Lipid peroxidation was determined by measuring the formation of Thiobarbituric acid reactive substances (TBARS) present in the test sample according to the method of Ottolenghi (1959) as reviewed by Alam, (2012).
An aliquot of 0.4 ml of the test sample was mixed with 1.6 ml of Tris-KCl buffer to which 0.5 ml of 30% TCA was added. Then 0.5 ml of 0.75% TBA was added and placed in a water bath for 45 minutes at 80oC. This was then cooled in ice to room temperature and centrifuged at 3000 rpm for 10 min . The clear supernatant was collected, and absorbance measured against a reference blank of distilled water at 532 nm.
I Absorbance x volume of mixture
Lipid peroxidation (mmol MDA/mg protein) ?=..... ....
Ебзгт « volume of sample x mg protein/ml
Where E 532nm is the molar extinction coefficient.
Evaluation of sperm parametersPreparation of Sperm Suspension
Sperm suspension was prepared according a to the method of Abu et al ., (2016).
Briefly, cauda epididymis was dissected from the testes and blotted free of blood. A 1: 20 dilution of sperm cells was carried out using 0.05% formol-saline and then incubated in a petrel dish at 37 ◦C for 30 min waiting for sperm liquefaction.
Sperm Motility
Determination of sperm motility was according to the method as described by Abu et al . (2016). Briefly, A drop of sperm of suspension was placed on a prewarmed grease free glass slide for microscopic evaluation of sperm. Individual sperm motility was assessed by counting motility at (x 40) magnification. Two hundred (200) sperm cells were examined and classified as progressive motile sperm across five different fields. Either motile or non-motile, assessment of motile sperm cells was expressed in percentage.
Sperm ConcentrationPreparation of Sperm Suspension
50 µL of semen was taken from the liquefaction and placed on a hemocytometer for determine of sperm concentration. The number of spermatozoa in an area of 2 sq mm was counted using (× 10) objective of the Olympus microscope after 3–5 minutes, Estimation of the number of spermatozoa in 1 mL of fluid was done by multiplying the number counted by 10 000.
Sperm Viability.
Sperm viability of sperm cells was determined using eosin-nigrosine according to the method of Blom (1973). Briefly, a drop of sperm suspension was mixed with a drop of eosin- nigrosine stain on a clean grease free glass slide, and the mixture was allowed to stand for 3 minutes. Then the smear was airdried and examined under the microscope (×100). Dead sperm cells were stained either partially or completely pink or red, and live spermatozoa appeared colorless. The hundred spermatozoa were randomly examined, and the percentage of live sperm cells was determined.
Sperm morphology
Sperm morphology was determined by using Giemsa stain smear made on a glass slide, fixed (absolute alcohol,5 mins) immersed in working Giemsa solution (35 mins) washed, air dried and viewed at 400x magnification, Sperm cells with normal heads and tails were counted as normal morphology, sperm cells with isolated heads, abnormal tails and fused sperm cells were counted as abnormal morphology, Estimation of the percentage of normal and abnormal morphology was done from the counting of two hundred spermatozoa Abu et al. (2016).
Acrosome Integrity
The method described by Abu et al. (2016) on the use of Giemsa stain was used to determine the acrosome integrity Two hundred spermatozoa were randomly examined across different fields and the percentage of spermatozoa with intact acrosomes was determined. The mean results were expressed as percent intact acrosome.
Histopathological Analysis
Representative samples of the testis were excised on 29th day post treatment from the normal control group of rats, untreated experimental control group rats and treated groups of rats. Testicular samples were flushed and fixed in 10% neutral buffered formalin for 72 hrs. Samples were trimmed and processed in serial grades of ethanol, cleared in Xylene, synthetic wax infiltration and embedding into Paraffin last tissue embedding media. 5μm tissue sections were cut by rotatory microtome, fixed into glass slides and stained by Hematoxylin and Eosin (H and E) stain, and examined by a histologist in blinded manner by using Full HD microscopic imaging system “(Leica Microsystems GmbH, Wentzler, Germany). All standard procedures for fixation, processing and staining of samples were done according to Culling, (2013).
Statistical Analysis
Data analysis was performed using statistical package for social sciences (SPSS) version 27 and expressed as means ± standard error of mean (SEM). One way analysis of variance (ANOVA), was used to determine statistical significance. Mean was separated using Duncan Multiple Range Test (DMRT), p≤ 0.05 was set as the level of statistical significance.
RESULTS
Percentage Extractive Yield;
The percentage yield of the aqueous-acetone extract of Raphia sudanica was found to be 16.8% w/ w = 16.8 g/ 100 g of the pulverized powder.
Phytochemical evaluation of Aqueous-Acetone Extract of Raphia sudanica (Mesocarp)
Phytochemical evaluation of crude extract of aqueous-acetone extract of Raphia sudanica (Mesocarp ) revealed the presence of phytoconstituents (Tables 1 and 2).
The extractable concentrations of total phenols, total flavonoids and tannins in fruit mesocarp extract of Raphia sudanica are presented in (Table 2).
Effect of Aqueous-Acetone Extract of Raphia Sudanica (Mesocarp) on Testicular Oxidative Stress Markers of STZ Induced Diabetic Male Rats
There was a significant (p<0.05) decrease in SOD, CAT activity and GPX activity in the diabetic control group 2 as compared to the non-diabetic group 1 and the treated groups 3, 4 and 5. (Table 3). The treatment of STZ-induced diabetes in rats with 100 mg/kg and 200 mg/kg b. w. of Raphia sudanica mesocarp extract show a significant (p<0.05) increased SOD, CAT, and GPX activities in the treated rats (Table 3). Similarly, there was a significant (p<0.05) increase in SOD, CAT activity and GPX activity in glibenclamide treated group as compared to STZ diabetic control group 2. Testicular MDA level was significantly (p<0.05) higher in the diabetic control group 2, compared to the normal control group 1 and the treated groups. The diabetic rats treated wth 100 mg/kg, 200mg/kg extract and 5 mg/kg glibenclamide significantly (p<0.05) lowered MDA level compared to the diabetic control group 2 (Table 3).
Effect of Aqueous-Acetone Extract of Rephia sudanica (Mersocarp) on Relative Reproduction Organ Weight of STZ Induced Diabetic Rats
There was a significant (p<0.05) reduction in the sizes of both right and left testes of the diabetic control group 2 as compared to the normal control group 1. There was a significant (P<0.05) reduction in the epididymal weight, weights of vas deferens, and seminal vesicles in the diabetic control 2 group when compared to the normal control (Table 4). Four weeks of treatment with 100 mg/kg and 200 mg/kg fruit mesocarp extract showed a significant (p<0.05) increase in the size of both testes, epididymis, vas deferens and seminal vesicles, when compared to the diabetic control group, while there was a significant (p<0.05) decrease in size of the prostate in the treated groups when compared to the diabetic control group. (Table 4). Similarly, the standard control rats, treated with glibenclamide (5 mg/kg), also showed a significant (p<0.05) increase in the size of both testes, epididymis, vas deferens and seminal vesicles, when compared to the diabetic control group, while there was a significant (p<0.05) decrease in size of the prostate in the treated groups when compared to the diabetic control group.
Effect of Aqueous- Acetone Raphia sudanica (Mesocarp) on Sperm Parameters
STZ Induced Diabetic Male Rats
There was a significant (p<0.05) decrease in the sperm motility of the diabetic control group 2 rats when compared to the normal control group 1 and groups 4 and 5 that received 100 and 200mg/kg crude extract of Raphia sudanica, mesocarp. Similarly, there was significant (p<0.05) decrease in sperm concentration and sperm viability in the untreated STZ induced diabetic rats’ group 2, when compared to the normal control and groups 3, 4 and 5 that received treatment. Also, there was a significant (p<0.05) decrease in normal sperm morphology with a high abnormal morphology expressed in the group 2 rats that was given STZ but did not receive treatment as compared to the normal control and groups 3, 4 and 5 that received treatment. These results also showed there was a significant (p<0.05) decrease in intact acrosome integrity with a corresponding increase in the not intact acrosome integrity of the untreated group 2 rats when compared with the normal control.
However, the treatment of STZ-induced diabetic rats with R. sudanica extract (100 mg/kg and 200 mg/kg b. w.) and glibenclamide significantly (p<0.05) improved the sperm motility, viability, concentration, morphology and acrosome integrity when compared with the untreated.
Effect of Aqueous-Acetone Extract of Raphia Sudanica (Mesocarp) on Testicular Histology of STZ Induced Diabetic Male Rats
Histological examination of different testicular tissue sections showed normal tubular morphology and germinal epithelial cell density in the normal control group On the other hand, Diabetic control group 2 showed abnormal tubular morphology characterized by severe loss of germinal epithelian (Fig. 3B) as compared to the normal control group1.The diabetic group 3 which received reference standard drug, glibenclamide (5mg/kg) showed mild exfoliation of spermatocytes aggregated in the luminal space and intact primary germ cells.(Fig. 4c) While the diabetic groups 4 and 5 which were treated 100mg/kg and 200mg/kg plant extract showed improved morphology and germinal cell density of the seminiferous tubules of the treated rats (Fig. 4D and 5E).
Table 1: Phytochemical Constituents of Aqueous-Acetone Extract of Raphia sudanica (Mesocarp)
|
Phytochemical Constituents |
Inference |
|
Alkaloids |
+ve |
|
Phenols |
++ ve |
|
Flavonoids |
++ve |
|
Tannins |
+ve |
|
Glycosides |
+ve |
|
Terpenoids |
+ve |
|
Reducing sugars |
+ve |
|
Steroids |
-ve |
|
Saponins |
++ve |
|
Volatile oils |
+ve |
(+v) denotes presence and (- v) denotes absence of phytochemical constituent
Table 2: Total Flavonoids, Phenolic, and Tannin Contents of Aqueous-Acetone Extract of Raphia sudanica (Mesocarp)
|
S/No |
Phytochemical |
Concentration (µg/ml) |
|
1 |
Flavonoids |
59.28 ± 0.38 |
|
2 |
Phenols |
50.62± 0.44 |
|
3 |
Tannins |
49.07± 1.30 |
Mean± SEM based on three observations
Table 3: Effect of Aqueous-Acetone Extract of Raphia sudanica (Mesocarp) on Testicular Oxidative Stress Markers of STZ Induced Diabetic Male Rats
|
Treatments groups |
SOD U/mg/ protein |
CAT Unit/mg/ protein |
GPx U/mg/ protein |
MDA mmol/mg/ protein |
|
1 Normal Control |
153.63 ± 25.07b |
1.35 ± 0.11c |
1.96 ± 0.20b |
4.20 ± 0.44b |
|
2 Diabetic Control |
54.44 ± 4.29a |
0.33 ± 0.01a |
0.47 ± 0.16a |
8.22 ± 0.58c |
|
3 Glibenclamid, 5 mg/kg |
131.51 ± 17.03b |
0.83 ± 0.05b |
1.50 ± 0.31ab |
2.66 ± 0.22a |
|
4 100 mg/kg |
151.68 ± 1.78b |
1.10 ± 0.12c |
1.17 ± 0.57ab |
2.49 ± 0.37a |
|
5 200 mg/kg |
158.07 ± 15.6b |
1.15 ± 0.04c |
1.41 ± 0.21ab |
3.87 ± 0.15a |
Values are mean ± standard error of mean (SEM), (n = 3). Values with different superscript alphabets in a column are significantly different at p<0.05.
SOD = Superoxide Dismutase GPx = Glutathione peroxidase MDA = Malondialdehyde. CAT= Catalase
Group 1 Normal Control Not induced with diabetes but received 1ml normal saline
Group 2 Disease Control Induced with STZ without treatment
Group 3 Induced with STZ and treated with 5 mg/kg glibenclamide
Group 4 Induced with STZ and treated with 100 mg/kg plant extract
Group 5 Induced with STZ and treated with 200 mg/kg plant extract
Table 4: Effect of Aqueous-Acetone Extract of Raphia sudanica (Mesocarp) on relative Reproductive Organ Weight of STZ Induced Diabetic Male Rats
|
Groups |
Reproductive organ weights |
Right testis (g) |
Left testis (g) |
Epididymis (g) |
Vas. deferens |
Seminal vesicles (g) |
Prostate (g) |
|
1 |
Normal Control |
0.562±0.047b |
0.660± 0.023c |
0.237±0.012c |
0.040±0.000b |
0.187±0.007bc |
0.050± 0.001a |
|
2 |
Diabetic Control |
0.370±0.030a |
0.376±0.030a |
0.136±0.007a |
0.014±0.002a |
0.128±0.007a |
0.131±0.009b |
|
3 |
Glibenclamide 5 mg/kg |
0.474±0.049ab |
0.510±0.050ab |
0.199±0.014b |
0.038±0.002b |
0.201±0.012c |
0.062±0.004a |
|
4 |
100 mg/kg |
0.562±0.081b |
0.594±0.076bc |
0.189±0.011b |
0.038±0.002b |
0.193±0.012bc |
0.069±0.014a |
|
5 |
200 mg/kg |
0.442±0.019ab |
0.502±0.025ab |
0.191±0.009b |
0.037±0.004b |
0.167±0.007b |
0.055±0.005a |
Table 5: Effect of Aqueous-Acetone Extract of Raphia sudanica (Mesocarp) on Sperm Parameters of Streptozotocin Induced Diabetic Male Rats
|
Treatment Groups |
Sperm motility (%) |
Sperm Cont. (×106)/ml |
Viability(%) |
Morphology (%) |
Acrosome Integrity % |
|||
|
Live Sperm |
Dead Sperm |
Normal |
Abnormal |
Intact |
Not Intact |
|||
|
I Normal Control |
87.60±2.37c |
38.3±9.68b |
79.90±3.49c |
18.10±2.51a |
89.24±2.59c |
14.80±2.66a |
82.70±1.70c |
16.30±1.42a |
|
2 Diabetic Control |
15.00±3.53a |
10.8±1.14a |
25.11±2.88a |
73.40±3.57d |
18.81±2.92a |
84.10±2.66d |
16.80±1.69a |
83.20± 1.69c |
|
3 Glibenclamide 5mg/kg |
69.00±3.67b |
32.0±1.44b |
67.48±1.29b |
32.39±2.21c |
89.66±1.87c |
22.70±1.57bc |
77.20±3.01bc |
22.80± 3.01ab |
|
4 100mg/kg |
75.00±1.58b |
39.4±2.15b |
74.40±25.60c |
25.60±1.07b |
89.56±2.85c |
17.70±0.98ab |
82.70±1.12c |
17.30± 1.12a |
|
5 200mg/kg |
67.00±1.22b |
30.8±1.30b |
66.00± 0.57b |
34.00±0.57c |
72.80±2.21b |
26.40±2.57c |
73.10±3.59b |
26.90± 3.59b |
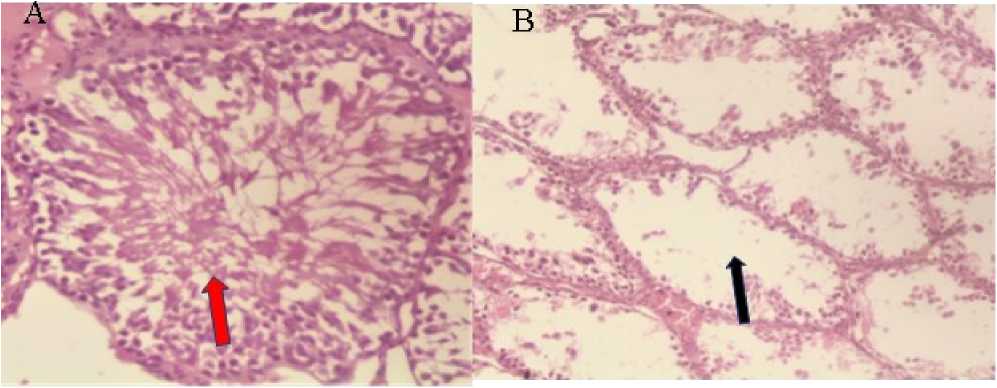
Figure 1: Photomicrograph of seminiferous tubules of the testis normal control rats in group 1 (A) showing ( red arrow) normal tubular morphology and germinal epithelial cell density. (H&E x400), and (B) Photomicrograph of the testis of STZ induced diabetic rats showing (black arrow) abnormal tubular morphology characterized by severe loss of germinal cells in diabetic control rats’ group 2. (H&E x400).
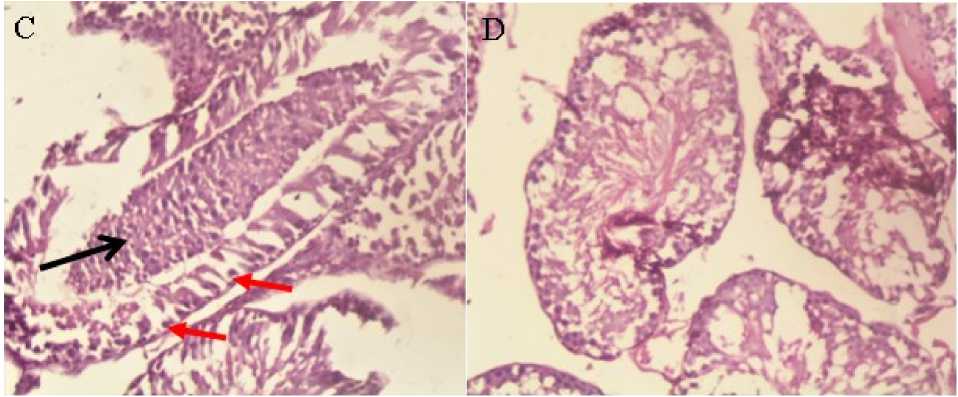
Figure 2: Photomicrographs of seminiferous tubules of the testis of STZ induced diabetic rats treated 5 mg/kg glibenclamide, group3 ( C ) showing mild exfoliation of spermatocytes (arrow) aggregated in the luminal space and intact primary germ cells (arrows) (H&E x400) and photomicrographs of testis showing normal tubular morphology and germinal cell density of the seminiferous tubules of STZ induced diabetic rats treated with 100 mg/kg of mesocarp extract group 4 ( D ) (H&E x400).
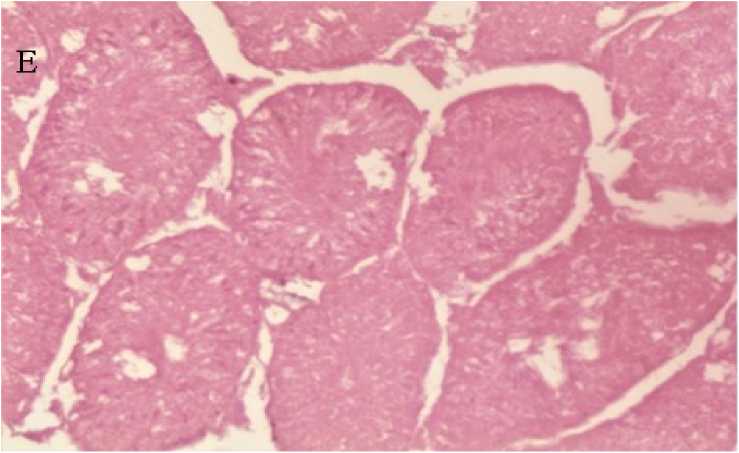
Figure 3: Photomicrograph of testis showing normal tubular morphology and germinal cell density of the seminiferous tubules of STZ induced diabetic rats treated with 200mg/kg of Raphia sudanica mesocarp extract, group 5 ( E ) (H&E x400)
DISCUSSION
This study reported the effect of aqueous acetone mesocarp extract of Raphia sudanica on sperm characteristics and oxidative stress in STZ- induced diabetic Wister rats.
Oxidative stress is implicated in the pathogenesis of diabetic spermatogenetic disease. Oxidative stress occurs when ROS overwhelm the endogenous antioxidants defense system (Birben et al ., 2012).
researchers have reported Signiant decrease in antioxidants enzymes in the testis of diabetic rats (Furukawa et al ., 2017). Studies have shown decrease testicular SOD, CAT, and GSH-Px activities in STZ – induced diabetic rats (Zheng et al ., 2023).
In the present study, the significant increase in antioxidant enzymes (SOD, CAT, and GSH-Px) activities in the group treated 100mg/kg and 200mg/kg extract of Raphia sudanica mesocarp may suggest that the possible ameliorative effect of the extract in improving testicular damage in STZ induced diabetic animals.
Malondialdehyde (MDA) is a common biomarker of lipid peroxidation status (Varsha et al ., 2014) Diabetes mellitus can result in an increase in the levels of testicular malondialdehyde (MDA) a product of lipid peroxidation, which could lead to oxidative injury (Kotian et al ., 2019). Elevation in MDA level indicates the degree of injury in biological tissues (Ochei and Kolhatkar 2008) It was observed that MDA level increased in the diabetic control group when compared with normal control. Treatment with 100mg/kg and 200mg/kg b.w of aqueous -acetone extract of Raphia sudanica mesocarp (Table 3) resulted in a significant fall of MDA and increase levels of SOD, CAT and GPx of the diabetic rats in response to the treatment with Raphia sudanica mesocarp extract is suggestive of its beneficial effect against oxidative stress. phytochemicals present in the extract (table 1) are important sources of antioxidants and are non-toxic. The high levels of phenols compounds in the extract are suggestive of its protective effect against oxidative stress and cellular damage. (Doe et al ., 2021).
Sperm characteristics, (counts, progressive motility, morphology) are considered as markers for testicular function. Diabetes disrupts spermatogenesis by the production of ROS and lipid peroxidation which adversely affect sperm motility and morphology. In the present study, we study we observed significant p < 0.05 weight reduction in the testes, epididymis, vas deferens, and seminal vesicles with an increase in the weight of prostate in the diabetic control rats, (Table 4). These findings agreed with Kotian et al. (2019) who reported that the gross weight of the testes and the epididymites were considerably reduced in the STZ-induced diabetic test group. Previous study by Guneli et al. (2008) showed that there was great reduction in the number of spermatogenic cells in the rats of the diabetic control groups when compared with the normal control group. The result of our study reveals significant (p < 0.05) improvement in the testicular weight, the weights of epididymis, vas deferens, and seminal vesicle and a reduction in the weight of prostate, following the treatment of STZ-induced diabetic rats with R. sudanica mesocarp extract. This may suggest that aqueous-acetone of Raphia sudanica mesocarp extract has protective effect against the adverse effects of STZ induced diabetes on the reproductive organs in male rats.
Our study further reveals a significant (p < 0.05) alterations in the biomarkers of reproductive parameters in male rats in the diabetic control group as compared to the normal control group and groups treated with 100 mg/kg and 200 mg/kg R. sudanica mesocarp extract. However, the result showed a significant improvement in sperm parameters following the treatment of STZ-exposed rats with aqueous-acetone extract of R. sudanica mesocarp (Table 5). This suggests that aqueous-acetone extract of R. sudanica mesocarp has protective effect against the adverse effects of STZ induced diabetes on the reproductive functions of male rats.
The histological studies done in the study showed a normal tubular morphology and germinal epithelial cell density in normal control group 1 (Fig. 1) (Smith et al ., 2022) and the testis of STZ induced diabetic rats showing abnormal tubular morphology characterized by severe loss of germinal cells in diabetic control rats’ group 2 as compared to the treated. Previous studies have shown that exposure to free radicals in diabetic rats leads to testicular damage (Smith et al ., 2022). The findings of the present study as revealed by the histopathological results confirm the protective effect of the extract against organ damage due to diabetes. The protective effect of the extract may be due to its free radical scavenging properties and subsequently reduction in oxidative damage of the testis.
CONCLUSIONS
In conclusion, the present study has shown that Raphia sudanica mesocarp extract has potential to reduce oxidative stress in diabetic male rats by enhancing the antioxidant capacity. In addition, our findings suggest that administration of the extract may protect against diabetes induced male reproductive dysfunction.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
ACKNOWLEDGEMENT
The authors acknowledged technical assistance of Mr. Daniel. W. Achanya of the Department of Veterinary Pharmacology and Toxicology, Joseph Sarwuan Tarka University , Makurdi.


