An influence of the kinetic parameters of the reaction on a size of obtained nanoparticles at the reduction of silver ions by hydrazine
Автор: Kytsya Andriy Romanovych, Hrynda Yuriy Mykolayovych, Bazylyak Liliya Igorevna, Zaikov Gennady Efremovich
Журнал: НБИ технологии @nbi-technologies
Рубрика: Социально-экономические инновации
Статья в выпуске: 2 (9), 2013 года.
Бесплатный доступ
Kinetics of the reaction of silver nitrate reduction by hydrazine in the presence of sodium citrate in alkaline medium was studied in a wide range of the reagents concentration variation. The orders of the reaction were determined and the effective constants of the silver nanoparticles nucleation process rate and of their propagation one were calculated. It was investigated the optical characteristics of the obtained sols of the silver nanoparticles. It was determined the empirical dependence of a size of the obtained silver nanoparticles on the kinetic parameters of a process.
Silver nanoparticles, nucleation, nanoparticles growth, kinetics, hydrazine
Короткий адрес: https://sciup.org/14968299
IDR: 14968299 | УДК: 537.5282
Текст научной статьи An influence of the kinetic parameters of the reaction on a size of obtained nanoparticles at the reduction of silver ions by hydrazine
1. Introduction a synthesis of the nanoparticles of noble metals,
2. Experimental Section
studies of their properties and practical application An exponential growth in a field of the is observed for the last decades. Silver fundamental and applied sciences connected with nanoparticles (Ag–NPs) are characterized by unique combination of the important physicalchemical properties, namely by excellent optical characteristics caused by the phenomena of the surface plasmon resonance [5], by ability to amplify the signal in spectroscopy of the combination dispersion and also by high antibacterial properties. Despite the fact, that there are a number of methods for the synthesis of different upon nature nanoparticles and nanomaterials describing in references [3; 4; 10; 11; 14; 15], however the kinetic peculiarities and regularities of the formation (nucleation and propagation) of nanoparticles studied insufficiently and has the episodical character [2; 6; 6].
That is why the aim of the presented work was to investigate an influence of the synthesis conditions on the kinetic parameters of a process and also on the form and on the size of the synthesized Ag–NPs.
Ag–NPs were obtained in accordance with the reaction (1) via reduction of silver nitrate by hydrazine in aqueous medium in the presence of sodium hydroxide at 20 0C:
4 AgNO3 + 4 NaOH + N2H4 =
3. Results and Discussion
= 4 Ag + 4 NaNO 3 + 4 H 2 O + N 2 - (1)
Sodium citrate has been used as the stabilizer at its concentration I x lO-4 mole/l in the reactive solution.
Kinetic of the reaction was studied by direct potentiometry method with the use of the ion-selective microelectrode «ELIS–131 Silver». The concentration of the silver ions was determined continuously during the reaction proceeding per change of the potential of the ion-selective electrode regarding to the chlorine-silver comparison electrode. In order to avoid the hit of the chlorine ions into the reactive mix the salt weak link with the potassium nitrate was used.
The form and the average diameter of the silver nanoparticles were estimated with the use of the scanning electron microscopy EVO–40XVP (Carl Zeiss) with a system of the X-ray microanalysis INCA Energy, XRD-analysis and also on a basis of the adsorption spectra of the surface plasmon resonance of Ag–NPs sols with the use of the single-beam spectrophotometer UV-visible range UVmini–1240 (P/N 206–89175–92; P/N 206–89175–38; Shimadzu Corp., Kyoto, Japan).
XRD–analysis was carried out with the use of XRD diffractometer DRON-3.0 with Cu-K a irradiation ( l = 1,5405 nm). Data have been analyzed by full-profile revision in accordance with the Ritveld’s method with the use of the simulation package GSAS (General Structure Analysis System).
In order to explain and to investigate the chemical process of the silver nanoparticles synthesis the kinetic characteristics have been investigated, namely the change of the concentration of the silver ions during the experiment. Typical kinetic curves of the silver concentration change via time are represented on Fig. 1.
As we can see, the starting section of the kinetic curve corresponds to the stage of the nucleus centers formation and the following sharp decrease of the concentration of silver ions corresponds to their growth stage. Therefore, the time (t0) of the initial section of the kinetic curve determines the Ag– NPs nucleation rate (W0 = 1/t0), and the tangent of the angle of inclination of its quick linear section determines the rate of the nucleus growth (W max ).
It was investigated an influence of the change of starting concentrations of silver nitrate, sodium hydroxide and hydrazine on kinetic parameters of a process. In order to increase the trustworthiness of the results, the series of investigations consisting of 7–10 experiments has been carried out for every concentration. Obtained data were averaged. The ratio error at the W0 and W max determination not exceeds 25 %.
Taking into account, that the reagents change concentration at the nucleation stage and also at the stage of the starting section of the silver NPs growth is insignificant, it can be used with some approximation the dependencies of the rates of a process on the starting concentrations of reactive mix components in order to determine the orders of the reaction.
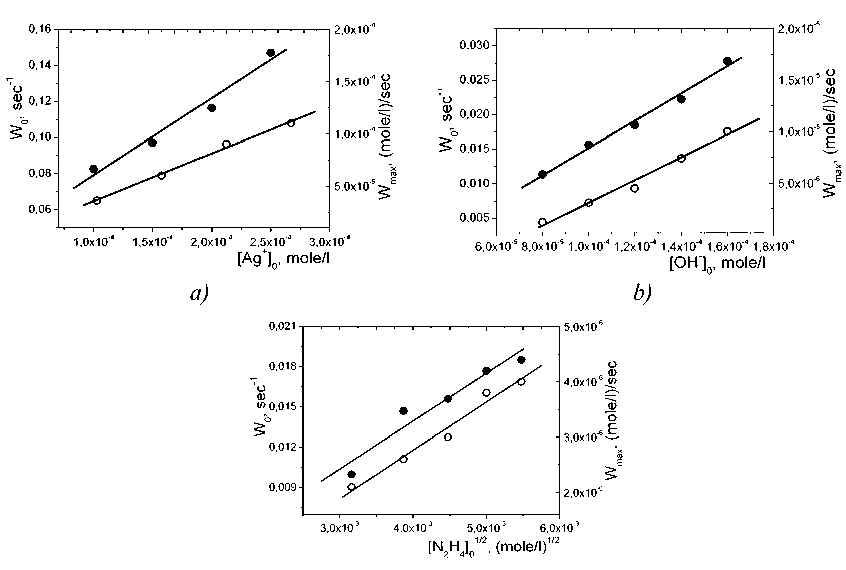
Presented on Fig. 2 data indicate that the orders of the reaction per every among reagents for the processes of silver nanoparticles nucleation and growth are agreed and are equal to 1, 1 and 1/2 for AgNO3, NaOH and N2H4 correspondingly.
Taking into account the determined orders of the reactions per every components, obtained experimental results can be represented in the coordinates of the acting masses law (2):
W = k # [Ag + ] 1 x [OH-]1 x [N 2 H 4 ]1 / 2 (2)
and the numerical values of the effective constants rates of the processes of new phase nucleation (kf# = (2,2±0,2)x108 (mole/l)-2-5xs-1) and its growing (kg# = (1,8±0,4)x105 (mole/l)-2xs-1) can be estimated (fig. 3).
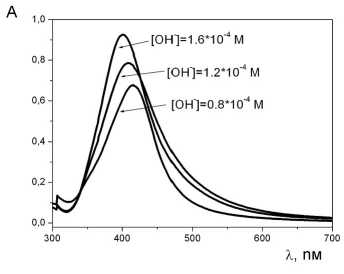
In order to identify the obtained silver nanoparticles, their spectral characteristics were investigated (Fig. 4 а). The all spectra of silver nanoparticles adsorption are characterized by one maximum corresponding to their spherical form [5]. Analyzing the references, it was discovered that the value of the square of wave frequency in adsorption maximum of the surface plasmon
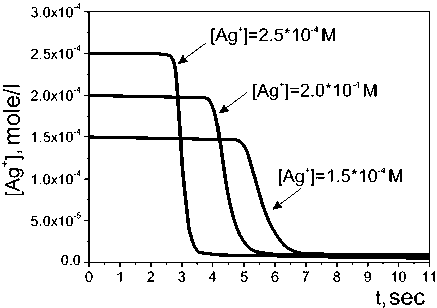
Fig. 1. Kinetic curves of the reduction reaction of silver ions by hydrazine at different starting concentrations of the silver nitrate
c)
Fig. 2. Dependencies of the silver nanoparticles nucleation rate W0 ( • ) and of the growth rate W max ( o ) on the initial concentrations of AgNO3 (а), NaOH (b) and N2H4(c).
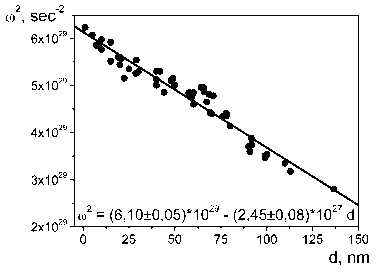
resonance of silver nanoparticles linearly depends on their size (Fig. 4, b), that gives the possibilities to calculate an average diameter of the obtained silver nanoparticles. Calculated values of the average diameter of silver nanoparticles consist of 13–35 nm depending on the synthesis conditions.
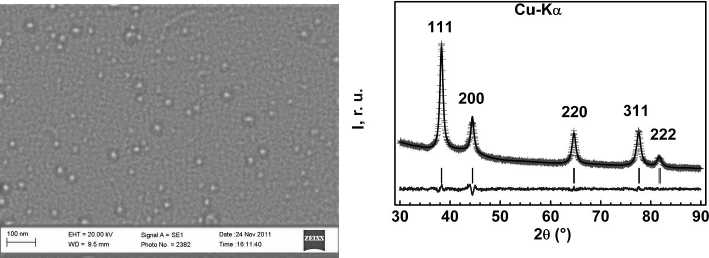
Silver nanoparticles obtained at 20 0С and starting concentrations of reagents [AgNO3]0 = 2,5 х 10-4 М, [NaOH]0 = 3,0 х 10-4 М, [N2H 4 ]0 = 7,5 х 10-5 М were investigated with the use of the SEM and XRD analysis methods for confirmation of the obtained calculations (Fig. 5). On a basis of the XRD–analysis results, an average size of the silver crystallites was calculated; it consists of DV = 9,3 nm; respectively the diameter of the spherical particle for monodisperse system is D = 4/3 and DV = 12,4 nm. Calculated accordingly to the location of the surface plasmon resonance adsorption maximum of the sol silver nanoparticles (Fig. 4 b) value of average diameter of silver nanoparticles obtained under such conditions consists of 13 nm.
At the analysis of the experimental data it was found that the size of the obtained silver nanoparticles depends on the ratio of the nuclear centers formation rate and the nuclear centers growth rate (Fig. 6).
Such dependence can be explained by fact that at the nuclear centers formation rate increasing not only the concentration of the nuclear centers is increased, but also their critical radius is decreased that leads to the decreasing of the average size of synthesized nanoparticles. Evidently, such dependence is the partial case and is determined by the conditions of the synthesis and by the choice of the reagents, however permits to obtain the silver nanoparticles by the controlled size.
4. Conclusions
The reaction of the silver ions reduction by hydrazine has been investigated in alkaline medium in the presence of the sodium citrate. It was
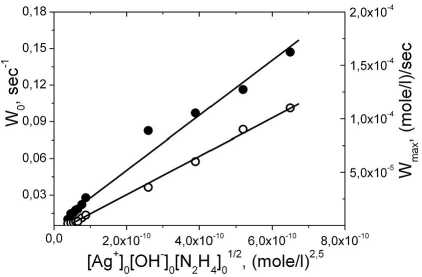
Fig. 3. Dependencies of W0 ( • ) and W max ( o ) on composition of the initial concentrations of the reagents in coordinates of the equation (2).
а )
b )
Fig. 4. Adsorption electron spectra of silver nanoparticles obtained at different starting concentrations of sodium hydroxide (а) and calibration plot for calculation of their average diameter (b) based on the references [6–7; 11–15]
а ) b )
Fig. 5. SEM image (а) and XRD–spectrum (b) of silver nanoparticles obtained at the starting concentrations [AgNO 3 ] 0 = 2,5 x 10-4 mole/l, [NaOH]0 = 3,0 x 10-4 mole/l, [N 2 H4]0 = 7,5 x 10-5 mole/l.
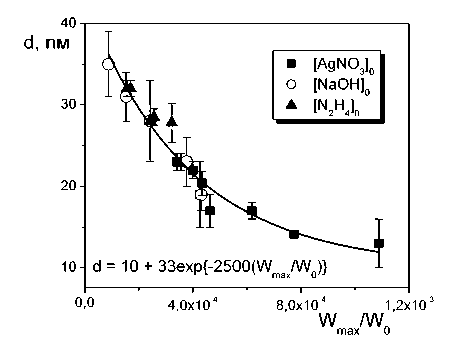
Fig. 6. Dependence of an average diameter of silver nanoparticles on kinetic parameters of the process: an average diameter of silver nanoparticles obtained at different starting concentrations of AgNO3 (?), NaOH ( o ) and N2H4 ( A ).
determined that the rates of the nucleation and of the growth of silver nanoparticles linearly depend on the initial concentrations of silver nitrate, sodium hydroxide and on concentration of hydrazine in degree 1/2. It was discovered the empirical dependence of a size of obtaining nanoparticles on the ratio of the rates of growth and nucleation of silver nanoparticles.
Acknowledgment
This work was performed via the framework of the Task Complex Program TCPSI «RESURS» (grant # Р 8.2–2013/K).
Список литературы An influence of the kinetic parameters of the reaction on a size of obtained nanoparticles at the reduction of silver ions by hydrazine
- Bryukhanov V.V., Tikhomirova N.S., Gorlov R.V, Sliezhkin V.A. An interaction of surface plasmons of silver nanoparticles on silokhrome with electron excited adsorbates of molecules of rhodamine 6Zh. Izviestiya KGTU. 2011, 23, 11-17.
- Chou K.S., Lu Y.C., Lee H.H. Effect of alkaline ion on the mechanism and kinetics of chemical reduction of silver. Materials Chemistry and Physics. 2005, 94, 429-433.
- David D. Evanoff Jr., Chumanov G. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. Chem. Phys. Chem., 2005, 6, 1221-1231.
- Egorova Е.М., Revina A.A. and ect. Bactericidal and catalytic properties of stable metallic nanoparticles in reverse micelles Vestn. Mosc. Univ. Ser. 2. Khimiya. 2001, 42 (5) 332-338.
- Henglein A. Small-Particle Research: Physicochemical Properties of Extremely Small Colloidal Metal and Semiconductor Particles. Chem. Rev. 1989, 89 (8), 1861-1873.
- Ignatyev A.I., Nashchyokin A.V., Nieviedomsky V.М., Podsvirov О.А., Sidorov A.I., Solovyov A.P., Usov О.А. Peculiarities of silver nanoparticles formation in photothermorefractive glasses at electron irradiation. Journal of technical physics. 2011, 81 (5), 75-80.
- Information about products. URL: http://nanocomposix.com/products/silver/spheres.
- Information about products. URL: http://www.sigmaaldrich.com/materials-science/nanomaterials/silverna-noparticles.html.
- Khan Z., Al-Thabaiti S.A., El-Mossalamy E.H., Obaid A.Y. Studies on the kinetics of growth of silver nanoparticles in different surfactant solutions. Colloids and Surfaces B: Biointerfaces. 2009, 73, 284-288.
- Krutyakov Y.A., Kudrinskiy A.A., Olenin A.Y., Lisichkin G.V. Synthesis and properties of silver nanoparticles: advances and prospects. Russ.Chem.Rev. 2008, 77(3), 233-258.
- Kryukov A.I., Zin’chuk N.N., Korzhak A.V., Kuchmii S.Ya. The effect of the conditions of catalytic synthesis of nanoparticles of metallic silver on their plasmon resonance. Theor. Exp. Chem. 2003, 39 (1), 9-14.
- Munro C.H., Smith W.E., Garner M., Clarkson J., White P.C. Characterization of the Surface of a Citrate-Reduced Colloid Optimized for Use as a Substrate for Surface-Enhanced Resonance Raman Scattering. Langmuir, 1995, 11, 3712-3720.
- Podlyegayeva L.N., Russakov D.M., Sozinov S.A., Morozova Т.V., Shvaiko I., Zvidentsova N.S., Kolesnikov L.V. An investigation of properties of silver nanoparticles obtained by the reduction from the solutions and by thermal sputtering in vacuum. Vestnik Kemerovskiego gossudarstvennogo universiteta. 2009, 2 (38), 91-96.
- Pomogaylo A.D., Rozenberg A.S., Uflyand A.S. Nanoparticles of metals in polymers. M., Khimiya, 2002, 672 p.
- Suzdalyev I.P. Nanotechnology: physico-chemistry of nanoclaters, nanostructures and nanomaterials.M., KomKniga, 2006, 592 p.


