Anaerobic germination potential in rice (Oryza sativa L.): role of amylases, alcohol deydrogenase and ethylene
Автор: Senapati Swetaleena, Kuanar Sandhya Rani, Sarkar Ramani Kumar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.15, 2019 года.
Бесплатный доступ
Rice is cultivated either through wet and dry direct seeding or transplanting. Under direct seeding seeds are sown in the field whereas under transplanting seedlings are planted. Though direct seeding is economical and environment friendly, it is riskier than transplanting due to soil flooding and other menaces. Anaerobic germination (AG) potential or tolerance to germination stage oxygen deficiency (GSOD) is a feature that enables rice to grow under soil flooding. In the present investigation, twenty-two rice genotypes were characterized for their AG potential / GSOD tolerance regarding different growth parameters as well as amylolytic and fermentative activities under different days of soil flooding. Significant genotypic variations were noticed for these parameters at different days of soil flooding. Differences between control and treatment were highly significant. The activities of these enzymes also varied significantly under different days of soil flooding. A-amylase and total-amylase activities showed significant positive association with seedling establishment under flooding...
Cultivation, direct seeded rice, germination and establishment, germination stage oxygen deficiency, soil flooding
Короткий адрес: https://sciup.org/143168571
IDR: 143168571
Текст научной статьи Anaerobic germination potential in rice (Oryza sativa L.): role of amylases, alcohol deydrogenase and ethylene
Cultivation of rice through direct seeding (DSR) have several benefits as compared to transplanting method of rice cultivation (TPR). However, on risk point of view, DSR is riskier than TPR. Under DSR seeds are sown either below the soil surface (dry seeding) or above the wet soil surface (germinated seed on the surface of puddled soil) or into standing water (seeds are broadcast on the surface of standing water). It is practiced throughout the globe. Direct seeding is the principal method of cultivation in Russia, and other European countries, Korea, and America (Akhgari, Kaviani, 2011). The main hurdle in direct seeding is to maintain proper plant stand. The seeds sown on the surface of the soil may be scattered due to torrential rain, may get damage due to the attack of birds and other raider. As rice and weed grow simultaneously under DSR, weed infestation is greater under DSR as compared to TPR. Seedlings may flow from one place to another place due to poor anchoring. Plant stand is sub-optimal that affects yield (Sarkar et al ., 1999; Sarkar, Das, 2003). Placing seeds below the soil surface may overcome the damage from birds and other predators, from splattering, however, may encounter serious threats due to untimely excess rain and soil flooding. Stagnation of water on soil surface deprive the germinating seeds from availability of oxygen (Ray et al ., 2016): the entire land looked to be barren due to lack of proper germination and plant establishment (Sarkar et al ., 1999). The setback is now more prominent due to climate change. Direct seeding of rice requires less labour, less energy and less water compared to transplanted rice (Kumar, Ladha, 2011; Liu et al ., 2015 ). Kuchanur et al . (2018) reported that farmer could earn Rupees 30,900/hectare (≈ $ 441, exchange rate Rupees 70/$) more by practicing DSR than TPR, yet the adoption rate of it in South-east Asian countries is not encouraging due to the problems associated with DSR. The problems described here related to DSR could be solved if rice possesses the characteristics to germinate and grow under deprived oxygen levels. Sowing seeds below the soil surface saves seeds from predators and torrential rains. Stagnation of water (5-10 cm) above the soil surface reduces weed infestation (Sarkar, 2012).
Though rice is comparatively more tolerant in anaerobic condition as compared to other cereals, yet all rice genotypes are not equally tolerant. Genotypic variation to flooding tolerance exists in rice (Sarkar et al., 1999; Ismail et al., 2009; Agbeleye et al., 2019). Depending on the stage of growth rice behaves differently to encounter the deleterious effects of excess water stress. Rice encounters excess water stress during germination stage through germination followed by elongation of coleoptile. This helps to push the leaf tip above the water surface (Das et al., 2004; Senapati et al., 2019). Submergence tolerance is somewhat different from it. Here elongation growth is checked and plant remains under water for 1-2 weeks and on desubmergence starts to grow (Panda et al., 2006; Xu et al., 2006). There is another strategy where with rising water level rice plant starts to elongate so that leaf tip remains above the water always and avoids submergence stress (Hattori et al., 2011). This strategy is required under very deep-water condition where stagnation of water exceeds more than 100 cm. Excessive elongation is not always beneficial. It helps in survival with yield penalty (Kuanar et al., 2017). Maintaining optimum tiller numbers per unit land is the key to get grater yield under partial submergence (Sarkar, 2016). Excess water stress affects rice growth differently at different growth stages by adopting different mechanisms (Xu et al., 2006; Hattori et al., 2011; Panda, Sarkar, 2013; Vijayan et al., 2018) and thus, the basis of tolerance worked out in relation to submergence / partial submergence is not totally applicable during germination stage excess water stress. Germination is an active process that requires greater energy to sustain growth. Soil flooding during germination stage restricts oxygen supply to the germinating seeds and therefore induces alcoholic fermentation. Alcohol dehydrogenase (ADH) is an important enzyme that supposed to increase under low oxygen level to sustain germination and growth (Takahashi et al., 2014; Vijayan et al., 2018). During germination non-structural carbohydrate (NSC) available in the endosperm is the sole supplier of energy. NSC is break-down due to the action of α-amylase, and other amylolytic enzymes (Panda et al., 2017). Germinating embryos received their energy requirement through the action of these hydrolytic enzymes. Therefore, to study the GSOD tolerance or AG potential in relation to the activities of these enzymes have great bearing to open up new insight. Submergence over-express the activities of these enzymes both in tolerant and susceptible genotypes (Takahashi et al., 2014; Panda et al., 2017; Vijayan et al., 2018). How far over-expression of these enzymes contribute to survival is not known as because most of the studies dealing with GSOD tolerance took 24 genotypes of extreme variability and drew the conclusion. In the present investigation large numbers of genotypes with different levels of sensitivity were taken, their growth parameters along with activities of amylases, fermentative enzyme and ethylene releasing capacity were determined. A time course study was made with the enzymes of interest and ethylene releasing capacity under different days of soil flooding. Grouping of genotypes were made based on Euclidean distance method so that genetic distance of different genotypes was apparent. The main goal of the investigation was to determine the contribution of the alcohol dehydrogenase and starch degrading enzymes and ethylene releasing capacity in imparting the seedling germination and establishment under soil flooding. Additional goal was to identify new genetic resources tolerant to germination stage oxygen deficiency. The new genotypes would be useful in developing high yielding rice suitable for direct seeding condition under excess water stress.
MATERIALS AND METHODS
Seed materials and ending of seed dormancy
The experiment was conducted with twenty two genotypes. Naveen, susceptible to GSOD and AC41620A, tolerant to GSOD were also included in this investigation (Vijayan et al ., 2018). The experiment was conducted with the seeds which were collected in the previous season. Dormancy of the seeds was broken by putting them in an oven at 48 ± 2 0C for five days.
Determination of AG potential
Polypropylene trays (37.5 x 35 x 13.0 cm) containing 2 cm depth of dried and pulverized soil were used to determine the AG potential of each genotype. Hundred seeds of each genotype were sown 1.0 cm below the soil surface. Seeds were covered properly with soil.
Submergence was provided with 10 cm depth of water immediately after sowing. A control set was run along with the treated set where instead of soil flooding saturated condition was maintained. Soil flooding treatment was given for 3 weeks. The seeds which germinated under water and successfully pushed their leaves / coleoptiles above the water surface under soil flooding were considered as successfully established plants (Ismail et al ., 2009). Seedling establishment (SE) % was calculated by multiplying with 100 of the ratio i.e. numbers of seedling emerged out from water or soil surface / numbers of seeds sown. The experiment was repeated thrice. Temperatures of the flood water varied from 25.6 to 32 0C, whereas oxygen levels varied from 2.3 to 4.7 mg L–1 during the entire period of experiment.
Determination of seedling length and dry weight and vigour index
Seedling length was measured with simple ruler taking measurement from the base to the tip of the plant and expressed in cm. Dry weight of shoot was taken by putting the harvested plant in an oven at 65 ± 2 0C. After 5 days dried sample was taken out from the oven and cooled down to room temperature by putting them in desiccators. Dry weight was taken using a precision balance. Vigour index was estimated following the method of Abdul-Baki, Anderson (1973).
Vigour Index (V.I.) = Seedling establishment (%) x Seedling length (cm)
Determination of amylases and alcohol dehydrogenase activity
The extraction and assay of the enzymes were done taking de-husked endosperm after 1, 3, 5, and 7 days of germination. The extraction was carried in ice-cold buffers. The extract was centrifuged at 15, 000g for 20 mins at 4oC. The supernatant was used to assay the activities of α-amylase, total-amylase and alcohol dehydrogenase following the methods of Doehlert, Duke (1983), Sarkar et al . (1982) and Ram et al . (2000), respectively. Calcium chloride (10 mM) was used for extraction of α-amylase and total-amylase whereas TRIS-HCl buffer (pH 7.5) containing 100 mM TRIS, 3 mM DTT, 2 mM MgCl 2 , 50 mM KCl and 1 mM EDTA was used for extraction of alcohol dehydrogenase.
The assay medium of alcohol dehydrogenase (ADH)
contained 50 mM TRIS-HCl (pH 7.5), 62.5 mM MgCl 2 , 3 mM NADH, 100 mM Acetaldehyde and 0.2 ml enzyme extract. Total volume of the assay medium was 3 ml. The absorbance was taken immediately after addition of NADH and after 3 min of reaction at 340 nm. The enzymes activities were expressed as unit min–1 mg–1 protein basis. One µ mole alcohol oxidized min–1 mg–1 protein was considered as one unit. Protein content was estimated following the method of Bradford (1976).
To measure α-amylase activity 2% starch azure (Sigma-Aldrich) was used as substrate. In a test tubes 4.5 ml of 2% starch azure (previously prepared by mixing 20 mM Sodium Phosphate Buffer with 50 mM Sodium Chloride, pH 7.0) was added to both blank and treatment, then 0.5 ml enzyme extract was added to continue the reaction (test set). In controlled set 2 ml 2.75 M acetic acid followed by 0.5 ml enzyme extract was added (blank set). All the sets were kept in a water bath at 37 oC for 1 hour with constant shaking. The reaction was terminated with the addition of 2 ml 2.75 M acetic acid. The suspension was filtered through hatman 54 filter paper. Optical density of the filtrate was measured at 595 using a UV-VIS a spectrophotometer (model SL 164, Elico, Hyderabad, India). A-amylase activity was expressed as unit min–1 mg–1 protein. Change in one unit of optical density min–1 mg–1 protein was considered as one unit.
Total amylolytic activities were measured taking one ml of enzyme extract plus one ml of 0.1 M acetate buffer (pH 4.7) containing 1% (w/v) soluble starch. The reaction mixture was incubated at 37 0C for 30 min. Two ml of dinitrosalicylic acid (DNSA) reagent was added to stop the reaction after designated time period. Thereafter, one ml 40% (w/v) sodium potassium tartarate solution was added. The mixtures were heated for 5 min in a boiling water bath to allow colour development, took back to room temperature and diluted to 25 ml with distilled water. Optical density of the diluted solution was measured at 560 nm. Total amylolytic activity was expressed as unit min–1 mg–1 protein. One µg maltose released min–1 mg–1 protein was considered as one unit (Sarkar et al ., 1982).
Determination of ethylene releasing capacity
Ethylene releasing capacity was measured after 1st,
3rd, 5th and 7th day of germination from both control and soil flooding sets following the method of Naik, Mohapatra (2000). Ethylene releasing capacity was measured in twelve selected rice genotypes. AG potential of these twelve genotypes varied from 25% to 86%. These genotypes belonged to different sensitivity group such as susceptible, moderately tolerant, tolerant and highly tolerant. The seedlings were properly cleansed with de-ionized water. The cleaned seedlings were put in a glass vial of the capacity 16 mL containing a piece of wet filter paper. The glass vials were sealed air tightly with rubber stopcock and kept in darkness at 30 oC for 2 h. The ethylene released during the process was drawn from headspace gas measuring 1 mL using an air tight syringe. Ethylene was estimated using a gas chromatograph (Chemito Instruments Pvt. Ltd., CERES 800 PLUS, Mumbai, India) where nitrogen used as carrier gas and hydrogen and oxygen gases were used for flame ionization detector. The data were expressed as ppm ethylene released h–1 mg–1 dry weight.
Statistical analysis
RESULTS
Establishment of seedlings, seedling length and dry weight
Seedling establishment significantly decreased due to soil flooding at germination stage (Table 1). Seedling establishment (%) did not vary significantly under control set, whereas wide variation of seedling establishment was observed under soil flooding. Out of 22 genotypes only six genotypes showed 60% or more than 60% of seedling establishment. These genotypes were AC41620A (86%), Rashpanjor (71%), AC34245 (69%), JRS196 and AC40346 (63%) and JRS8 (60%). In other genotypes seedling establishment (%) varied between 15% (Ravana) and 51% (JRS182). Seedling length significantly differed among the genotypes not only under soil flooding but also under control condition. The length of seedling varied from 20.0 cm to 41.8 at control set whereas length of seedling varied from 17.0 cm to 39.5 cm at treatment set. The seedling length was minimum in FR13A whereas was maximum in Ravana at both the conditions. The effect of treatment on seedling length showed mixed reaction. In some genotypes soil flooding significantly decreased the seedling length as compared to control whereas in some other genotypes the effect was non-significant. Significant genotypic variation was observed for shoot dry weight (SD ) both under control and soil flooding conditions. SD varied from 23.3 mg plant–1 (Kamini) to 67.4 m plant–1 (Talmugra) and 13.9 mg plant–1 to 37.1 mg plant–1 (Paloi), respectively under control and soil flooding conditions. Under control SD was maximum in Talmugra (67.4 mg plant–1), followed by Paloi (62.0 mg plant–1), Ravana (60.6 mg plant–1), Pokkali (57.8 mg plant–1) and JRS21 (57.5 mg plant–1), whereas under soil flooding SD significantly higher in Paloi (37.1 mg plant–1), followed by AC41647 (32.4 mg plant–1), Talmugra (31.8 mg plant–1), JRS20 (29.8 mg plant–1) and JS155 (29.2 mg plant–1). The genotypes, which showed greater establishment % under soil flooding neither accumulated greater quantities of shoot dry weight nor exhibited more seedling length as compared to these moderately tolerant genotypes. However, performance of tolerant genotypes in respect of seedling length and SD was better as compared to susceptible genotypes FR13A and Naveen.
Enzymatic activities in germinating seeds
Flooding had greater impact in altering the activities of alcohol dehydrogenase, α-amylase and total-amylase (Fig. 1). The activities of these enzymes were significantly greater under soil flooding treatment as compared to saturated soil condition. The increase of the activities of these enzymes was 19.5%, 31.7% and
199.4%, respectively for alcohol dehydrogenase, α-amylase and total-amylase.
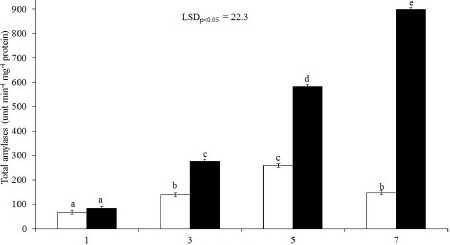
Alcohol dehydrogenase activity increased after 3 days of germination as compared to 1 day of germination (Fig. 2A). ADH activity started to decrease after 5 days in control whereas it declined after 7 days of germination in treatment. The activity of it was more under treatment compared to control. Maximum activity of it was notice after 3 and 5 days of germination under soil flooding. The activity of both amylases started to increase after 1 day of germination. The increase continued up to 5 days, then declined in control set. However, the increase of the activities of both amylases continued up to the study period i.e. 7 days. The activity of α-amylase was significantly low under treatment as compared to control after 1 day of germination whereas the activity of it was significantly greater after 3rd, 5th and 7th days of germination in treatment set as compared to control (Fig. 2B). In the case of total-amylase the activity of it was not statistically different after 1 day of germination between the treatments; thereafter, the differences were significant (Fig. 2C).
During germination a section of genotypes showed an upsurge of ADH activity due to soil flooding as compared to control (Fig. 3). The increase of ADH activity due to soil flooding was statistically different in ten genotypes namely, JRS21, JRS182, JRS196, AC34245, AC40346, Pantara, Panikekua, Talmugra, Ravana and AC41620A (Fig. 3A). In two genotypes such as AC41647 and Naveen, ADH activity decreased under soil flooding. In rest of the genotypes the differences in the activity of ADH were non-significant. In contrast to ADH activity, the activity of both α-amylase and total-amylase increased in most of the genotypes due to soil flooding (Fig. 3B, 3C). The activity of α-amylase and total-amylase decreased significantly in Naveen and Panikekua, respectively under soil flooding as compared to soil saturation condition. The α-amylase activity remained unchanged in two genotypes such as AC39416A and FR13A, whereas total-amylase activity was at par between control and soil flooding in three genotypes namely, AC39416A, Kamini and Naveen. In six tolerant to highly tolerant genotypes such as JRS8, JRS196, AC34245, AC40346, Rashpanjor and AC41620A the activity of both α-amylase and total- amylase increased significantly under soil flooding as compared to soil saturation condition (Fig. 3B, 3C), whereas the activity of ADH significantly increased in the case of four genotypes such as JRS196, AC34245, AC40346, AC41620A. In other two genotypes the differences were non-significant (Fig. 3A).
Ethylene releasing capacity in germinating seedlings
Ethylene releasing capacity increased in germinating seedlings up to seven days (study period) of germination both under control and soli flooding conditions (Fig. 4A). The rate of increase was greater under soil flooding as compared to soil saturation condition. The percentage increase after 1, 3, 5, and 7 days of germination was 16.1%, 36.0%, 15.3%, and 40.7%, respectively. The differences of ethylene concentrations were highly significant in all the days between soil saturation and soil flooding conditions. Comparing the ethylene releasing capacity of different genotypes revealed that except in two genotypes such as AC41647 and FR13A, the differences between control and treatment were significant (Fig. 4B). In susceptible genotype Naveen, ethylene releasing capacity was significantly less under soil flooding as compared to soil saturation conditions. Ethylene releasing capacity showed highly significant positive association with seedling establishment (%) under soil flooding (Fig. 4C).
Correlation among different growth parameters and grouping of genotypes based on average linkage
Seedling establishment (%) showed highly significant association with α-amylase (r = 0.607**, p<0.01) and total-amylase (r = 0.797***, p<0.001) and vigour index (r = 0.971**, p<0.001) under soil flooding (Table 2). Stability of the enzymes i.e. activity under flooding / activity under control also showed significant association with seedling establishment (%) of these two enzymes. Alcohol dehydrogenase activity under flooding did not show any significant association with seedling establishment (%). Seedling length did not show any significant association with establishment (%), enzyme activities and seedling dry weight. There was significant positive association between shoot dry weight and totalamylase activity under flooding. Vigour index as measured through the multiplication between seedling establishment (%) and seedling length showed significant positive association with α-amylase (r = 0.680***, p<0.001), total-amylase (r = 0.749***, p<0.001) and stability of α-amylase activity (r = 0.585**, p<0.01). Regression analysis revealed that total amylolytic activities explained the seedling establishment under flooding more strongly than α-amylase. The adjusted vales of R2 were 61.7% and 33.7%, respectively in total amylase and α-amylase. Total-amylase along with α-amylase, however, explained 70.4% of variability in germination under soil flooding.
The grouping of genotypes was done through proximity analysis based on Euclidean distance (Table 1). Twenty-two genotypes were divided into two major clusters ( Fig. 5 ). The cluster A was comprised of twenty-one genotypes belonged to susceptible to tolerant types whereas the cluster B contained only one highly tolerant genotype. Analysis of cluster A revealed that the group was further divided into two major sub-groups such A1 and A2. Sub-group A2 was comprised of seven highly susceptible genotypes whereas sub-group A1 was comprised of susceptible and moderately tolerant (A1-1) and tolerant (A1-2) genotypes. One tolerant genotype such as AC40346 was however represented in group A1-1.
DISCUSSION
Establishment of seedlings, seedling length and dry weight
Greater seedling vigour is a useful attribute in direct seeded rice (Vu et al ., 2016). After germination fast seedling growth suppresses weed infestation and pushes the coleoptiles above the water surface very quickly. This improves the crop stand. An optimum crop stand is the guaranty of good yield (Sarkar, 2012). The present investigation showed that out of 22 genotypes, six genotypes were highly capable to germinate under soil flooding. Seedling establishment % varied from 60 to 86% in these genotypes. Likely, there was great variation in seedling length and dry weight. New genetic resources open up new avenues for crop improvement programme. Prospects of identification of new genes/quantitative trait loci (QTLs) have been increased that ultimately help to develop stress tolerant crop (Burke et al ., 2009).
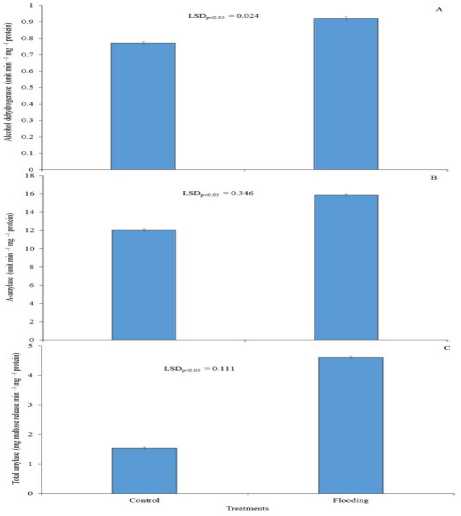
Figure 1. Soil flooding (Treatment) during germination stage improves the activity of alcohol dehydrogenase (A), α-amylase (B) and total-amylase (C) as compared to soil saturation condition (Control) in rice. LSD, least significant difference at P = 0.05. Bar represents standard error.
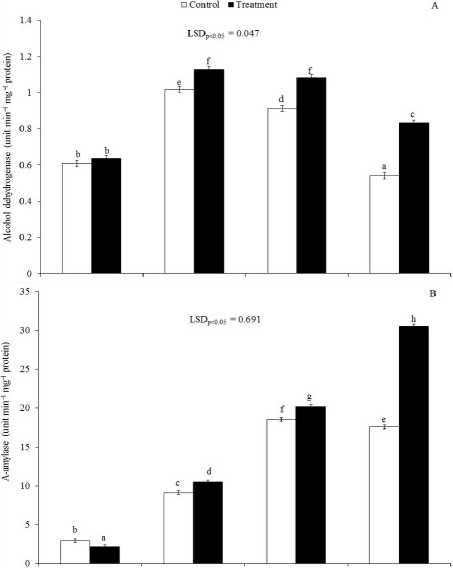
Figure 2. Changes of alcohol dehydrogenase (A), α-amylase (B) and total-amylase (C) with progression of germination under soil flooding (Treatment) and soil saturation condition (Control) in rice. LSD, least significant difference at P = 0.05. Bar represents standard error. Dissimilar letter implies significant difference whereas similar alphabet denotes nonsignificant.
Days after submergence
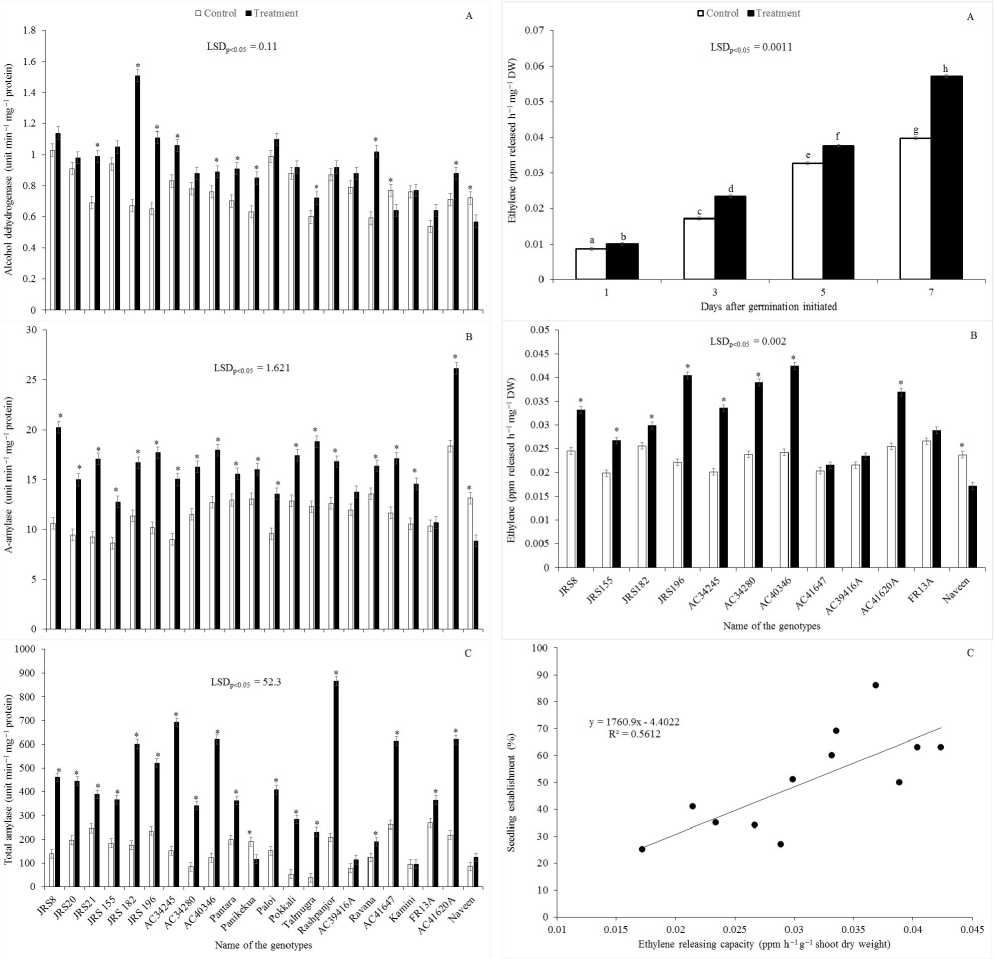
Figure 3. Genotypic variation of the activity of alcohol dehydrogenase (A), α-amylase (B) and totalamylase (C) under soil flooding (Treatment) and soil saturation condition (Control) in rice. LSD, least significant difference at P = 0.05. Bar represents standard error. Star (*) represents significant difference between treatment and control of the same genotype, otherwise the difference is non-significant.
Figure 4. (A) Ethylene releasing capacity at different days of germination under soil flooding (Treatment) and soil saturation condition (Control) in rice. (B) Genotypic variation of ethylene releasing capacity. (C) Regression analysis between ethylene releasing capacity under soil flooding and seedling establishment therein. LSD, least significant difference at P = 0.05. Bar represents standard error. Dissimilar letter implies significant difference whereas similar alphabet denotes nonsignificant (A). Star (*) represents significant difference between treatment and control of the same genotype, otherwise the difference is nonsignificant (B).
JRS 155
Pantar a
Talmugra
AC39416 A
JRS 182
AC40346
AC41 647
JRS21
AC34280
JRS20
JRS8
JRS 196
AC34245
Rashpanjor
Ravana
Kamini
Naveen
Panikekua
Pokkali
FR13A
Paloi
AC41620A
Dendrogram using Average Linkage (Between Groups)
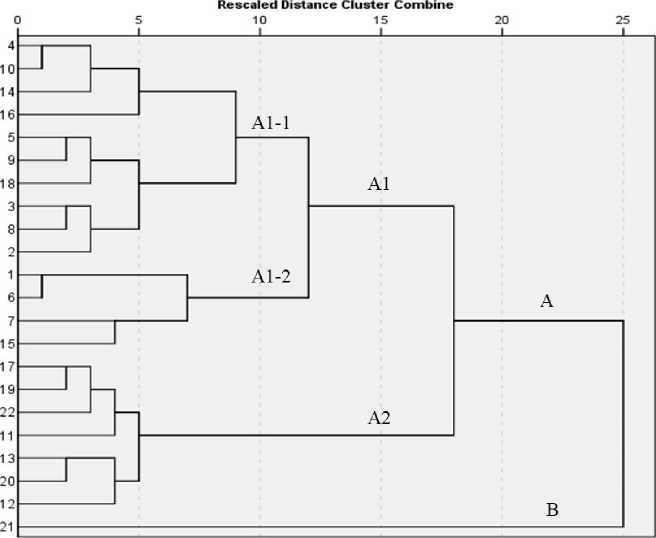
Figure 5. Dendogram using average linkage between groups. Two major groups were formed (A) and (B). The group (A) was further divided into sub-set A1 and A2 and so on. Group B comprised of one highly tolerant genotype and no sub-set was formed.
Table 1. Changes of seedling establishment, length and dry weight due to germination stage submergence
|
Genotypes |
Seedling Establishment (%) |
Seedling Length (cm) |
Seedling Dry eight (mg plant –1) |
Vigour Index |
||||||
|
C |
T |
T/C |
C |
T |
T/C |
C |
T |
T/C |
||
|
JRS8 |
99 |
60 |
0.61 |
33.3 |
30.9 |
0.93 |
45.8 |
25.3 |
0.55 |
1854 |
|
JRS20 |
94 |
46 |
0.49 |
30.4 |
30.0 |
0.99 |
55.7 |
29.8 |
0.54 |
1380 |
|
JRS21 |
98 |
48 |
0.49 |
37.0 |
31.0 |
0.84 |
57.5 |
24.3 |
0.42 |
1488 |
|
JRS 155 |
94 |
34 |
0.36 |
36.0 |
32.0 |
0.89 |
49.8 |
29.2 |
0.59 |
1088 |
|
JRS 182 |
99 |
51 |
0.52 |
33.8 |
30.5 |
0.90 |
53.6 |
31.2 |
0.58 |
1556 |
|
JRS 196 |
99 |
63 |
0.64 |
32.4 |
29.3 |
0.90 |
43.4 |
27.5 |
0.63 |
1846 |
|
AC34245 |
99 |
69 |
0.70 |
34.1 |
30.3 |
0.89 |
44.7 |
27.5 |
0.62 |
2091 |
|
AC34280 |
99 |
50 |
0.51 |
32.9 |
31.6 |
0.96 |
48.8 |
24.8 |
0.51 |
1580 |
|
AC40346 |
99 |
63 |
0.64 |
27.5 |
25.9 |
0.94 |
33.6 |
24.5 |
0.73 |
1632 |
|
Pantara |
99 |
38 |
0.38 |
29.1 |
28.8 |
0.99 |
42.7 |
22.3 |
0.52 |
1094 |
|
Panikekua |
99 |
23 |
0.23 |
38.3 |
33.3 |
0.87 |
53.7 |
25.9 |
0.48 |
766 |
|
Paloi |
96 |
21 |
0.22 |
38.9 |
31.7 |
0.81 |
62.0 |
37.1 |
0.60 |
666 |
|
Pokkali |
95 |
17 |
0.18 |
36.0 |
28.4 |
0.79 |
57.8 |
15.9 |
0.28 |
483 |
|
Talmugra |
96 |
34 |
0.35 |
30.9 |
33.4 |
1.08 |
67.4 |
31.8 |
0.47 |
1136 |
|
Rashpanjor |
96 |
71 |
0.74 |
40.0 |
27.5 |
0.69 |
50.1 |
27.5 |
0.55 |
1953 |
|
AC39416A |
96 |
35 |
0.36 |
41.3 |
35.4 |
0.86 |
53.3 |
26.6 |
0.50 |
1239 |
|
Ravana |
98 |
15 |
0.15 |
41.8 |
39.5 |
0.94 |
60.6 |
13.9 |
0.23 |
593 |
|
AC41647 |
97 |
41 |
0.42 |
38.5 |
34.8 |
0.90 |
50.6 |
32.4 |
0.64 |
1427 |
|
Kamini |
99 |
22 |
0.22 |
36.6 |
28.5 |
0.78 |
23.3 |
15.9 |
0.68 |
627 |
|
FR13A |
98 |
27 |
0.28 |
20.0 |
17.0 |
0.85 |
42.5 |
24.0 |
0.56 |
459 |
|
AC41620A |
99 |
86 |
0.87 |
29.3 |
29.9 |
1.02 |
46.5 |
25.1 |
0.54 |
2571 |
|
Naveen |
99 |
25 |
0.25 |
33.5 |
20.0 |
0.60 |
36.0 |
18.0 |
0.50 |
500 |
|
LSD p<0.05 |
G = 9, T = 3, G x T = 12 |
G = 2.1, T = 0.64, G x T = 2.99 |
G |
= 3.41, T = 1.03, G x T = 4.82 |
G = 20 |
|||||
Genotype, G; Control, C; Treatment, T.
Table 2. Correlation coefficient among different physiological parameters and seedling establishment (%) under germination stage submergence
|
Parameters |
SL |
D |
ADH |
α-Amy |
t-Amy |
T/C: ADH |
T/C: α- Amy |
T/C: t- Amy |
Vigour index |
|
SE (%) |
-0.066 |
0.307 |
0.204 |
0.607** |
0.797** |
0.137 |
0.490* |
0.361 |
0.971** |
|
SL |
--- |
0.184 |
0.263 |
0.381 |
-0.105 |
0.262 |
0.386 |
0.017 |
0.156 |
|
D |
--- |
0.277 |
0.089 |
0.454* |
0.056 |
0.384 |
0.209 |
0.368 |
|
|
ADH |
--- |
0.173 |
0.306 |
0.791** |
0.342 |
0.151 |
0.256 |
||
|
α-Amy |
--- |
0.405 |
0.221 |
0.553** |
0.397 |
0.680** |
|||
|
t-Amy |
--- |
0.141 |
0.436* |
0.445* |
0.749** |
||||
|
T/C: ADH |
--- |
0.249 |
-0.031 |
0.174 |
|||||
|
T/C:α-Amy |
--- |
0.320 |
0.585** |
||||||
|
T/C: t-Amy |
-- |
0.352 |
SE (%) = ̶ 13.737+ 3.503 * (α-Amylase)
[ F-value , 11.686; R2 , 36.9 %; adjusted R2 , 33.7 %; Probability , 0.003] SE (%) = 12.738+ 0.075 * total-Amylase
[ F-value , 34.846; R2 , 63.5 %; adjusted R2 , 61.7 %; Probability , 0.000] SE (%) = ̶ 13.687 + 1.962 * (α-Amylase) + 0.062 * (t-Amylase)
[ F-value , 25.956; R2 , 73.2 %; adjusted R2 , 70.4 %; Probability , 0.000]
SE (%), seedling establishment (%); SL, seedling length (cm); D , dry weight of shoot (mg seedling ̶ 1); ADH, alcohol dehydrogenase under submergence (unit min ̶ 1 mg ̶ 1 protein); α-Amy, α-Amylase (unit min ̶ 1 mg ̶ 1 protein) during submergence; t-Amy, total amylase (unit min ̶ 1 mg ̶ 1 protein) during submergence; T/C, activity at treatment/activity at control. *, **, significance at 0.05 and 0.01 level at 20 degrees of freedom (n = 22).
New QTLs have been identified from rice genetic resources, which imparted tolerance to drought (Bernier et al ., 2007), submergence (Sarkar et al ., 2009), salinity (Chattopadhyay et al ., 2014) and many more other abiotic stresses. Genotypes tolerance to germination stage oxygen deficiency (GSOD) or with greater AG potential were identified earlier (Yamauchi et al ., 1993; Sarkar et al ., 1999; Angaji et al ., 2010; Barik et al ., 2019), however, identification of robust QTLs is not yet achieved (Kim, Reinke, 2018). These new genetic resources may further enhance the discovery of new genes/QTLs associated with greater AG potential.
Enzymatic activities in germinating seeds
Soil flooding restricts the supply of oxygen to germinating seeds. Germination is an active process and requires lots of energy (Ota, 1982; Vartapetian, 2005; Ray et al ., 2016). So, to get the required energy germinating seeds switch over from aerobic respiration to ethanolic fermentation under soil flooding (Vijayan et al ., 2018). Among the different ethanolic fermentative enzymes, ADH is the most important enzyme, which was upregulated under oxygen deficiency (Kato-Noguchi, 2006; Miro, Ismail, 2013). Over expression of
ADH activity was noticed under soil flooding as compared to soil saturation condition ( Fig. 2A, 3A ). Rice is capable to germinate under the absence of oxygen (Magneschi, Perata, 2009). Amylases are synthesized in such conditions in rice and initiate the breakdown of starch to simple sugar. In the present investigation it was observed that the activity of both α-amylase and total-amylase increased under soil flooding. Over expression of amylases were greater in tolerant genotypes as compared to susceptible genotypes (Fig. 2B, 2C, 3B, 3C).
Ethylene releasing capacity in germinating seedlings
Ethylene, a phytohormone is involved in controlling the extension growth under submergence (Magneschi, Perata, 2009). Ethylene production is suppressed under submergence in submergence tolerant rice by the action of submergence tolerant gene SUB1-1A (Xu et al., 2006; Fukao, Bailey-Serres, 2008), whereas, ethylene production augments to boost up elongation growth under deep-water conditions by the action of Snorkel genes SK1 and SK2 to avoid drowning (Hattori et al., 2009; Yang et al., 2015). Ethylene production increased due to soil flooding as we observed in the present investigation. Hsu, Tung (2015) found increase ethylene production and greater coleoptiles elongation under oxygen deficiency. Vijayan et al. (2018) reported greater production of ethylene in tolerant genotype compared to susceptible genotype (Fig. 4). Ethylene releasing capacity under soil flooding showed significant positive association (r = 0.749**, p<0.01, n = 12) with seedling establishment (%) under flooding.
Correlation among different growth parameters and grouping of genotypes based on average linkage
Establishment % did not show any significant association with seedling length and dry matter accumulation per plant. This showed that establishment % under water was the main hurdle rather than seedling length and dry weight (Miro et al ., 2017). Among the 22 genotypes, vigour index varied significantly. Yamauchi and inn (1996) found highly linear correlation between establishment % in field or in laboratory with vigour index (Table 2). The genetic distance between tolerant and susceptible genotypes was wider (Fig. 5). Highly tolerant genotype was in another group as compared to the other tolerant genotypes. The offspring is more heterogeneous due to transgressive segregation if the parents are more dissimilar as compared closely related parents (Rieseberg et al . 2003). The present investigation showed that numbers of genotypes though were tolerant to GSOD was distantly related.
Increase of the activities of ADH and amylases were important to supply the required energy to the germinating seeds (Takahashi et al., 2014). Vijayan et al. (2018) reported upsurge of ADH and amylolytic activities in tolerant genotypes as compared to susceptible genotypes (Fig. 2, 3). In this investigation, when we compared the data of highly tolerant genotypes with that of susceptible genotypes, activities of all the enzymes were greater in tolerant genotypes e.g. activities of these enzymes were more in AC41620A (tolerant type) as compared to Naveen (susceptible type). Their conclusion was based on the data of 2-4 numbers of genotypes. In the present investigation we took more numbers of genotypes, estimated the activities of enzymes in all the enzymes and a correlation study was done. The data showed that α-amylase and total-amylase were more imperative in determining the ability of AG potential of rice as compared to ADH. Between α-amylase and totalamylase, the impact of latter found more as evident from regression analysis (Table 2).
CONCLUSION
Rice cultivation under direct seeded conditions is economical and environment friendly, however, due to some intrinsic problem farmers encounter greater loss under DSR as compared to TPR. Rice genotypes tolerant to germination stage oxygen deficiency / with greater AG potential can remove the hurdle of direct seeding. Growth parameters such as seedling length, and shoot dry weight under soil flooding changed in the same direction both in tolerant and susceptible genotypes and could not show any significant relation with seedling establishment. In contrary tolerant genotypes maintained greater vigour index than susceptible genotypes under soil flooding. The genotypes studied in this investigation showed a high degree of genetic diversity to GSOD tolerance. Enzymes involved in supplying energy to germinating seeds, totalamylase played greater role followed by α-amylase and alcohol dehydrogenase. Total-amylase cans alone explain 61.7% of variability in germination under soil flooding. In addition of total amylolytic activities of germinating seeds, ethylene releasing capacity of growing embryo positively support the seedling establishment under flooding. Total amylolytic activities of endosperm and ethylene releasing capacity of growing embryo are two important physiological parameters determine greatly the success of a genotype under soil flooding.
ACKNOWLEDGEMENT
Список литературы Anaerobic germination potential in rice (Oryza sativa L.): role of amylases, alcohol deydrogenase and ethylene
- Abdul-Baki A.A. and Anderson J.D. (1973) Vigor determination in soybean seed by multiple criteria. Crop Sci., 13, 630-633
- Agbeleye O.A., Olubiyi M.R., Ehirim B.O., Shittu A.O., Jolayemi O.L., Adetimirin V.O., Ariyo O.J., Sanni K.A., and Venuprasad R. (2019) Screening African rice (O. glaberrima Steud.) for tolerance to abiotic stress. III. Flooding. SABRAO J. Breed. Genet., 51, 128-150
- Akhgari H. and Kaviani B. (2011) Assessment of direct seeded and transplanting methods of rice cultivars in the northern part of Iran. African J. Agric. Res., 6, 6492-6498
- Angaji S., Septiningsih E.M., Mackill D.J. and Ismail A.M. (2010) QTLs associated with tolerance of anaerobic conditions during germination in rice (Oryza sativa L.). Euphytica, 172, 159-168
- Barik J., Kumar V., Lenka S.K. and Panda D. (2019) Genetic potentiality of lowland indigenous indica rice (Oryza sativa L.) landraces to anaerobic germination potential. Indian J. Plant Physiol., 24, 249-261
- Bernier J., Kumar A., Ramaiah V., Spaner D. and Atlin G. (2007) A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Sci., 47, 507-516
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248-254
- Burke M.B., Lobell D.V. and Guarino L. (2009) Shifts in African crop climates by 2050, and the implications for crop improvement and genetic resources conservation. Global Environ. Change, 19, 317-325
- Chattopadhyay K., Nath D., Mohanta R.L., Bhattacharyya S., Marndi B.C., Nayak A.K., Singh D.P., Sarkar R.K. and Singh O.N. (2014) Diversity and validation of microsatellite markers in Saltol QTL region in contrasting rice genotypes for salt tolerance at the early vegetative stage. Aust. J. Crop Sci., 8, 356-362
- Das K.K., Panda D., Nagaraju M., Sharma S.G. and Sarkar R.K. (2004) Antioxidant enzymes and aldehyde releasing capacity of rice cultivars (Oryza sativa L.) as determinants of anaerobic seedling establishment capacity. Bulg. J. Plant Physiol., 30, 34-44
- Doehlert D.C and Duke S.H. (1983) Specific determination of α-amylase activity in crude plant extracts containing β-amylase. Plant Physiol., 71, 229-234
- Fukao T. and Bailey-Serres J. (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. (USA), 105, 16814-16819
- Hattori Y., Nagai K. and Ashikari M. (2011) Rice growth adapting to deep water. Curr. Opin. Plant Biol., 14, 100-105
- Hattori Y., Nagai K., Furukawa S., Song X.L., Kawano R. and Sakakibara H. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460, 1026-1030
- Hsu S.K. and Tung C.W. (2015) Genetic mapping of anaerobic germination-associated QTLs controlling coleoptile elongation in rice. rice 8, 1-12
- Ismail A.M., Ella E.S., Vergara G.V. and Mackill D.J. (2009) Mechanisms associated with tolerance for flooding during germination and early seedling growth in rice (Oryza sativa). Ann. Bot., 103, 197-209
- Kato-Noguchi H. (2006) Pyruvate metabolism in rice coleoptiles under anaerobiosis. Plant Growth Regul., 50, 41-46
- Kim S-M. and Reinke R.F. (2018) Identification of QTLs for tolerance to hypoxia during germination in rice. Euphytica, 214, 160-169
- Kuanar S.R., Ray A., Sethi S.K., Chattopadhyay K. and Sarkar R.K. (2017) Physiological basis of stagnant flooding tolerance in rice. Rice Sci., 24, 73-84
- Kuchanur P., Singh Y.K., Rajakumar R.H., Anand S.R., Patil S.G., Jat M.L. and van Loon G.W. (2018). Resource conservation through direct seeded rice. Agri. Res. Tech.: Open Access J., 14, ID. 555905
- Kumar V. and Ladha J.K. (2011) Direct seeding of rice: recent developments and future research needs. Adv. Agron., 111, 297-413
- Liu H., Hussain S., Zheng M., Peng S., Huang J., Cui K. and Nie L. (2015) Dry direct-seeded rice as an alternative to transplanted-flooded rice in Central China. Agro. Sustain. Dev., 35, 285-294
- Magneschi L. and Perata P. (2009) Rice germination and seedling growth in the absence of oxygen. Ann. Bot., 103, 181-196
- Miro B., Longkumer T., Entila F.D., Kohli A. and Ismail A.M. (2017) Rice seed germination underwater: Morpho-physiological responses and the bases of differential expression of alcoholic fermentation enzymes. Front. Plant Sci., 8(1857): 1-17
- Miro B. and Ismail A.M. (2013) Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front. Plant Sci., 4(269): 1-18
- Naik P.K. and Mohapatra P.K. (2000) Ethylene inhibitors enhanced sucrose synthase activity and promoted grain filling of basal rice kernels. Func. Plant Biol., 27, 997-1008
- Ota Y. (1982) Promotion of emergence and establishment of rice seedlings by using calcium peroxide-coated seeds in direct sowing on flooded paddy field. Jap. Agric. Res. Quart. 15, 221-226
- Panda D., Rao D.N., Das K.K. and Sarkar R.K. (2017) Role of starch hydrolytic enzymes and phosphatases in relation to under water seedling establishment in rice. Indian J Plant Physiol., 22, 279-286
- Panda D., Rao D. N., Sharma S.G., Strasser R.J. and Sarkar R.K. (2006) Submergence effects on rice genotypes during seedling stage: Probing of submergence driven changes of photosystem 2 by chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica, 44, 69-75
- Panda D. and Sarkar R.K. (2013) Structural carbohydrates and lignifications associated with submergence tolerance in rice (Oryza sativa L.). J. Stress Physiol. Biochem., 9, 299-306
- Ram P.C., Lal R.K. and Chaturvedi G.S. (2000) Laoratory Manual for Physiological and Environmental Studies. Department of Plant Physiology, NDUAT, Faizabad, India
- Ray S., Vijayan J. and Sarkar R.K. (2016) Germination stage oxygen deficiency (GSOD): An emerging stress in the era of changing trends in climate and rice cultivation practice. Front. Plant Sci., 7(671): 1-4
- Rieseberg L.H., Widmer A., Arntz A.M. and Burke J.M. (2003) The genetic architecture necessary for transgressive segregation is common in both natural and domesticated populations. Phil. Trans. R. Soc. Lond. B 358, 1141-1147
- Sarkar RK (2012) Seed priming improves agronomic trait performance under flooding and non-flooding conditions in rice with QTL SUB1. Rice Sci 19: 286-294
- Sarkar R.K. (2016) Stagnant flooding tolerance in rice: endeavours and achievements. NRRI Research Bulletin No. 11. ICAR-National Rice Research Institute Cuttack, Odisha-753006 India
- Sarkar R.K., Bera S.K. and De R.N. (1999) Rice (Oryza sativa) cultivars for anaerobic seeding. Indian J. Agric. Sci., 69, 73-76
- Sarkar R.K. and Das S. (2003) Yield of rainfed lowland rice with medium water depth under anaerobic direct seeding and transplanting. Trop. Sci., 43, 192-198
- Sarkar R.K., Panda D., Reddy J.N. and Sarkar R.K. (2009) Performance of submergence tolerant rice (Oryza sativa) genotypes carrying the Sub1 quantitative trait locus under stressed and non-stressed natural field conditions. Indian J Agric Sci., 79, 876-883
- Sarkar R. K., Banerjee A. and Mukherji S. (1982) Effects of toxic concentrations of Natrium fluoride on growth and enzyme activities of rice (Oryza sativa L.) and jute (Corchorous olitorius L.) seedlings. Biol. Plant., 24, 34-38
- Senapati S., Kuanar S.R. and Sarkar R.K. (2019) Improvement in anaerobic germination potential and grain yield of rice (Oryza sativa) through seed priming. SAARC J. Agric., 17, 37-48
- Takahashi H., Greenway H., Matsumura H., Tsutsumi N. and Nakazono M. (2014) Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Ann. Bot., 113, 851-859
- Vartapetian B. (2005) Plant anaerobic stress as a novel trend in ecological physiology, biochemistry, and molecular biology: 1. Establishment of a new scientific discipline. Russ. J. Plant Physiol., 52, 826-844
- Vijayan J., Senapati S., Ray S., Chakraborty K., Molla K.A., Basak N., Pradhan B., Yeasmin L., Chattopadhyay K. and Sarkar R.K. (2018) Transcriptomic and physiological studies identify cues for germination stage oxygen deficiency tolerance in rice. Environ. Exp. Bot., 147, 15-248
- Vu H.T.T., Nguyen H.T.T., Tran K.D., Khuat T.H. and Nakamura C. (2016) Genetic diversity of Vietnamese lowland rice germplasms as revealed by SSR markers in relation to seedling vigour under submergence. Biotech. Biotechnol. Equip. 30(1): 17-25
- Xu K., Xia X., Fukao T., Canlas P., Maghirang-Rodriguez R., Heuer S., Ismail A.M., Bailey-Serres J., Ronald P.C. and Mackill D.J. (2006) Sub1A is an ethylene response factor-like gene that confers submergence tolerance to rice. Nature, 44, 705-708
- Yamauchi M., Aguilar A.M., Vaughan D.A. and Seshu D.V. (1993) Rice (Oryza sativa L.) germplasm suitable for direct sowing under flooded soil surface. Euphytica, 67, 177-184
- Yamauchi M. and Winn T. (1996) Rice seed vigor and seedling establishment in anaerobic soil. Crop Sci., 36, 680-686
- Yang C., Lu X., Ma B., Chen S.-Y., and Zhang J.-S. (2015) Ethylene signalling in rice and arabidopsis: Conserved and diverged aspects. Mol. Plant, 8, 495-505


