Antidiabetic Activity of Methanolic Leaves Extract of Transformed Soybean Plantlets in Streptozotocin (STZ) Induced Diabetic Rats
Автор: C. Janani, B. Sundararajan, Anil Kumar Moola, B. D. Ranjitha Kumari
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.17, 2021 года.
Бесплатный доступ
Diabetes is expanding quickly around the world, which produces unsettling influences in the digestion of sugar, protein, and lipid. Proof suggested that the normal drugs beginning from plant source may speak to a socially applicable correlative treatment for diabetes. This examination planned to assess the cell reinforcement and antidiabetic exercises of the methanolic leaves extracts of transformed and non-transformed soybean in streptozotocin induced diabetic rats. The enlistment of diabetes with a single dose of streptozotocin and afterward the rats are treated with single dose of methanolic leaves extracts of transformed and non-transformed soybean (50 mg/kg body weight) for 21 days. Glibenclamide is utilized as a standard medication (0.5 mg/kg b.w.). The degree of glucose, lipid peroxidation, glutathione peroxidase, decreased glutathione, superoxide dismutase and catalase were resolved in diabetic rats after the treatment. The administration of methanolic leaves extracts of transformed soybean saw a critical reduction in levels of glucose and lipid peroxidation. The significant increased was found in glutathione, glutathione peroxidase, superoxide dismutase and catalase levels in the liver when contrasted and diabetic control rats after medicines. The examination demonstrated that the methanolic leaves concentrates of transformed soybean rewarded rats successfully manage the cell reinforcement exercises in STZ induced diabetic rewarded rats. This examination presumed that the transformed (PPARγ gamma) crude methanolic leaves extracts have huge hypoglycemic, hypolipidemic and cell antioxidant potential.
Antioxidant, Diabetes, Glibenclamide, Streptozotocin
Короткий адрес: https://sciup.org/143173890
IDR: 143173890
Текст научной статьи Antidiabetic Activity of Methanolic Leaves Extract of Transformed Soybean Plantlets in Streptozotocin (STZ) Induced Diabetic Rats
Individuals with diabetes are a metabolic issue with the dynamic articulation of pathological Instability, resulting in various micro and macro vascular complications. Because of the inablity of the cells to use glucose appropriately, it is described by hyperglycemia (West 2000). Diabetes for the most part crosses the periods of harm which prompts decrease in secretion of insulin, impaired levels of glucose, hyperinsulinemia, excessive adipogenesis, insulin resistance and modulation of nuclear peroxisome proliferator-activated receptor (PPAR) (Groop 1999; Porte and Kahn 2001). The radicals initiated oxidative pressure, which accepted that May as a factor for different ailments (Batool et al. , 2010). Glucose height made oxidative pressure which aftereffects of expanded creation of responsive oxygen species (ROS), non-enzymatic glycation of proteins and glucose auto-oxidation (Dewanjee et al. , 2009). The diminished in cancer prevention agents, and expanded oxidative pressure might be identified with diabetes. The adjustments in free radical rummaging guard systems may prompt insufficient searching of receptive oxygen species, bringing about oxidative harm and tissue injury in diabetes mellitus. The cancer prevention agents are liable for forestalling or restraining the malicious results of oxidative worry for flavors and herbs. The flavors and herbs which go about as free extreme foragers because of essence flavonoids and polyphenols (Dewanjee et al. , 2009; Arunachalam and Parimelazhagan 2013). Synthetic substances, which is accessible with free radical scavengers and antioxidant properties may use to protect pancreas and in the β-cells recovery against streptozotocin cytotoxic impacts (Alvarez et al. , 2004; Coskun et al. , 2005).
Streptozotocin is an anti-toxin and utilized in the field of medicine for treating the Islets of Langerhans. It goes into the β-cells through a glucose transporter of GL T2 and causes alkylation of DNA. Artificially it is glucosamine-nitroso urea and poisonous to the β-cells of the pancreas which produce insulin in well evolved creatures by destructing the DNA. The ribosylation of poly ADP by DNA harm prompts exhaustion of ATP and NAD+. The substrate for xanthine oxidase discharges after treatment of streptozotocin bringing about the arrangement of superoxide radicals, hydrogen peroxide and hydroxyl radicals because of improved ATP dephosphorylation. Streptozotocin liberates nitric oxide that takes an interest in DNA harm and restrains aconitase action. The streptozotocin activity brought about rots and causes β-cell annihilation. Overwhelming portions of β-cell poisons like streptozotocin instigate insulin insufficiency and cause type 1 diabetes mellitus with ketosis. The pulverization of β-cell mass used to create insulin lacking of type 2 diabetes mellitus (Zou et. al., 2014). This study was aimed to assess the viability of transformed and non transformed methanolic leaves concentrates of soybean (50 mg/kg) for antidiabetic movement utilizing a lower portion of glibenclamide (0.5 mg/kg) bringing down body weight, blood glucose levels, cancer prevention agent, biochemical lipid profile and histopathological considers.
MATERIALS AND METHODS
Soybean ( Glycine max L. Merr.) variety of JS335 seeds were obtained from Indian Agricultural Research Institute (IARI), New Delhi, India. The binary vector of pBI121 was constructed with RnPPARγ quality (1428 bp) of Rattus norvegicus under CaMV35s promoter. The transformation was done and confirmed that JS335 variety of soybean harboring pBI121- RnPPARγ gene by using strain EHA105. Glibenclamide (drug) were from Sanofi India Ltd. India and Streptozotocin (STZ) were purchased from Hi-media Laboratories Pvt. Ltd., India.
Preparation of Methanolic Leaves Extracts:
The transformed soybean with RnPPARγ quality and non-transformed soybean leaves were gathered and conceal dried. Leaves of 10 g were separated utilizing 50 ml of methanol in an orbital shaker (LabTech RC2100) at room temperature for 60 minutes. The removed leaves were centrifuged at 10,000 rpm for 10 min (Eppendorf 5830) at that point sifted utilizing a microfilter (Behnaz et al. , 2013) with certain changes.
tilizing a vacuum evaporator filtrate was dissipated at 80°C, and unrefined methanolic leaves separates were utilized for additional examination.
Animals
These animal studies were carried out in the animal house of Kovai Medical College and Hospital
Coimbatore and it was affirmed by the Institutional Ethical Committee (KMCRET/Ph.D./09/2016-2017). The creatures were isolated into five Groups; each Group was fixed with six male rats. In this examination, 200250 g of male pale skinned person Wistar rats was utilized. Rats were kept up under the states of 12 h light or dark cycles at 25-28°C with relative mugginess 55–60 % in the standard lab and were taken care of a standard pellet diet and water. The animals were acclimatized to research center condition for one week before beginning the investigation.
Diabetes Induction
Tentatively, six grown-up Wistar male rats (200-250 g) caused to get diabetic with a solitary portion of 50 mg/kg of body weight by infusing intravenously. As indicated by Itankar et al. (2011), streptozotocin was newly arranged in chilly 0.1 M citrate cradle (pH 4.5). A single dose of 50 mg/kg was injected into the rats for two days. Initial blood glucose levels were resolved on the third day of streptozotocin infused, for the 18-hour fasted rats by estimating their blood glucose levels in blood tests acquired from the tails of rats utilizing a blood glucometer (Roche Symptomatic, Indianapolis, IN).
Treatment
After induction of diabetes, the rats were divided into five groups every group separated with six animals. Considered the first group, which served as normal (non-diabetic) control rats, rest of all other groups were consists of diabetic rats. Group two served as STZ induced diabetic control rats. Third Group served as reference drug which provided glibenclamide (0.5 mg/kg body weight) daily and groups of four and five received methanolic leaves extract of non-transformed soybean and transformed soybean with RnPPARγ gene (50 mg/kg body weight) respectively (Naskar et al. , 2011). Group I – Non-diabetic rats (Control) with Saline (10 ml/kg)
Group II – STZ induced diabetic rats with Saline (10 ml/kg)
Group III – STZ induced diabetic rats were treated with Glibenclamide (0.5 mg/kg)
Group IV – STZ induced diabetic rats treated with non- transformed soybean methanolic leaves extracts (50 mg/kg).
Group V – STZ induced diabetic rats treated with transformed soybean methanolic leaves extracts (50 mg/kg).
Level of Blood Glucose and Body Weight
The alteration in blood glucose levels of all the rats was recorded at regular intervals (0, 7, 14 and 21 days) by using a glucometer (Roche Diagnostic, Indianapolis, IN). The changes in body weight were monitored at regular intervals (0, 7, 14 and 21 days) using standard digital weight balance and estimated as follows.
Body Weight Changes = [(final body weight — initial body weight) / (initial body weight)] × 100 %.
Biochemical Parameters and Antioxidant Estimation
After the 24 hours of a final dose, blood samples were collected and sacrificed all the animals (Noor et al. , 2008) then the liver was excised and washed with phosphate buffer saline in ice-cold condition. The serum lipid profiles like Total Cholesterol (TC), low-density lipoprotein (LDL), High-Density Lipoprotein (HDL) and Triglycerides (TG) were estimated using commercially available kits. The very low-density lipoprotein (VLDL) levels of serum were calculated using formula: VLDL = TG/5; LDL = TC - (HDL + VLDL) levels were measured according to the manufacturer’s instructions on an autoanalyzer (Agappe Diagnostics, India). The liver homogenate (10%) was mixed with 0.15 M Tris-HCl buffer (pH 7.4) then centrifuged (REMI C-24) at 2000 rpm for 20 minutes at 4°C and the cell debris removed then the supernatant was centrifuged at 12000 rpm for 1 hour at 4°C. The evaluation was carried out by measuring the level of total protein, lipid peroxidation and antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), non-enzymatic antioxidant, i.e. reduced glutathione (GSH) (Rajesh and Perumal 2014 ).
Histopathological Studies
Finally, the rats were sacrificed, from each animal liver and adipose tissue were separated and rinsed in normal saline then fixed with 10% of formalin and sections of 3-5 μm thickness were cut and stained routinely with acidic dye eosin and basic dye haematoxylin to differentiate the cytoplasm and nucleus (Premanath et al., 2015). The sections were studied at 10x and 40x magnifications for the characteristics using a binocular compound microscope.
Statistical Analysis
Statistical analysis was carried out by one-way ANOVA as in Standard Statistical Software Package of Social Science (SPSS) version 16.0 and P≤0.05 are considered as level significances.
RESULTS AND DISCUSSION
Effect of Transformed and Non-transformed Methanolic Leaves Extract on Body Weight and Blood Glucose
In the current investigation, STZ instigated diabetic control rats (Group II) body weight was diminished essentially contrasted with normal control rats (Group I). Diabetic control rats saw that shed pounds while Transformed and non-Transformed methanolic leaves extracts at 50 mg/kg portion demonstrated noteworthy improvement (P ≤ 0.05) in body weight contrasted with STZ actuated diabetic control (Table 1). During insulin inadequacy, STZ initiates diabetes is related with loss of body weight because of high catabolism of protein to give a; mino acids to gluconeogenesis brings about squandering of muscle and loss of weight in control rats (Kumar et al., 2011). In the current investigation, the organization of methanolic leaves concentrates of Transformed soybean (50 mg/kg b.w.) orally demonstrated a huge decrease in body weight when contrasted with STZ incited diabetic control rats (Table 1). Some different reports hypothesize that decline in levels of blood glucose are related with isoflavones which proposed to diminish the glucose level by improving the insulin discharge (Potter 1995; Nilausen and Meinertz 1999; Anderson and Major 2002). The role of dietary filaments were indicated for antihyperglycemic action (Anderson et al., 1999; Quan et al., 2003), while Gupta and Sharma (2012) study, toward the end of extraction (containing fiber), left the buildup, when directed orally to rats, found to diminish blood glucose levels to 5.97% as it were. Also, the stifled fat tissue PPARγ, GL T4, adiponectin, and insulin receptor β- subunit mRNA articulations were upregulated while the raised fat tissue resistin articulation was downregulated in diabetic rodents because of treatment with the leaf and strip separate (Abdel Aziz et al., 2020).
The increase in body weight was seen in the Transformed, and non-Transformed methanolic leaves extracts in the portion of 50 mg/kg as 128.8 g and 127.2 g separately when contrasted with diabetic control (131.3 g/b.w.) on the 21st day. The impact of oral administration of Transformed and non-Transformed leaves extracts in STZ actuated diabetic control on levels of blood glucose is introduced in Table (2). In this examination, organization of dosages of 50 mg/kg Transformed and non-Transformed leaves remove in STZ actuated diabetic control rats caused the decrease of blood glucose levels altogether. Maximum reduction observed on the 21st day is 334.0 mg/dl for nontransformed and 246.0 mg/dl for transformed soybean. However, in comparison to this, aqueous extract of 12 hours germinated soybean seeds when orally produced 15.6% fall in blood glucose levels after administration. Along these lines, Gupta and Sharma (2012) demonstrated that fiber is not the essential part for creating antihyperglycemic action and the basic segment is accessible in the watery concentrate of soybean seeds. Das and Chhetry (2016) demonstrated that the methanolic crude extracts have powerful antidiabetic movement and cancer prevention agent action in streptozotocin-incited diabetic rats, so the current examination uncovered the oral organization of methanolic leaves concentrates of Transformed soybean (50 mg/kg b.w.) had a noteworthy decrease in glucose levels when contrasted with STZ actuated diabetic control rats (Table 2).
Effect of Transformed and Non-transformed Methanolic Leaves Extracts on Lipid Profiles
The Total Cholesterol (TC), Triglycerides (TC) and Very low-density lipoprotein (VLDL) levels were increased, and High-density lipoprotein (HDL) level was diminished in STZ instigated diabetic control rats contrasted with healthy control rats. Administration of transformed and non-transformed methanolic leaves extract at doses of 50 mg/kg in diabetic rats showed (P ≤ 0.05) reduction of TC, TG and VLDL levels significantly which compared with STZ induced normal rats. The level of HDL was increased in STZ induced diabetic rats. When rats were treated with transformed, non-transformed leaves extracts and glibenclamide in diabetic control rats and the HDL level was reduced (Table 3). In diabetes, the cholesterol statement in the fringe tissue is conveyed by LDL and VLDL, fringe tissue to endure and afterward discharge of cholesterol is finished by HDL. The expanded degree of LDL and VLDL is atherogenic (Jothi et al., 2016). The serum lipids level was raised twice more when contrasted with the ordinary control rats. Treatment of methanolic leaves concentrates of Transformed soybean fundamentally controls the expanded degree of serum lipids (Triglyceride, LDL and VLDL) and essentially expanded the degree of HDL in diabetic control rats. The STZ created abundance free unsaturated fats brings down the removal of insulin-intervened glucose and advance the transformation of overabundance free unsaturated fats into cholesterol and phospholipids in the liver. The substances alongside the abundance level of triglycerides framed in the liver might be released into the blood as lipoprotein (Bopanna et al., 1997). In the other atherogenic familial sort blended (combined) hyperlipidemia, which happens at a recurrence of 15/5000, there is a lessening in high-thickness lipoprotein (HDL) cholesterol and an expansion in non-HDL cholesterol (LDL and VLDL) indicating hypercholesterolemia and hypertriglyceridemia. It is additionally named Type III-hyperlipidemia, dysbetalipoproteinemia, or wide beta illness. It is a hereditary issue portrayed by aggregation, in the plasma, of remainder chylomicrons from intestinal lipoproteins and VLDL leftovers got from hepatic lipoproteins bringing about the improvement of untimely atherosclerosis (Al-Shamma 2020; Boot et al., 2019; Zhu et al., 2018). Hepatic creation of triglycerides was expanded, and diminished fringe expulsion has been shown hypercholesterolemia and hypertriglyceridemia have been accounted for in diabetic rats (Pusparaj et al., 2000). This may be ascribed because of absence of insulin which enacts the lipase compounds, hydrolyzing the put away TG and discharging a lot of unsaturated fats and glycerol in the coursing blood (Naskar et al.,
2011). The degrees of absolute cholesterol and LDL cholesterol higher are significant hazard factors for cardiovascular ailments, though expanded HDL cholesterol is related with abatement in cardiovascular malady chance (Ali et al. , 2012). The post-treatment results for methanolic leaves concentrate of soybean rewarded diabetic rats has demonstrated a decrease in serum TG, cholesterol, LDL cholesterol level with the checked increment in HDL levels. These outcomes show that the methanolic leaves concentrate of Transformed soybean has a lipid-bringing down impact in the diabetic rats.
Antioxidant Activity of Transformed and Nontransformed Leaves Extracts
The CAT, SOD, LPO, TP and GPx levels of the activities in normal and STZ induced diabetic rats were shown in Table (4). The reduction in the activities of CAT, SOD, GPx, GSH and increase in LPO and TP levels were observed in STZ induced diabetic rats. However, oral administration of transformed and nontransformed leaves extracts at 50 mg/kg the values were similar to glibenclamide (0.5 mg/kg) treated rats. The protein content in liver was altogether diminished in the methanolic leaves concentrate of Transformed soybean rewarded bunches contrasted with diabetic benchmark group. The sound liver can play out its ordinary capacity, for example, protein digestion. STZ-prompted hepatotoxicity in diabetic rats (Ohaeri 2001) cause harm to the cell layer, change chemical movement lastly initiates hepatic injury or rot. Accordingly, liver is not equipped for playing out its typical capacities where the protein digestion is influenced. The deamination of amino acids so as to breakdown the protein in the liver neglects to emerge bringing about the protein aggregation (Mitchell et al., 1973). In diabetic rats, the levels are essentially decreased, and the levels are recovered after the treatment of methanolic leaves concentrates of Transformed soybean rewarded Groups. In the cancer prevention agent guard instrument, Grass is most significant compounds (Curtis JJ, and Mortiz 1972) and which changes over the superoxide anion into hydrogen peroxide, it likewise evacuates superoxide anion. It impedes the poisonous impacts brought about by radical. The oxidative worry in diabetic rats initiated with the bringing down movement of this chemical (Kaleem et al., 2006).
In diabetes mellitus, a few reports have uncovered that the lower cancer prevention agent had improved peroxidative status (Punitha et al. , 2005; Pari and Suman 2010). Feline, cancer prevention agent compounds present in the tissues and assume a basic job in the hydrogen peroxide atom deterioration and furthermore the tissues are kept from the receptive hydroxyl radicals (Chance and Greenstein 1992). STZ hinders the movement of Feline and restraint of this protein may incite the arrangement of hydroxyl radical and it harms the cell. In the current investigation, the degree of the Feline chemical is diminished in the STZ rewarded rats, and the degrees of this compound are expanded in the methanolic leaves concentrates of Transformed soybean rewarded Groups and this level is like the standard medication rewarded Group. This examination saw the degree of GPx is brought down in the STZ rewarded Group and expanded in the methanolic leaves concentrates of Transformed soybean rewarded Groups. The exercises of plant separate rewarded Groups of rats are like a medication rewarded Group of rats. It annihilates the peroxides and assumes a fundamental job as a cell reinforcement barrier. GPx engaged with the hydrogen peroxide disposal (Chen and Schopfer 1999). GPx is bringing down the movement; the LPO is gathered and expanded the oxidative worry in diabetic rats (Kaleem et al. , 2006).
The degree of GSH is diminished in the diabetic rewarded Group, and the degrees of GSH are typical at the methanolic leaves concentrates of Transformed soybean rewarded Groups, and the concentrates rewarded bunch is like the standard medication rewarded Group. It is the intracellular free extreme scrounger. It assumes an essential job in the upkeep of cell reinforcement status in plasma, and it is the few compounds of the cofactor. GSH diminished level is diminished in numerous models of diabetes. Diminished GSH level is diminished in the mesangial cells (Catherwood et al., 2002) and muscle cells (Sharpe et al., 1998; Hamilton et al., 2003) when presented to high grouping of glucose. The lower substance of GSH may discard the phones, to bring down the guard action against the oxidative worry during diabetes (Gopalakrishnan et al., 2013). The layers are harmed, and it expands the degree of lipid peroxidation (Giugliano et al., 1996). The lipid peroxidations are exceptionally receptive, and it prompts harm the protein and DNA, at last causes different diabetes intervened entanglements (Gopalakrishnan et al., 2013). Tissue harm affected by free radicals relies upon the endogenous cancer prevention agent obstruction component and the age of free radicals (Davi et al., 2005). In this examination, the expanded LPO levels were seen in the STZ initiated diabetic rats, and the levels are decreased after the treatment of methanolic leaves concentrates of Transformed leaves rewarded Group. These qualities are like the standard medication rewarded Group. As needs be, expanded lipid peroxidation in the film of the liver (Srivastava et al., 2001) is accounted for in diabetic cases. In diabetics level of MDA Expanded recommends that peroxidative injury might be engaged with the improvement of diabetics related with vascular entanglements (Gopalakrishnan et al., 2013). Increment in lipid peroxidation brings about tissue harm (Gopalakrishnan et al., 2013).
Out of the Transformed and non-Transformed methanolic leaves concentrates of soybean (50 mg/kg b.w.), Transformed soybean is by all accounts the best cell reinforcement movement against STZ incited hyperglycemia and helpful as the antidiabetic specialist, this might be because of the impact of the various sorts of auxiliary metabolites. Organization of the methanolic leaves concentrates of Transformed soybean indicated a diminishing in the degree of blood glucose. An improved cell reinforcement potential appeared as confirm by diminished LPO and a huge increment in the action of Feline, Turf, GPx and GSH (Soman et al. , 2010).
Study of Histopathological Liver
Fig. (1 a) is the photomicrographs of the liver of a normal rat demonstrating standard architecture and Fig. (1 b) is the photomicrographs of the liver of the untreated diabetic rats. The pulverization of the liver was seen as little and contracted. Fig. (1 c) is photomicrographs of the liver of diabetic rodent rewarded with glibenclamide and saw with no harm. Eosin and hematoxylin segments of diabetic rat liver with Transformed soybean methanolic leaves removes are appeared in Fig. (1 d). In creatures rewarded with 50 mg/kg body weight of methanolic leaves concentrates of Transformed soybean, was seen with no harm contrasted with creatures rewarded with nonTransformed soybean. In creatures rewarded with 50 mg/kg body weight of methanolic leaves concentrates of non-Transformed soybean, there were degenerative changes in tissue architecture of liver (Fig. 1 e).
Histopathological investigations of tissue of liver were embraced, and it was discovered that the methanolic leaves concentrates of Transformed soybean was nontoxic and recovered the poisonous impact of STZ. Fat tissue is an organ plays a basic prerequisite of the living being (Lafontan et al. , 2009). Abundance vitality admission and decreased vitality use brings about irregular unnecessary development of White Fat Tissue (WAT) which can prompt the improvement of weight (Jo et al. , 2009). After oral organization of Transformed methanolic leaves removes, the fat tissue was essentially diminished contrasted with the diabetic rats. These outcomes recommend that concentrates may forestall the Group of WAT in the diabetic rodent.
Histopathological Study of Adipose Tissue
Photomicrographs of the histological of White Adipose Tissue (WAT) of the control and rewarded of STZ initiated diabetic rats toward the finish of the examination are appeared in Fig. (2). The WAT histology demonstrated various adipocytes firmly pressed together and an expansion in cell size of adipocytes in the diabetic rats contrasted and the control which indicated typical conveyance of adipocytes and the cells of ordinary sizes. However, extracts supplemented diet indicated histology similar to control suggesting inhibition of the hyperplastic growth of the adipocytes. The effects of drug-supplement were mild on the histological architecture of the adipose tissue. These observations of the adipose tissue histology correlate to body weight results.
The fat consumption of stomach WAT noted in PPARE mice with lipodystrophy contrasts from different models for lipodystrophy in the mouse (Reue and Peterfy 2000; Garg 2016; Reitman et al. , 2000). The heaviness of the epididymal fat cushions in the PPARE mice associated with the substance of hepatic fatty oils i.e., the more modest the WAT weight, the lower the liver TG content (Olswang et al. , 2002). Reliable with the nonattendance of clear greasy liver in these mice, this relationship unequivocally proposes that the fat tissue-determined FFA contribute fundamentally to the amalgamation of hepatic fatty substances.
Table 1. Effect of Transformed and Non-transformed Soybean Leaves Extracts on Body Weight of Streptozotocin (STZ) Induced Diabetic Rats.
Body Weight (g)
|
Treatments |
Initial |
7th day |
14th day |
21st day |
|
Control (Normal Saline) Rats |
128±0.6 |
136.4±0.3 |
153.4±0.3 |
144.0±0.2 |
|
STZ induced Diabetic control Rats |
135.2±0.4 |
147.4±0.5 |
124.0±0.6 |
131.2±0.5 |
|
Glibenclamide (0.5 mg/kg) |
137.8±0.5 |
146.8±0.2 |
124.0±0.4 |
131.6±0.3 |
|
MLENT (50 mg/kg) |
118.8±0.1 |
131.2±0.5 |
126.2±0.5 |
128.8±0.4 |
|
MLET (50 mg/kg) |
121.2±0.3 |
134.2±0.4 |
129.6±0.4 |
127.2±0.1 |
MLENT – Methanolic leaves extract of non-transformed; MLET – Methanolic leaves extract of transformed; Values are mean of three replicates ±Standard Error (n = 6), P ≤ 0.05, compared with control group.
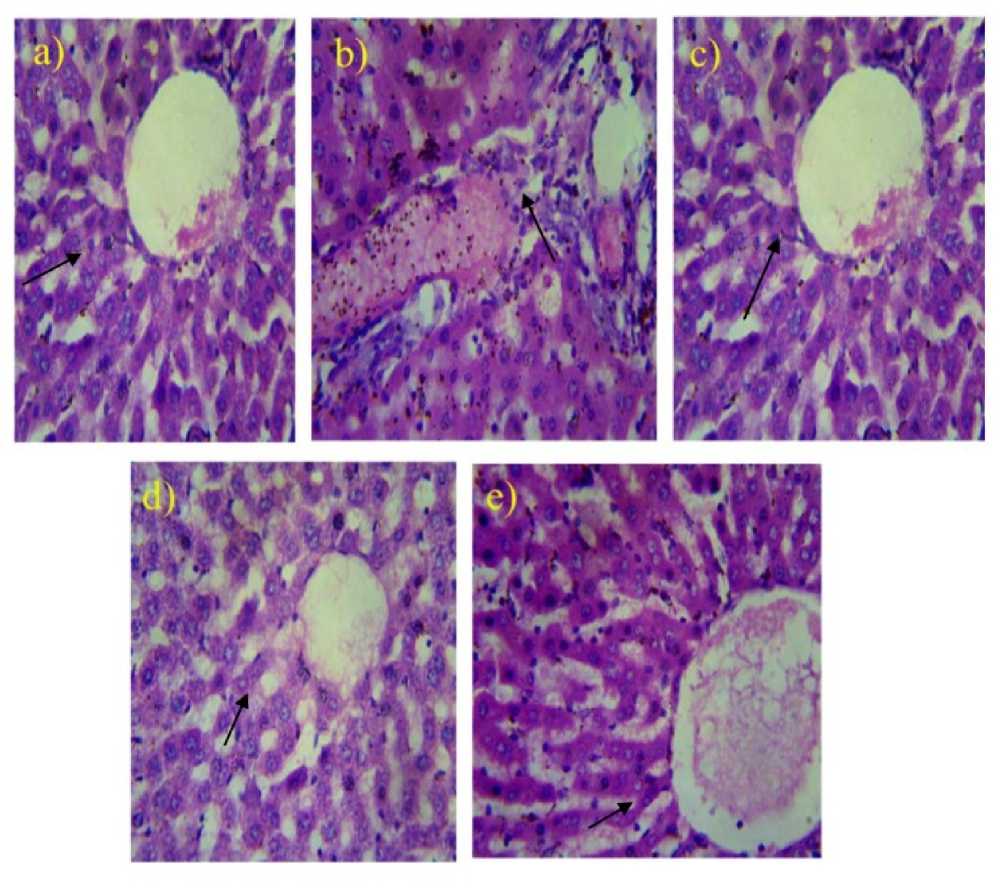
Figure 1: Histological Section of a Liver of Control and Experimental Rats: (a) Normal rats : Shows normal central vein; (b) STZ induced diabetic rats: shows periportal fatty infiltration with focal fatty necrosis; (c) Glibenclamide treated rats: shows normal Portal vein; (d) Transformed soybean methanolic leaves extracts treated rats: shows normal central vein; (e) Non-transformed soybean methanolic leaves extracts treated rat: shows normal central vein congestion.
Table 2. Effect of Transformed and Non-transformed Soybean Leaves Extracts on Blood Glucose Level of Streptozotocin (STZ) Induced Diabetic Rats.
|
Treatments |
Blood Glucose Levels (mg/dl) |
|||
|
Initial |
7th day |
14th day |
21st day |
|
|
Control (Normal Saline) Rats |
86.0±0.7 |
86.0±0.9 |
85.0±0.2 |
82.6±0.1 |
|
STZ induced Diabetic control Rats |
75.6±0.5 |
490.0±0.5 |
530.0±0.3 |
496.0±0.5 |
|
Glibenclamide (0.5 mg/kg) |
79.2±0.3 |
444.0±0.2 |
406.0±0.9 |
288.0±0.2 |
|
MLENT (50 mg/kg) |
76.0±0.9 |
464.0±0.8 |
452.0±0.7 |
334.0±0.7 |
|
MLET (50 mg/kg) |
78.6±0.6 |
448.0±0.9 |
336.0±0.1 |
246.0±0.5 |
MLENT – Methanolic leaves extract of non-transformed; MLET – Methanolic leaves extract of transformed;
Values are mean of three replicates ±standard error (n = 6).
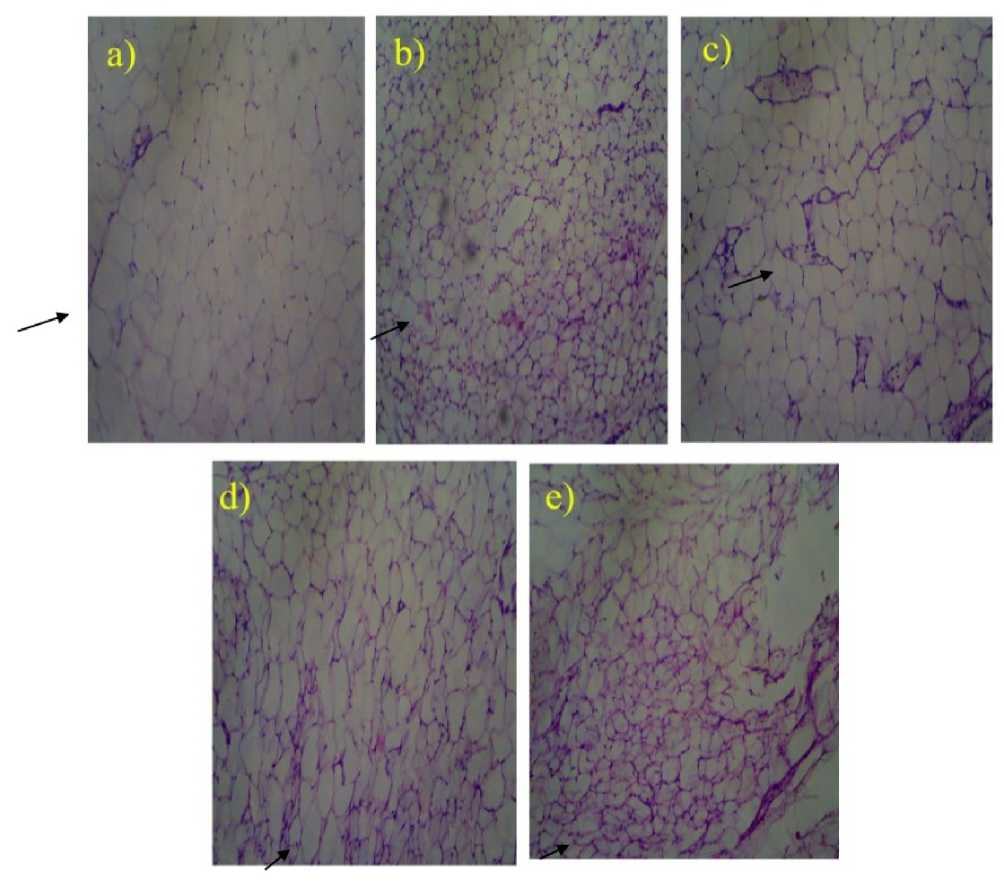
Figure 2: Histological Section of Adipose Tissue of Control and Experimental Rats: Histological sections of (a)Normal rats, (b)STZ induced diabetic rats, (c) Glibenclamide treated rats, (d) Non-transformed soybean methanolic leaves extracts treated rats, and (e) Transformed soybean methanolic leaves extracts treated rats.
Table 3: Comparisons of Serum Lipid Level of Transformed and Non-transformed Leaves Extracts of Soybean on Streptozotocin (STZ) Induced Diabetic Rats.
|
Treatments |
Total Cholesterol mg/dl |
Triglycerides mg/dl |
HDL mg/dl |
VLDL mg/dl |
|
Control (Normal Saline) Rats |
55.62±3.0 |
66.83±13.0 |
38.33±1.2 |
13.36 ± 2.5 |
|
STZ induced Diabetic control Rats |
74.89±0.7 |
150.73±10.7 |
25.9±2.3 |
20.14 ± 2.1 |
|
Glibenclamide (0.5 mg/kg) |
51.39±4.1 |
88.93±9.8 |
29.13333 |
17.78 ± 1.9 |
|
MLENT (50 mg/kg) |
65.97±3.2 |
98.6±15.2 |
38.3±1.6 |
31.32 ± 3.0 |
|
MLET (50 mg/kg) |
53.5±2.0 |
87.73±6.1 |
42.46±3.6 |
17.54 ± 1.2 |
HDL – high density lipoprotein; VLDL – very low density lipoprotein;
MLENT – Methanolic Leaves extract of non-transformed; MLET – Methanolic Leaves extract of transformed; Data are presented as means ± standard errors
Table 4. Antioxidant Activities of Transformed and Non-transformed Methanolic Leaves Extracts of Soybean on Liver in Streptozotocin (STZ) Induced Diabetic Rats.
|
Treatment |
SOD units/mg protein |
CAT ( µ mole of H 2 O 2 consumed/min/mg of protein) |
GPx ( µg of GSH utilized/mg protein) |
LPO ( µ mole of MDA/mg protein) |
TP (g/dl) |
|
Control (Normal Saline) Rats |
0.50±0.04 |
0.28±0.01 |
0.14±0.01 |
0.51±0.01 |
1.24±0.13 |
|
STZ induced Diabetic control Rats |
0.68±0.02 |
0.34±0.01 |
0.22±0.00 |
0.77±0.03 |
1.90±0.30 |
|
Glibenclamide (0.5 mg/kg) |
0.37±0.04 |
0.20±0.01 |
0.15±0.02 |
0.33±0.03 |
0.81±0.01 |
|
MLENT (50 mg/kg) |
0.47±0.03 |
0.25±0.00 |
0.16±0.02 |
0.44±0.02 |
1.02±0.15 |
|
MLET (50 mg/kg) |
0.48±0.05 |
0.30±0.01 |
0.16±0.01 |
0.53±0.04 |
0.81±0.14 |
SOD – superoxide dismutase; CAT – catalase; GPx – glutathione peroxidase; GSH – reduced glutathione; LPO – lipid peroxidation; MLENT – Methanolic Leaves extract of non-transformed; MLET – Methanolic Leaves extract of transformed; Data are presented as means ± standard errors.
CONCLUSION
This investigation uncovered that the Transformed (PPARγ quality) unrefined methanolic leaves concentrates of soybean had a significant action of hypoglycemic, hypolipidemic and antioxidant potential with the capacity of restored the altered serum glucose level, body weight, all out protein substance and cancer prevention agent catalysts close to typical rats. The components behind these perceptions require further examination so as to decide whether there is a connection between methanolic leaves concentrate of soybean with oxidized lipids, PPARγ action and glucose prejudice in a creature model. This examination further takes into consideration the plan of future human investigations to recognize different advantages and dangers related with this diabetes. The test of things to come is to decide if isoflavones with various PPARγ potencies and their parent botanicals have any upgraded advantage hazard profiles for the administration of the plague of diabetes, dyslipidemia and the metabolic condition.
CONFLICTS OF INTEREST
Список литературы Antidiabetic Activity of Methanolic Leaves Extract of Transformed Soybean Plantlets in Streptozotocin (STZ) Induced Diabetic Rats
- Abdel Aziz, S. M., Ahmed, O. M., Abd EL-Twab, S. M., Al-Muzafar, H. M., Amin, K. A., & Abdel-Gabbar, M. 2020. Antihyperglycemic Effects and Mode of Actions of Musa paradisiaca Leaf and Fruit Peel Hydroethanolic Extracts in Nicotinamide/Streptozotocin-Induced Diabetic Rats. Evidence-Based Complementary and Alternative Medicine, 2020.
- Ali K.M., Wonnerth A., Huber K. and Wojta J. 2012. Cardiovascular disease risk reduction by raising HDL cholesterol–current therapies and future opportunities. Br. J. Pharmacol; 167(6):1177-1194.
- Al-Shamma, G. 2020. Serum Lipid Again, and Always. Baghdad Journal of Biochemistry and Applied Biological Sciences, 1(01), 1-4.
- Alvarez J.F., Barbera A, Nadal B, Barcelo-Batllori S, Piquer S, Claret M, Guinovart JJ, and Gomis R, 2004. Stable and functional regeneration of pancreatic beta-cell population in STZ-rats treated with tungstate. Diabetologia. 47: 470–477.
- Anderson J, Anthony M, Messina M and Garner S. 1999. Effects of phyto-oestrogens on tissues. Nutr. Res. Rev.. 12. 75-116.
- Anderson JW, and Major AW, 2002. Pulses and lipaemia, short and long-term effect: potential in the prevention of cardiovascular disease. Br. J. Nutr.; 88:S263-271.
- Arunachalam K, and Parimelazhagan T, 2013. Antidiabetic activity of Ficus amplissima Smith. Bark extract in streptozotocin induced diabetic rats. J. Ethnopharmacol.; 147: 302-310.
- Batool F, Sabir SM, Rocha, JBT, Shah AH, Saify ZS and Ahmed SD, 2010. Evaluation of Antioxidant and free radical Scavenging activities of fruit extract from Zanthoxylum alatum: a commonly used Spice from Pakistan. Pak. J. Bot.; 42(6):4299-4311.
- Behnaz M, Davood EA and Atena A. 2013. Diurnal change in rutin content in Capparis spinose growing wild in Tafresh/Iran. Europ J Experimen Biol.; 3(3): 30-34.
- Boot CS, Middling E, Allen J, Neely RDG. 2019. Evaluation of the Non-HDL Cholesterol to Apolipoprotein B Ratio as a Screening Test for Dysbetalipoproteinemia. Clinical Chemistry.;65(2):313–320
- Bopanna KN, Kannan J, Sushma G, Balaraman R and Rathod SP. 1997. Antidiabetic and antihyperlipidemic effects of neem seed kernel powder on alloxan diabetic rabbits. Indian J Pharmacol.; 29:162-7.
- Catherwood MA, Powell LA, Anderson P, Mcmaster D, 2002. Sharpe PC and Trimble ER, Glucoseinduced oxidative stress in mesangial cells. Kidney Int.; 61(2): 599-608.
- Chance B, and Greenstein DS, 1992. The mechanism of catalase actions–steady state analysis. Arch. Biochem. Biophys.; 37(2): 301-339.
- Chen S, and Schopfer P, 1999. Hydroxyl radical production in physiological reaction. A novel function of peroxidise. Eur. J. Biochem.; 260(3): 726-735.
- Coskun O, Kanter M, Korkmaz A, and Oter S, 2005. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and b-cell damage in rat pancreas. Pharmacol Res.; 51: 117–123.
- Curtis JJ, and Mortiz M, 1972. Serum enzymes derived from liver cell fraction and response to carbon tetrachloride intoxication in rats. Gastroenterol.; 62(5): 84-92.
- Das A, and Chhetry TK, 2016. Evaluation of Anti-Diabetic Activity of Mahonia nepalensis in STZ Induced Rat Model. Int J Pharmacogn Phytochem Res.; 8(7):1104-1110.
- Davi G, Falco A, and Patrono C, 2005. Lipid peroxidation in diabetes mellitus. Antioxid. Redox. Signal.; 7(1-2): 256-258.
- Dewanjee S, Das AK, Sahu R, and Gangopadhyay M, 2009. Antidiabetic activity of Diospyros peregrine fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol.; 47: 2679–2685.
- Garg, A. 2016. Lipodystrophies. In Genetic Diagnosis of Endocrine Disorders (pp. 325-339). Academic Press.
- Giugliano D, Ceriello A, and Paolisso G, 1996. Oxidative stress and diabetic vascular level and the PE fraction showed a moderate increase in GSH complications. Diabetes Care.; 19(1): 257-67.
- Gopalakrishnan G, and Dhanapal CK, 2013. Evaluation of in vivo antioxidant activity of ethanolic extract of Coleus vettiveroides Jacob in streptozotocin induced oxidative stress in rats. Int. J. Pharm. Pharm. Sci.; 6(1): 590-592.
- Groop LC, 1999. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes. Metab.; 1:S1–S7.
- Gupta R, and Sharma SB, 2012. Effect of germinated Glycine max seeds on glycemic control in STZ+ NAD induced type 2 diabetic models: a preliminary study. J. Exp. Integr. Med.; 2(2), 155-160.
- Hamilton JS, Powell LA, Mcmaster C, Mcmaster D, and Trimble ER, 2003. Interaction of glucose and long chain fatty acids (C18) on antioxidant defences and free radical damage in porcine vascular smooth muscle cells in vitro. Diabetologia.; 46(1): 106-14.
- Itankar PR, Lokhande SJ, Verma PR, Arora SK, Sahu RA and Patil AT, 2011. Antidiabetic potential of unripe Carissa carandas Linn. fruit extract, J Ethnopharmacol.; 135(2): 430-3.
- Jo J, Gavrilova O, and Pack S, 2009. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput. Biol.; 5(3): e1000324.
- Jothi A, Parameswari CS, and Vincent S, 2016. Antidiabetic, Hypolipidemic and histopathological analysis of Zingerone in Streptozotocin Induced Diabetic Rats. Asian J. Pharm. Clin. Res.; 220-224.
- Kaleem M, Asif M, Ahmed OU, and Bano N, 2006. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin induced diabetic rats. Singapore Med. ; 47(8): 670-675.
- Kumar A, Lingadurai S, Shrivastava TP, Bhattacharya S and Haldar PK, 2011. Hypoglycemic activity of Erythrina variegata leaf in streptozotocin-induced diabetic rats. Pharm Biol,; 49(6): 577-82.
- Lafontan M, and Langin D, 2009. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid. Res.; 48(5): 275–297.
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR and Brodie BB, 1973. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther,; 87(1): 185-94.
- Naskar S, Mazumder UK, Pramanik G, Bala A, Haldar PK, Islam A and Gupta M. 2011. Comparative in vitro antioxidant activity of different parts of Cocos nucifera (linn.) on reactive oxygen and nitrogen species. Int J Pharm Pharm Sci:; 3(3), 104-107.
- Nilausen K, and Meinertz H, 1999. Lipoprotein (a) and dietary proteins: casein lowers lipoprotein(a) concentrations as compared with soy protein. Am. J. Clin. Nutr.; 69: 419-425.
- Noor A, Gunasekaran S, Manickam AS, and Vijayalakshmi MA, 2008. Antidiabetic activity of Aloe Vera and histology of organs in STZ induced diabetic rats. Curr Sci.; 94: 1070-1076.
- Ohaeri OC, 2001. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci. Rep.; 21(1): 19-24.
- Olswang, Yael & Cohen, Hannah & Papo, Orit and Cassuto, Hanoch & Croniger, Colleen & Hakimi, Parvin & Tilghman, Shirley & Hanson, Richard & Reshef, Lea. 2002. A mutation in the peroxisome proliferator-activated receptor -binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proceedings of the National Academy of Sciences of the United States of America. 99. 625-30.
- Pari L, and Suman S, 2010. Antihyperglycemic and antilipidperoxidative effects of flavanoid natringin in streptozotocin–nicotinamide induced diabetic rats. International. J. Biol. Med. Res.; 1: 206-210.
- Porte D, and Kahn SE. 2001. β-Cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes.; 50:S160–S163.
- Potter SM, 1995. Overview of proposed mechanism for the hypocholesterolemic effect of soy. J. Nutr.; 125: 606-611.
- Premanath, Ramya & Lakshmidevi Nanjaiah. 2015. Antidiabetic and Antioxidant potential of A. paniculata Nees. Leaf ethanol extract in STZ induced diabetic rats. J App Pharm Sci,; 5 (01): 069-076.
- Punitha IR, Rajendra K, Shirwaikar A, and Shiwaikar A, 2005. Alcoholic stem extract of Cascinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin nicotine amide induced diabetic rats. Evid. Based Complement Alternate Med.; 2(3): 375-81.
- Pusparaj P, Tan CH, and Tan BK, 2000. Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J. Ethnopharmacol.; 72(1-2): 69-76.
- Quan J, Yin X, Jin M, Shen M, 2003. Study on the inhibition of alpha-glucosidase by soyasaponins. Zhong yao cai= Zhongyaocai J. Chi. Med. Mat.; 26: 654-6.
- Rajesh V, and Perumal P. 2014, In vivo assessment of antidiabetic and antioxidant activities of methanol extract of Smilax zeylanica leaves in wistar rats. Orient. Pharm. Exp. Med.; 14(2), 127-144.
- Reitman, M. L., Arioglu, E., Gavrilova, O., & Taylor, S. I. 2000. Lipoatrophy revisited. Trends in Endocrinology & Metabolism, 11(10), 410-416.
- Reue, K., Peterfy, M. 2000. Mouse models of lipodystrophy. Curr Atheroscler Rep 2, 390–396.
- Sharpe PC, Yue KK, Catherwood MA, Mcmaster D, and Trimble ER, 1998. The effects of glucose-induced oxidative stress on growth and extracellular matrix gene expression of vascular smooth muscle cells. Diabetologia.; 41(10): 1210-9.
- Soman S, Rauf AA, Indira M, and Rajamanickam C, 2010. Antioxidant and antiglycative potential of ethyl acetate fraction of Psidium guajava leaf extract in streptozotocin-induced diabetic rats. Plant Foods Hum. Nutr.; 65(4): 386-91.
- Srivastava S, Conklin DJ, Liu SQ, Prakash N, Boor PJ, Srivastava SK, and Bhatnagar A, 2001. Identification of biochemical pathways for the metabolism of oxidized low-density lipoprotein derived aldehyde-4-hydroxy trans-2-nonenal in vascular smooth muscle cells. Atherosclerosis.; 158(2): 339-350.
- West IC, 2000. Radicals and oxidative stress in diabetes. Diabet Med.. 17: 171–180.
- Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, 2018. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a populationbased crosssectional study in China. Lipids in Health and Disease.;17(1):37–37.
- Zou CY, Gong Y, Liang J. 2014. Metabolic signaling of insulin secretion by pancreatic beta-cell and its derangement in type 2 diabetes. Eur Rev Med Pharmacol Sci. ;18(15):2215–2227.


