Antigen specific immune responses in mice subjected to infrared heat stress
Автор: Sukumaran M.K., Manjunath R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.8, 2012 года.
Бесплатный доступ
Short exposures to infrared heat are generally used to facilitate tail-vein bleeding of experimental mice as an alternative to retro-orbital bleeding for the purpose of obtaining serum samples. Altered temperatures have been shown to influence immune responses in a variable manner. This study evaluates the effects of infrared heat on the immune response. After confirming the efficacy of heat exposure as measured by alterations in body temperatures, the exposed mice were evaluated for antigen-specific antibody responses and allogeneic cytotoxic T lymphocytic (CTL) responses as readouts for humoral and cellular immune responses respectively. Antigen-specific serum antibody titers to lysozyme, bovine serum albumin, ovalbumin, diphtheria toxin and rabbit IgG antigens were analyzed in infrared heat exposed and unexposed control C57BL/6 mice that were immunized with the corresponding antigen. Significant decreases in antigen-specific antibody titers were observed only when heat exposed C57BL/6 mice were immunized with lysozyme or BSA but not with other antigens tested. These alterations were not seen in heat exposed BALB/c mice. Dialyzed serum prepared from such heat exposed C57BL/6 was also found to inhibit CTL generation in vitro and inhibited IL-2 stimulated proliferation of CTLL-2 cells. These studies suggest that the procedure of infrared heat exposure prior to tail vein bleeding may influence some immune parameters although this may not be true for all strains of mice and all antigens.
Infrared heat, serum antibodies, ctl
Короткий адрес: https://sciup.org/14323589
IDR: 14323589
Текст научной статьи Antigen specific immune responses in mice subjected to infrared heat stress
Environmental stress such as heat stress is a stimulus that disturbs physiological equilibrium (Sareh et al., 2011; Salak-Johnson and McGlone, 2007; Harikai et al., 2003) and elevated temperature is known to alter immune responses (Wetsel, 2011; Morrow-Tesch et al., 1994; Yoshioka et al., 1990). Exposure of rats to heat stress resulted in suppressed DTH response to keyhole limpet haemocyanin, decreased number of peripheral blood lymphocytes, total T cells, T helper cells as well as thymus size. On the other hand, serum anti-tetanus toxoid IgG antibodies were enhanced while proliferative response of Con-A stimulated splenic lymphocytes remained unaltered (Chayoth et al., 1988). Acute hyperthermia (rectal temperature 42°C) enhanced lymphocyte functions, proliferative responses to alloantigens, PHA and Con-A, while chronic hyperthermia was associated with suppressed T cell proliferative responses that returned to normal levels 40 days after induction of hyperthermia (Anderson and Kuhn., 1989). PHA and Con-A induced blastogenesis of PBL was considerably decreased in heat stressed sheep. Addition of serum from heat stressed sheep also significantly suppressed the blastogenesis of lymphocytes obtained from human, bovine and sheep donors suggesting that the suppressive effect was species non-specific. Susceptibility of animals to infectious agents has also been shown to alter under stressful conditions (Jin et al., 2011; Klein, 1993).
Experimental mice are routinely bled from the retro-orbital sinus or tail vein for the purpose of obtaining serum samples. Tail vein bleeding is preferred when damage to the eyes needs to be avoided. Short exposures to infrared heat have been used to enhance blood flow to facilitate tail vein bleeding by increasing body temperature and infrared heat treatment has been shown to alter food intake in rats (Hu et al., 2011; Joseph et al., 1991). We show in this report that lysozyme-specific antibody responses are lowered in C57BL/6 but not BALB/c mice that were subjected to infrared heat exposure prior to tail vein bleeding. We further show that this does not occur with all antigens suggesting that the effects of infrared heat exposure employed for tail vein bleeding may be strain and antigen-dependent.
MATERIALS AND METHODS
Reagents
Ovalbumin, electrophoresis reagents and RPMI 1640 were purchased from Sigma chemical company, USA. Minimum Essential Medium (MEM), gentamycin, streptomycin, penicillin, sodium pyruvate, L-glutamine, non essential amino acids
(NEAA), Coomasie Brilliant Blue R 250 and Lysozyme were purchased from Hi-Media Pvt. Ltd., India. Fetal Bovine Serum ( FBS) was from GIBCO, USA. Tissue culture ware was purchased from Nunc, Netherlands. Cellulose Acetate membranes used for sterile filtration of media were purchased from Millipore, India and Advanced microdevices, India. Na 2 51CrO 4 (specific activity usually ranging between 65-175 g Ci/mg in different batches) and Tritiated thymidine (specific activity 6,500 mCi/mMole) were purchased from Board of Radiation Isotope Technology (BRIT), India.
Cells and Media
P815 (H-2d), a DBA/2 derived mastocytoma and EL-4 (H-2b), a C57BL/6 mouse derived T cell lymphoma were obtained from National Facility for Animal Tissue and Cell Culture, Pune, India. All cultures were done at 37°C in a 5% CO 2 incubator.
Media used routinely to maintain cell lines was RPMI 1640 supplemented with antibiotics (100 units/ml penicillin, 250 g g/ml streptomycin and 50 g g/ml gentamycin), L-glutamine (0.32 mg/ml) and 5% FBS. Mixed lymphocyte culture (MLC) medium containing RPMI 1640 supplemented with sodium pyruvate (0.12 mg/ml), non essential amino acids (0.1 mg/ml), 2-mercaptoethanol (5 x 10-4M) and 5% FBS was used for cytotoxic T lymphocyte (CTL) cultures. CTLL-2 cells were routinely grown in RPMI 1640 medium that was supplemented with 5% FBS and exogenously added 20U/ml recombinant IL-2.
Antibodies
Biotinylated Goat anti-mouse IgG (whole molecule), Rabbit anti-mouse IgG (whole molecule), Avidin peroxidase, Rabbit anti-mouse IgG (whole molecule) peroxidase conjugate were purchased from Bangalore Genei, Bangalore.
Mice and blood collection
Inbred strains of C57BL/6 and BALB/c mice were obtained from the Central animal facility, Indian Institute of Science and immunized subcutaneously (s.c.) or intraperitoneally (i.p.) with antigen (20µg/mouse) after emulsification with crude Freund’s adjuvant (CFA) on day 0 and 21 unless otherwise stated. Sera were collected from control and exposed mice on day 0, 14, 21, 28 and 35. Heat exposure of mice was carried out on day 1, 15, 22 and 29. All mice were subjected to light ether anesthesia prior to retro-orbital bleeding. For testing in CTL cultures, serum was obtained by tail vein bleeding immediately or after different times of heat exposure from an infrared source for 4 min as given below.
Heat Exposure
Mice were enclosed in a 500 ml glass beaker and exposed to heat for a period of 4 min. The heat was generated from an infrared lamp. Each mouse was positioned in the inverted beaker such that the tail protruded through the spout of the beaker. The tail was held tight not only to curtail panic-induced sudden movements, but also to ensure direct frontal exposure of the animal. Control animals were handled similarly using a fresh glass beaker but the infrared lamp was switched off.
Measurement of internal body temperature in mice
Rectal temperature was measured with a digital thermometer (LDC portable digital multistem thermometer with external sensing probe, Singapore) every two min over a period of 15 min. The temperature measured before initiation of stress was taken as the normal body temperature. The average of three readings was taken for each measurement using three mice per group.
ELISA
Antibody titres in sera of mice were determined by Avidin-Biotin micro ELISA. All assays were carried out in 96 well flat bottomed polystyrene plates (Costar, USA). 0.1 ml of antigen at a concentration of 5 µ g/ml in 0.1M sodium carbonate-bicarbonate buffer, pH 9.6, was coated onto each well and the plate was incubated for 30 min at 37°C. After incubation, excess antigen was removed from the well by washing with rinse buffer (PBS with 0.1% tween 20). Non-specific sites were blocked by addition of 0.3 ml serum diluent buffer (SDB, containing PBS with 0.1% tween-20 and 5% goat serum) into each well and incubated for 1 hour 15 min at 37°C. After washing, 0.1 ml of appropriately diluted antiserum was added to each well and the plate was incubated for 30 min at 37°C. Excess antiserum was removed by washing the wells and 0.1 ml of 1:1000 diluted secondary antibody (goat anti-mouse IgG biotinylated) was added to each well and the plate was incubated for 30 min at 37°C. Wells were washed and 0.1 ml of 1:1000 diluted Avidin-HRP conjugate was added to each well was incubated for 8 min at 37°C. After incubation, wells were washed and 0.1 ml of substrate (0.5 mg OPD/ml PO 4 buffer 0.2 M, pH 7.0/1 µ l of 6% H 2 O 2 ) was added to each well and the plate was incubated for 25 min at room temperature. The reaction was terminated by addition of 0.1 ml of 2N HCl and the color developed was read at 490 nm in a microplate autoreader (Bio-Tek instrument) ELISA Reader.
Centricon dialysis and concentration
Serum obtained from control and heat exposed mice were subjected to centrifugal concentration and dialysis on 30 kDa Centricon membrane filters (Amicon Inc, Beverly, MA 01915, USA). Briefly, 0.2 ml of serum along with 0.4 ml MLC medium
(without FBS) was subjected to Centricon centrifugal concentration at 6000 rpm for 1 hr in a Kubota centrifuge (Model Kubota KR-20000 T). After centrifugation, 0.4 ml of medium was added to the serum component retained above the membrane filter (retentate) and centrifuged for a further period of 1 hr. This step was repeated once again. The filtrate passing through the filter was collected and stored separately in a tissue culture tube at -20°C until use. The retentate cup was reverse spun at 2000 rpm for 2 min to collect the retained protein fraction. 0.6 ml of medium was added to wash the top of the membrane filter and reverse spun again to collect the washings. Retentate thus obtained contained serum components that were more than 30 kDa and was stored at -20°C until further use. Both retentate and filtrate fractions (less than 30 kDa) were tested at various concentrations that were expressed as the final serum equivalents from which they were prepared.
Allogeneic Cytotoxic T Lymphocyte (CTL) generation and assay
C57BL/6 (6-8 weeks old) mice were primed in vivo by immunizing them with 1x107 BALB/c splenocytes intraperitoneally (i.p) on day 0. Animals were sacrificed after a 12 day period and an in vitro allogeneic CTL culture was set up by the co-culture of 5x106 responder C57BL/6 splenocytes with 2x106 irradiated BALB/c splenocytes in 2 ml MLC culture medium per well in 24-well Linbro plates for 5 days at 37° C in a CO 2 incubator.
Effector cells generated during the 5 day culture period were assayed for their cytotoxic activity in a 45 hr Chromium release assay. Varying number of effector cells were incubated with 2x104 target cells (P815, EL-4) in a 96 well V bottomed plate for 4 hr at 37°C and 5% CO2 atmosphere after spinning the plates at 400 rpm for 4 min. Target cells were prepared by preincubating cells with 100 aCi of Na251CrO4 for 1 hr at 37°C. Excess chromium was removed by washing the cells thrice. At the end of the incubation period plates were spun down at 1200 rpm for 5 min and 0.1ml of cell free supernatant was counted in a LKB Minigamma counter. Percent lysis was calculated using the formula: (cpm released in the presence of effectors minus spontaneous release/total cpm released with detergent minus spontaneous release) x 100.
CTLL-2 Proliferation assay
The effect of serum filtrate and retentate obtained from control and heat exposed C57BL/6 was tested on IL-2 induced proliferation of CTLL-2 cells (a cloned murine cytotoxic T cell line). CTLL-2 cells cultured in IL-2 medium were washed twice with medium to remove residual IL-2 and added to 96 well plates. Cells (1x104/well) in MLC medium containing 0.2 U/ml IL-2 and appropriately diluted samples to be tested were cultured at 37 ° C and 5 % CO 2 atmosphere in a 96 well flat bottomed tissue culture plate. Control well contained cells only. Two hours before harvesting, the cultures were pulsed with 4x105 ([3H]-thymidine) cpm per well. At the end of incubation period, cells were harvested onto glass fiber filters using Nunc semi automated cell harvester. Samples were counted in a LKB Rack Beta counter using scintillation fluid (0.5 % PPO and 0.025% POPOP in toluene). The standard proliferation curve obtained from the 36 hrs culture with added IL-2 was linear over the range of 1.5 units to 14 units yielding 5000 to 10,000 cpm. Data represent mean + SD of IL-2 U/ml of culture supernatants.
Table 1: Anti-lysozyme response in C57BL/6 mice
ELISA OD 490 nm
cMice were bled under light ether anesthesia and tested at 1:500 serum dilution.
Exposure Time (Min)
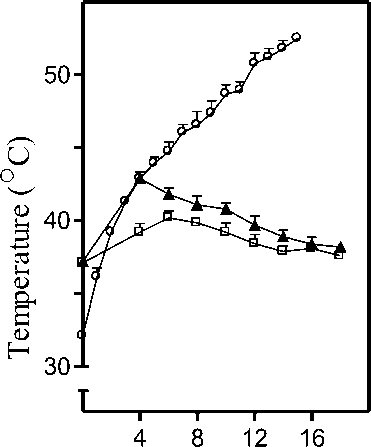
Figure 1. Measurement of body temperature. C57BL/6 (▲) or BALB/c ( □ ) mice were subjected to infrared lamp heat exposure for 4 min as described in Materials and Methods . Rectal temperature for both strains of mice was recorded thereafter at regular intervals over a period of 14 min after cessation of exposure. Temperature inside the empty inverted glass beaker ( ο ) was also recorded for a period of 16 min with the thermometer inside the empty beaker while the infrared lamp was switched on continuously. Data is represented as mean rectal temperature recorded at each time interval ± SD for 3 mice per group.
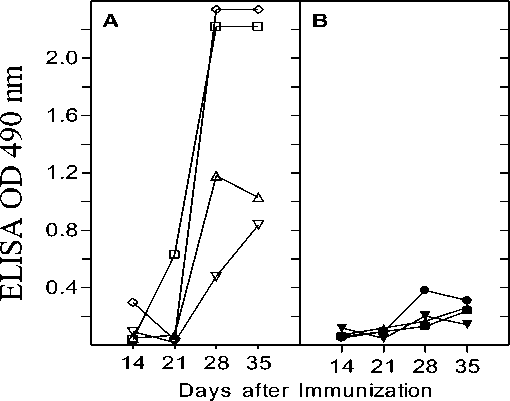
Figure 2. Lysozyme-specific antibody response in C57Bl/6 mice. Mice immunized (i.p) with lysozyme on day 0 and 21 were subjected to heat exposure on day 1, 15, 22 and 29. Anti lysozyme IgG antibody titers were measured in serum of control (A) and heat exposed (B) mice that were obtained on day 14, 21, 28 and 35 as given in Materials and Methods section. ELISA was performed at several antiserum dilutions but absorbance values at 490 nm are given only for one representative antiserum dilution (1:500). Each curve represents the values obtained for individual mice at different time points. All mice were bled retro-orbitally.
1.6
1.2
0.8
Е о 0.4
о>
■у-
Q
О 1.6
<
— 1.2
ш
0.8
0.4
Days after Immunization
14 21 28 35
14 21 28 35
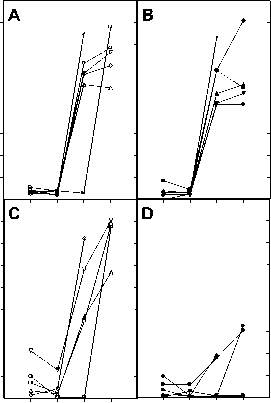
Figure 3. Antibody responses to BSA in heat exposed mice. BALB/c (A and B) and C57BL/6 (C and D) mice were immunized (s.c.) on day 0 and 21 with BSA and subjected to heat exposure on day 1, 15, 22 and 29. Anti BSA IgG antibody titers were measured in serum of control (Panels A and C) and heat exposed (Panels B and D) mice that were obtained on day 14, 21, 28 and 35 as given in Materials and Methods section. ELISA was performed at several antiserum dilutions but the absorbance values at 490 nm are given only for one representative antiserum dilution (1:20,000 for C57BL/6 and BALB/c mice). Each curve represents the values obtained for individual mice at different time points. All mice were bled retro-orbitally. Panel A: control BALB/c mice; Panel B: heat exposed BALB/c mice; Panel C: control C57BL/6 mice; Panel D: heat exposed C57BL/6 mice.
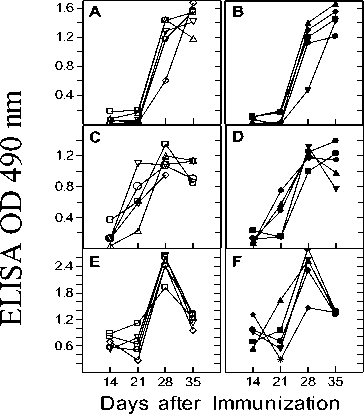
Figure 4. Antibody response in heat exposed C57BL/6 mice. C57BL/6 mice were immunized (s.c) on day 0 and 21 with ovalbumin, diphtheria toxin and rabbit IgG. Antigen-specific antibody titers were measured by standard Avidin-Biotin ELISA in the serum of control (Panels A, C, E) and heat exposed (Panels B, D, F) mice on day 7, 14, 21, 28 and 35. Heat exposure was carried out on day 1, 15, 22 and 29. Antibody titers were determined at several antiserum dilutions but the values obtained for one representative antiserum dilution (1:400 in A and B; 1:2000 in C and D; 1:10000 in E and F) are plotted. Each curve represents the values obtained for individual mice at different time points. All mice were bled retro-orbitally. Panels A and B: Anti ovalbumin response in control and heat exposed mice; Panels C and D: Anti diphtheria toxin response in control and heat exposed mice; Panels E and F: Anti rabbit IgG response in control and heat exposed mice.
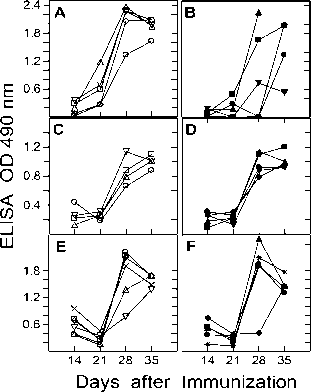
Figure 5. Antibody response in heat exposed BALB/c mice. BALB/c mice were immunized (s.c) on day 0 and 21 with ovalbumin, diphtheria toxin and rabbit IgG. Antigen-specific antibody titers were measured by standard Avidin-Biotin ELISA in the serum of control (Panels A, C, E) and heat exposed (Panels B, D, F) on day 7, 14, 21, 28 and 35. Heat exposure was carried out on day 1, 15, 22 and 29. Antibody titers were determined at several antiserum dilutions but the values obtained for one representative antiserum dilution (1:2000 in A and B; 1:2000 in C and D; 1:10000 in E and F) are plotted. Each curve represents the values obtained for individual mice at different time points. All mice were bled retro-orbitally. Panels A and B: Anti ovalbumin response in control and heat exposed mice; Panels C and D: Anti diphtheria toxin response in control and heat exposed mice; Panels E and F: Anti rabbit IgG response in control and heat exposed mice.
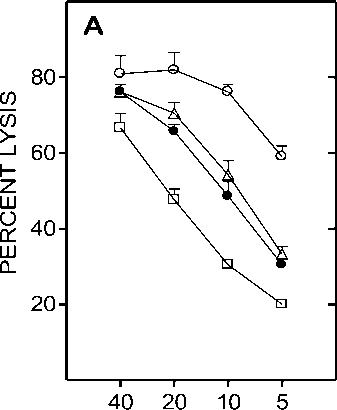
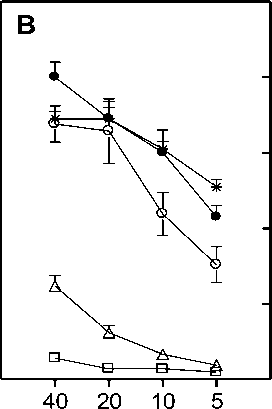
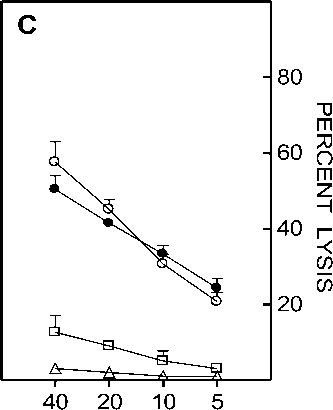
Figure 6. Effect of centricon dialyzed serum from heat exposed mice on CTL generation. Spleen cells from C57BL/6 mice that were primed with BALB/c splenocytes 12 days earlier were restimulated in vitro with irradiated BALB/c splenocytes in the presence of medium alone ( ○ ), medium supplemented with 1.5% ( • ), 3% ( A ), 4.5% ( □ ) equivalent serum retentate or 4.5% equivalent serum filtrate ( * ) prepared from normal (Panel A) or heat exposed (Panel B) C57BL/6 mouse serum. Panel C represents the lysis obtained when the retentate added to the culture was prepared from serum obtained at 0 ( A ), 6 ( □ ) or 12 ( • ) hr after heat exposure of C57BL/6 mice. Effector cells obtained after 5 day culture period were assayed on P815 targets in a 5 hr chromium release assay. Data is represented as percent lysis obtained at four different E:T (Effector:Target) ratios ± SD.
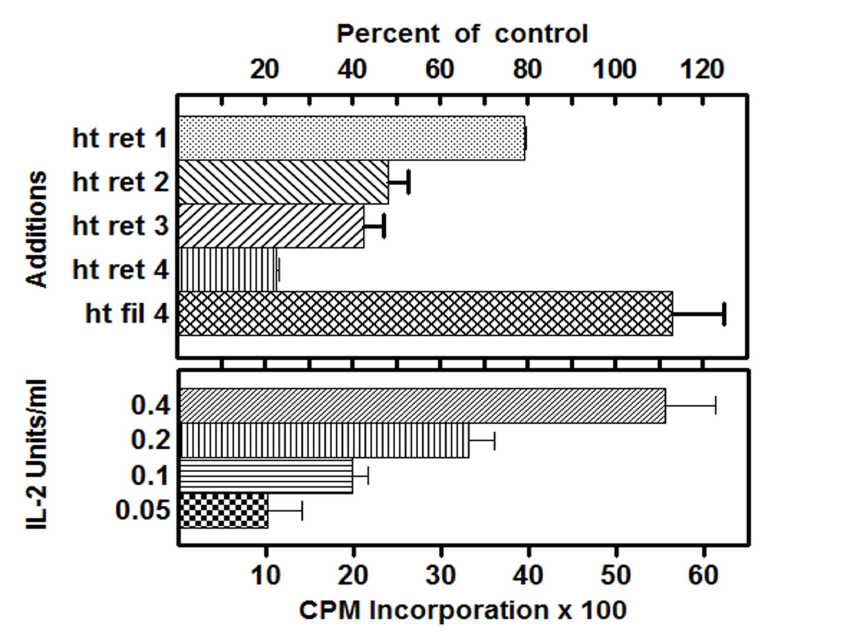
Figure 7. Effect of centricon dialyzed serum on CTLL-2 proliferation. Top panel: CTLL-2 cells were cultured in medium containing 0.2 U/ml IL-2, either in the absence (control) or presence of retentate or filtrate prepared from serum of heat exposed mice. Histograms marked ht ret 1, 2, 3 and 4 represent the addition of 1x, 2x, 3x and 4x concentrations of retentate where 4x was equivalent to 9% serum and ht fil 4 represents the corresponding filtrate control. Bottom panel: Dose response of IL-2 on CTLL-2 cell proliferation. CTLL-2 cells were cultured in the presence of medium supplemented with increasing concentrations of IL-2 as given. All cells were pulsed with 3[H] thymidine and harvested as described in Materials and Methods section. All assays were performed in triplicates and results are expressed as percent of control response.
RESULTS
Список литературы Antigen specific immune responses in mice subjected to infrared heat stress
- Anderson, K.J. and Kuhn, R.E. (1989). Elevated environmental temperature enhances immunity in experimental Chagas' disease. Infect Immun., 57, 13-7.
- Chayoth, R., Christou, N.V., Nohr, C.W., Yale, J.F., Poussier, P., Grose, M., Montambault, M., Chan, W. and Marliss, E.B. (1988). Immunological responses to chronic heat exposure and food restriction in rats. Am J Clin Nutr., 48, 361-7.
- Cheng, G.J., Morrow-Tesch, J.L., Beller, D.I., Levy, E.M. and Black, P.H. (1990). Immunosuppression in mice induced by cold water stress. Brain Behav Immun., 4, 278-91.
- Dhabhar, F.S., Miller, A.H., McEwen, B.S. and Spencer, R.L. (1996). Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol., 157, 1638-44.
- Fujiwara, R. and Orita, K. (1987). The enhancement of the immune response by pain stimulation in mice. I. The enhancement effect on PFC production via sympathetic nervous system in vivo and in vitro. J Immunol., 138, 3699-703.
- Gautam, S.C., Hilfiker, M.L. and Battisto, J.R. (1983). In vivo development of cytolytic T lymphocytes (CTL) to hapten-altered self: MIs-disparate cells facilitate the response by neutralizing IL 2 inhibitor. J Immunol., 130, 533-7.
- Harikai, N., Tomogane, K., Miyamoto, M., Shimada, K., Onodera, S. and Tashiro, S. (2003). Dynamic responses to acute heat stress between 34 degrees C and 38.5 degrees C, and characteristics of heat stress response in mice. Biol Pharm Bull., 26, 701-8.
- Hardt, C., Rцllinghoff, M., Pfizenmaier, K., Mosmann, H. and Wagner, H. (1981). Lyt-23+ cyclophosphamide-sensitive T cells regulate the activity of an interleukin 2 inhibitor in vivo. J Exp Med., 154, 262-74.
- Honda, M., Chan, C. and Shevach, E.M. (1985). Characterization and partial purification of a specific interleukin 2 inhibitor. J Immunol., 135, 1834-9.
- Hu, J., Choo, H.J. and Ma, S.X. (2011). Infrared heat treatment reduces food intake and modifies expressions of TRPV3-POMC in the dorsal medulla of obesity prone rats. Int J Hyperthermia., 27, 708-16.
- Jasnic, N., Korac, A., Velickovic, K., Golic, I., Djordjevic, J., Djurasevic, S., Djordjevic, I., Vujovic, P. and Cvijic, G. (2010). The effect of acute heat exposure on rat pituitary corticotroph activation: the role of vasopressin. Folia Histochem Cytobiol., 48, 507-12.
- Jin, Y., Hu, Y., Han, D. and Wang, M. (2011). Chronic heat stress weakened the innate immunity and increased the virulence of highly pathogenic avian influenza virus H5N1 in mice. J Biomed Biotechnol., 2011:367846. Epub 2011 May 29.
- Joseph, I.M., Suthanthirarajan, N. and Namasivayam, A. (1991). Effect of acute heat stress on certain immunological parameters in albino rats. Indian J Physiol Pharmacol., 35, 269-71.
- Kelley, K.W. (1980). Stress and immune function: a bibliographic review. Ann Rech Vet., 11, 445-78.
- Klein, T.W. (1993). Stress and infections. J Fla Med Assoc., 80, 409-11.
- Lelchuk, R. and Playfair, J.H. (1985). Serum IL-2 inhibitor in mice. I. Increase during infection. Immunology., 56, 113-8.
- Levine. S., Sowinski. R. and Steinetz. B. (1980). Effects of experimental allergic encephalomyelitis on thymus and adrenal: relation to remission and relapse. Proc Soc Exp Biol Med., 165, 218-24.
- Mason, D., MacPhee, I. and Antoni, F. (1990). The role of the neuroendocrine system in determining genetic susceptibility to experimental allergic encephalomyelitis in the rat. Immunology., 70, 1-5.
- Miossec, P., Elhamiani, M., Chichehian, B., D'Angeac, A.D., Sany, J. and Hirn, M. (1990) Interleukin 2 (IL 2) inhibitor in rheumatoid synovial fluid: correlation with prognosis and soluble IL 2 receptor levels. J Clin Immunol., 10, 115-20.
- Morrow-Tesch, J.L., McGlone, J.J. and Salak-Johnson, J.L. (1994). Heat and social stress effects on pig immune measures. J Anim Sci., 72, 2599-609.
- Niwano, Y., Becker, B.A., Mitra, R., Caldwell, C.W., Abdalla, E.B. and Johnson, H.D. (1990). Suppressed peripheral blood lymphocyte blastogenesis in pre-and postpartal sheep by chronic heat-stress, and suppressive property of heat-stressed sheep serum on lymphocytes. Dev Comp Immunol., 14, 139-49.
- Regnier, J.A. and Kelley, K.W. (1981). Heat-and cold-stress suppresses in vivo and in vitro cellular immune responses of chickens. Am J Vet Res., 42, 294-9.
- Riley, R.L., Wilson, L.D., Germain, R.N. and Benjamin, D.C. (1982). Immune responses to complex protein antigens I. MHC control of immune responses to bovine albumin. J Immunol., 129, 1553-8.
- Sadegh-Nasseri, S., Kipp, D.E., Taylor, B.A., Miller, A. and Sercarz, E. (1984). Selective reversal of H-2 linked genetic unresponsiveness to lysozymes. I. Non-H-2 gene(s) closely linked to the Ir-2 locus on chromosome 2 permit(s) an antilysozyme response in H-2b mice. Immunogenetics., 20, 535-46.
- Sapolsky, R.M. and Donnelly, T.M. (1985). Vulnerability to stress-induced tumor growth increases with age in rats: role of glucocorticoids. Endocrinology., 117, 662-6.
- Salak-Johnson, J.L. and McGlone, J.J. (2007). Making sense of apparently conflicting data: stress and immunity in swine and cattle. J Anim Sci., 85, E81-8.
- Sareh H., Tulapurkar, M.E., Shah, N.G., Singh, I.S. and Hasday, J.D. (2011). Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperones., 16, 297-307.
- Sei, Y., Skolnick, P. and Arora, P.K. (1992). Strain variation in immune response and behavior following the death of cage cohorts. Int J Neurosci., 65, 247-58.
- Shavit, Y., Depaulis, A., Martin, F.C., Terman, G.W., Pechnick, R.N., Zane, C.J, Gale, R.P. and Liebeskind, J.C. (1986). Involvement of brain opiate receptors in the immune-suppressive effect of morphine. Proc Natl Acad Sci U S A., 83, 7114-7.
- Solov'ev, A.S. (1992). The effect of a high external temperature on cellular immunity. Biull Eksp Biol Med., 114, 382-3.
- Steplewski, Z. and Vogel, W.H. (1986). Total leukocytes, T cell subpopulation and natural killer (NK) cell activity in rats exposed to restraint stress. Life Sci., 38, 2419-27.
- Tecoma, E.S. and Huey, L.Y. (1985). Psychic distress and the immune response. Life Sci., 36, 1799-812.
- Wetsel, W.C. (2011). Hyperthermic effects on behavior. Int J Hyperthermia., 27, 353-73.
- Yoshioka, A., Miyachi, Y., Toda, K., Imamura, S., Hiraoka, M. and Abe, M. (1990). Effects of local hyperthermia on natural killer activity in mice. Int J Hyperthermia., 6, 261-7.


