Antistress property of Glycyrrhiza glabra (Athimadhura) on stress induced Drosophila melanogaster
Автор: Sowmya M., Sathish Kumar B.Y.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.6, 2010 года.
Бесплатный доступ
Stress is defined as a condition that disturbs the normal function of the biological system or a condition that decreases fitness. The present study was to evaluate the antistress property of Glycyrrhiza glabra (Athimadura). Here the Antistress property was experimented on Drosophila melanogaster. Stress was induced by adding methotrixate (MTX) to the media. The 4 groups of Drosophila melanogaster were cultured in the laboratory. In the first group only control flies. In the second group MTX induced flies, in the third group MTX along with plant powder induced flies. In the fourth group only plant powder induced flies were cultured. Stress related enzymes like Catalase (CAT) and Super Oxide Dismutase (SOD) are most widely used paradigm for the evaluation of enzyme activity. SOD and CAT Activity in Stress induced flies was increased compared to that of normal flies. After incorporation of the plant powder to the media fed for Drosophila melanogaster, both SOD and CAT Activity was decreased indicating the reduction in Stress by the plant powder. Thus Glycyrrhiza glabra may have Antistress property, as it has reduced stress in Drosophila melanogaster induced by MTX at different concentration.
Stress, drosophila, catalase, superoxide dismutase
Короткий адрес: https://sciup.org/14323498
IDR: 14323498
Текст научной статьи Antistress property of Glycyrrhiza glabra (Athimadhura) on stress induced Drosophila melanogaster
In the daily life, most of us experience stress at one time or another. Without stress, there would be no life. However, excessive or prolonged stress can be harmful. Stress symptoms commonly include a state of alarm and adrenaline production, short-term resistance as a coping mechanism, and exhaustion, as well as irritability, muscular tension, inability to concentrate and a variety of physiological reactions such as headache and elevated heart rate. During the stress many Reactive Oxygen Species [ROS] are released in our body, to antagonize this ROS our body has certain cellular defensive enzymes like Catalase and Super oxide Dismutase which take part in capturing the anions. During times of environmental stress ROS levels can increase dramatically, which can result in significant damage to cell structures. This cumulates into a situation known as oxidative stress. A particularly destructive aspect of oxidative stress is the production of reactive oxygen species, which include free radicals and peroxides. Some of the less reactive of these species (such as superoxide) can be converted by oxidoreduction reactions with transition metals or other redox cycling compounds (including quinones) into more aggressive radical species that can cause extensive cellular damage (Valko et al., 2005). The major portion of long term effects is inflicted by damage on DNA (Evans and Cooke, 2004). Most of these oxygen-derived species are produced at a low level by normal aerobic metabolism and the damage they cause to cells is constantly repaired. However, under the severe levels of oxidative stress that cause necrosis, the damage causes ATP depletion, preventing controlled apoptotic death and causing the cell to simply fall apart. (Lelli et al.,1998). (Lee and Shacter, 1999)
Effects of ROS on cell metabolism have been well documented in a variety of species. These include not only roles in apoptosis (programmed cell death), but also positive effects such as the induction of host defense (Rada and Leto, 2008) (Conner et al., 2002) . Genes and mobilization of ion transport systems. Superoxide dismutase (SOD) is a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen. Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it functions to catalyze the decomposition of hydrogen peroxide to water and oxygen. (Chelikani et al., 2004).
Catalase has one of the highest turnover numbers of all enzymes; one molecule of catalase can convert millions of molecules of hydrogen peroxide to water and oxygen per second (Goodsell, 2004). Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long. (Boon et al., 2007). The optimum temperature also varies by species (Toner et al,. 2007). Deleting antioxidant enzymes generally yields shorter lifespan, though overexpression studies have not (with some recent exceptions), consistently extended lifespan. (Muller et al,. 2007)
In order to evaluate antistress property first we induced stress to the flies by adding Methotrexate [MTX]. It acts by inhibiting the metabolism of folic acid. Methotrexate competitively inhibits dihydrofolate reductase (DHFR), an enzyme that participates in the tetrahydrofolate synthesis. The affinity of methotrexate for DHFR is about one thousand-fold that of folate for DHFR. Dihydrofolate reductase catalyses the conversion of dihydrofolate to the active tetrahydrofolate. Folic acid is needed for the de novo synthesis of the nucleoside thymidine, required for DNA synthesis. Also, folate is needed for purine base synthesis, so all purine synthesis will be inhibited. Methotrexate, therefore, inhibits the synthesis of DNA, RNA, thymidylates, and proteins. (Johnston et al,. 2005). Analogues of folic acid were in development, and by 1950, methotrexate (then known as amethopterin ) was being proposed as a treatment for leukemia (Meyer et al,. 1950). A specific reason for integrating work on osmotic and metabolic or oxidative stress is that both trigger some of the same stress responses. One physiological response to stress is the increased activity of enzymes Catalase and Superoxide Dismutase enzymes are present in all living species .They play an important role for the maintenance of homeostasis in the cell.
Though there is a long list of drugs to handle stress the list of their side effects like headaches, back ache, neck pain, frozen shoulders, and other ailments is equally long. So everyone is on the look out for natural stress relief techniques which are very effective and at the same time don't have many side effects. Herbal treatment always has a significant chunk of followers. Herbs are natural stress relievers with little or no side effects. Herbs are used as single herb product or a combination product in the stress management therapy.
The result of the present study showed that the plant powder may have the anti-stress property as it reduced the stress, which was demonstrated by the reduced activities of enzymes like SOD and Catalase in stress induced Drosophila melanogaster .
MATERIALS AND METHODS
Fly rearing:
Flies were reared on ‘Rava-Agar Medium’.The DROSOPHILA STOCK CENTRE, Department of Zoology, University of Mysore, provided us the stocks of wild type Drosophila melanogaster , further, the stocks were cultured in our laboratory.The flies were maintained in an temperature between 20-25 degrees. The optimum temperature for the maintenance of Drosophila is between 20-25 degree.As the temperature decreases the development time increases. (Ashburner and Thompson 1978) (Ashburner et al,. 2005). Drosophila transfer and examinations are made while the flies are anesthetized using Di-ethyl-ether. Stocks are successfully cultured by periodic mass transfer of adults to fresh food. Stocks kept at room temperature should be transferred to fresh food every 20-30 days.
Stress induction
Any alteration in the food creates stress in an organism. Methotrexate was used and mixed along with the media in different concentration in range of 5ppm, 10ppm, 15ppm, and 20ppm and 25ppm. Later the stress induction was found by the estimation of enzyme activity were stress was found to be more in flies of all the different concentrations. The assayed stress enzymes are Catalase and superoxide dismutase.
Enzyme prepration:
Five adult flies each from Control flies, stresses induced Methotrexate flies of different concentrations (5ppm-25ppm), Stress induced along with plant powder and only plant extract flies were taken in a different Ependroff tubes. 200 microlitres of 50mM Phosphate buffer of pH 7 for Catalase assay, 200 microlitres of 250 mM Phosphate buffer of pH 7.8 for SOD Assay were added and crushed using tissue homogenizer in cold ice condition and centrifuged at 8000rpm for 20 min in a cooling Microfuge. After centrifuge, supernatant is removed and pooled to another ependroff tubes. 0.1ml/100ul of enzyme was used for the assay.
Catalase Activity Assay
Catalase enzyme (EC 1.11.1.6) was determined by the method of (Beers and Sizer 1952). Enzyme extract were mixed with 2.9 ml of 30% hydrogen peroxide (Freshly prepared using 50 mM phosphate buffer).Decrease in the absorbance due to decomposition of hydrogen peroxide was monitored at 240 nm in a spectrophotometer. Total protein estimation was done by Lowry’s method and the activity was expressed in units /mg of protein.
SOD Activity assay
SOD enzyme (EC 1.15.1.1)was assayed using a slightly modified procedure originally described by (Beauchamp and Fridovich 1971) .Enzyme extract were mixed with cocktail of solutions containing 250mM phosphate buffer(0.8 ml),100mM
Methionine (1 ml),100mM Riboflavin (0.5 ml), 5mM EDTA (0.1 ML), 750mM NBT (0.1 ML) .The volume was made upto 3 ml with distilled water . The cocktail solution without the supernatant (enzyme) and NBT was prepared to serve as blank .Control solution was prepared with the presence of NBT but no enzyme. Reduced NBT to formazone was read at 560 nm. The total protein content of enzyme was estimated by Lowry’s method and the activity was expressed in units /mg of protein.
RESULTS
Rearing of flies on media containing Methotrexate resulted in the increased activity of SOD and Catalase. These enzymes are the marker enzymes for the oxidative stress. The activity of SOD and Catalase increases, with respect to the increased concentration of Methotrexate in the media (Fig 1 and 2, Table -1) , when compared to control flies on normal media (no Methotrexate i.e; 0 ppm )
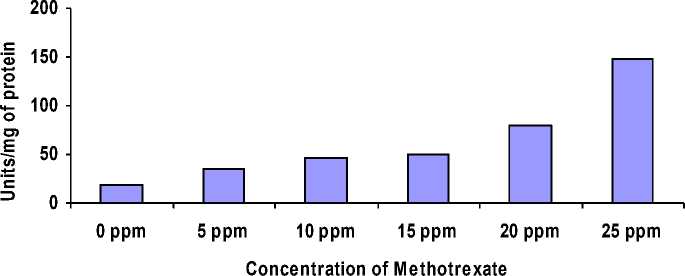
Figure 1: Activity of Catalase increases gradually in flies reared on media containing different concentration of Methotrexate when compared with control flies.
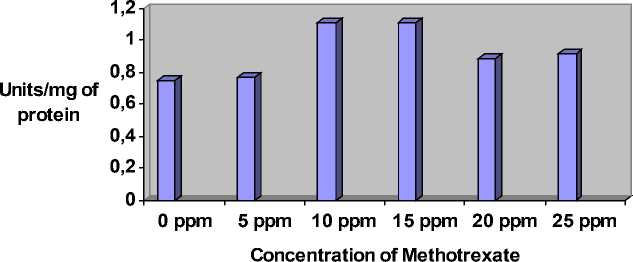
Figure 2: The flies reared on media containing different concentration of Methotrexate. Activity of SOD increases gradually till 15 ppm and slightly decreased in 20 ppm and 25 ppm.
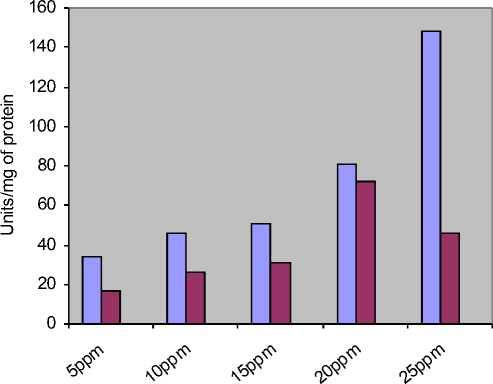
-
□ Catalase activity of flies treated with Methotrexate alone
-
□ Catalase Activity of flies treated with both Methotrexate and plant sample
Concentration of Methotrexate with 0.5 gms of plant sample
Figure 3: The increased activity in stress induced flies is reduced when treated with plant sample at a constant range
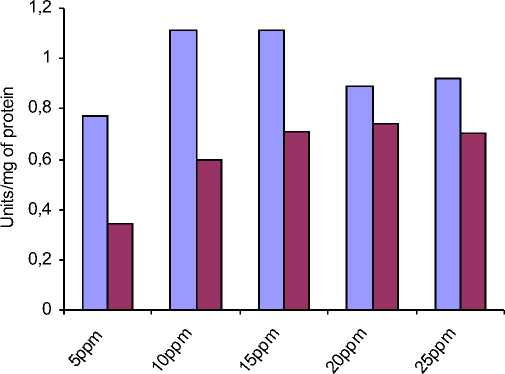
□ SOD Activity of flies treated with Methotrexate alone
□ SOD Activity if flies treated with both Methotrexate and plant sample
Concentration of Methotrexate with 0.5 gms of plant sample
Figure 4: The increased activity of Catalase in D.melanogaster is reduced when treated with plant sample at a constant range.
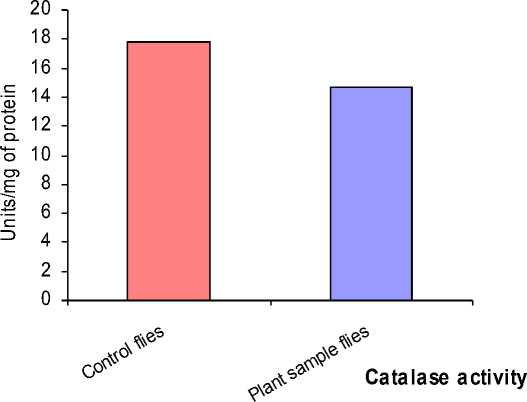
Figure 5: Compared with the Catalase activity of the control flies, the activity in the flies treated with plant sample alone was decreased.
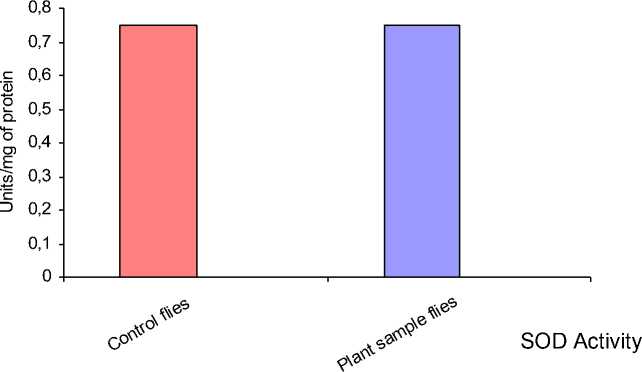
Figure 6: The SOD activity of both the control flies and the flies treated with plant sample alone was found to be same.
Enzyme activity in stress induced flies treated with plant sample.
The enzyme activity was differed in the flies reared on the media containing only 0.5 gm of plant sample. There was a decreased Catalase activity compared with control flies (Fig 5) and the SOD activity was found to be same as the control flies (Fig 6) (Table – 3).
Table.1 Increased SOD and Catalase activity in flies exposed to different concentration of
Methotrexate.
|
0 ppm (control) |
5 ppm |
10 ppm |
15 ppm |
20 ppm |
25 ppm |
|
|
Catalase Activity in (Units/mg of protein) |
17.6 |
34.40 |
46 |
50.30 |
80.42 |
147.76 |
|
SOD Activity in (Units/mg of protein) |
0.75 |
0.77 |
1.113 |
1.11 |
0.89 |
0.92 |
Table 2: Comparision table of Catalase and SOD activity of stress induced (MTX) alone Flies and MTX +0.5 gm of plant sample at different concentrations.
|
Concentration of MTX |
5ppm |
10ppm |
15ppm |
20ppm |
25ppm |
|
|
Catalase Activity (Units/mg protein) |
MTX alone |
34.40 |
46 |
50.30 |
80.42 |
147.76 |
|
MTX+0.5gm plant sample |
16.76 |
26.16 |
31.11 |
71.87 |
46 |
|
|
SOD Activity (Units/mg protein) |
MTX alone |
0.77 |
1.113 |
1.11 |
0.89 |
0.92 |
|
MTX+0.5gm plant sample |
0.34 |
0.60 |
0.71 |
0.74 |
0.7 |
Table 3. Variation of ezyme activity in normal D.melanogaster flies and the flies treated with Plant sample alone.
|
Catalase Activity in Units/mg of protein. |
SOD Activity in Units/mg of protein. |
|
|
Control flies |
17.96 |
0.75 |
|
Flies treated with plant sample alone |
14.63 |
0.75 |
DISCUSSION observed in humans after long term exposure
Long-term exposure to multiple stressors can cause depression in humans. Induction of depression using CMS is considered as the most congruent animal model of depressive conditions to multiple stressors (Willner, 1986). The results of the current study demonstrated decreased activity of the stress related marker enzymes in stress induced Drosophila melanogaster flies.
SOD, which dismutases the highly reactive superoxide anion to the less reactive species H 2 O 2 (Teixeira et al ., 1998). Catalase, a haeme containing enzyme, which scavenges hydrogen peroxide to water and molecular oxygen (Mates & Sanchez-Jimenez, 1999) and non-enzymatic ascorbic acid, which is a water-soluble antioxidant forage free radical protect the biological system from oxidative stress (Beyer, 1994).
The activity of Catalase and SOD increases significantly in a concentration dependent manner after inducing stress. One possible reason is that the stress inducing agent MTX may cause oxidative stress and much ROS is produced. In order to antagonize ROS, defensive enzymes such as Catalase and SOD activities are involved. The stress induction in the Drosophila was confirmed by the increased activity of cellular defensive enzymes like Catalase and SOD.
As per our results, the plant powder was found to reduce the stress induced by the stress against Methotrexate in D . melanogaster . The Catalase and SOD activity were increased in the Drosophila , which were grown in the media containing different concentrations of MTX. This was confirmed by comparing the activity of enzymes with that of control flies. Simultaneously the activity of the enzymes were decreased in the stress induced flies, when they were reared on the media containing 0.5 gm of plant sample along with different concentrations of MTX. Some of the flies were reared in the media, in the presence of only 0.5 gm. of plant sample. The Catalase activity in these flies was found to be decreased slightly, indicating that the plant sample is effective in suppressing the Catalase activity .In SOD activity in this group of flies was found to be same as the control, indicating that the plant sample may not be effective in suppressing the SOD activity in Drosophila .
Further investigations into the identification of compounds of Glycyrrhiza glabra is needed to better elucidate their Antistress property.
CONCLUSION
The result of the present study showed that the plant powder may have the anti-stress property as it reduced the stress, which was demonstrated by the reduced activities of marker enzymes like SOD and Catalase in stress induced Drosophila melanogaster .
Therefore, Catalase and SOD are thought to limit the accumulation of reactive oxygen species. The plant sample used as an anti-stress agent can be used for stress related disorders. the Anti- stress property was confirmed by employing the fall in Catalase and SOD activity compared to the stressor induced group i.e., the Anti-stressor group showed decreased catalase and SOD activity.
Acknowledgement
Список литературы Antistress property of Glycyrrhiza glabra (Athimadhura) on stress induced Drosophila melanogaster
- Ashburner, M. and Thompson, J. N. (1978) The laboratory culture of Drosophila in Ashburner M, Wright TRF. The genetics and biology of Drosophila. 2A, Academic Press, 1-81.
- Ashburner, M., Golic, K. G. and Hawley, R. S. (2005) Drosophila: A Laboratory Handbook. (2nd ed.). Cold Spring Harbor Laboratory Press. pp. 162-4.
- Beauchamp, C. O. and Fridovich, I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem.,44, 276-287.
- Beers, R. F., Jr., and Sizer, I. W. (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Biol. Chem., 195(1), 133-140.
- Beyer, R.E. (1994) The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J. Bioenerg Biomembr. 26, 349-358.
- Boon, E. M., Downs, A. and Marcey, D. (2007) Catalase: H2O2: H2O2 Oxidoreductase. Catalase Structural Tutorial Text.
- Chelikani, P., Fita, I. and Loewen, P. C. (2004) Diversity of structures and properties among catalases. Cell. Mol. Life Sci., 61 (2), 192-208.
- Conner, G. E., Salathe, M. and Forteza, R. (2002) Lactoperoxidase and Hydrogen Peroxide Metabolism in the Airway. Am J Respir Crit Care Med 166 (12), S57.
- Evans, M. D., Cooke, M. S. (2004) Factors contributing to the outcome of oxidative damage to nucleic acids. Bioessays 26(5), 533-42.
- Goodsell, D. S. (2004) Catalase. Molecule of the Month. RCSB Protein Data Bank.
- Johnston, A., Gudjonsson, J. E., Sigmundsdottir, H., Ludviksson, B. R. and Valdimarsson, H. (2005) The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 114, 154-163.
- Lee, Y. J., Shacter, E. (1999). Oxidative stress inhibits apoptosis in human lymphoma cells. J.Biol. Chem. 274 (28), 19792-8.
- Lelli, J. L., Becks, L. L., Dabrowska, M. I. and Hinshaw, D. B. (1998) ATP converts necrosis to apoptosis in oxidant-injured endothelial cells. Free Radic. Biol. Med. 25 (6), 694-702.
- Mates, J.M., and F. Sanchez-Jimenez (1999) Antioxidant enzymes and their implications in pathophysiologic processes. J. Front Biosci. 4, 339-345.
- Meyer, L. M., Miller, F. R., Rowen, M. J., Bock, G. and Rutzky, J. (1950) Treatment of acute leukemia with amethopterin (4-amino, 10-methyl pteroyl glutamic acid). Acta Haematologica 4 (3), 157-67.
- Muller, F. L., Lustgarten, M. S., Jang, Y., Richardson, A. and Van Remmen, H. (2007) Trends in oxidative aging theories. Free Radic. Biol. Med. 43 (4), 477-503.
- Rada, B., Leto, T. L., (2008). Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol 15, 164-87.
- Teixeira, H.D., R.I. Schumacher, and R. Meneghini (1998) Lower intracellular hydrogen peroxide levels in cells overexpressing CuZn-superoxide dismutase. Proc Natl Acad Sci USA. 95, 7872-7875.
- Toner, K., Sojka, G. and Ellis, R.(2007) A Quantitative Enzyme Study, catalase.bucknell.edu.
- Valko, M., Morris, H. and Cronin, M. T. (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem 12 (10), 1161-208.


