Application of DNA (RAPD) and ultrastructure to detect the effect of cadmium stress in Egyptian clover and Sudan grass plantlets
Автор: Aly Amina A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.8, 2012 года.
Бесплатный доступ
Background In recent years, several plant species have been used as bioindicators to evaluate the toxicity of environmental contaminants on vegetal organisms. In this study, Egyptian clover and Sudan grass seedlings were grown in four cadmium (Cd) concentration levels (0.0, 25, 50 and 100 µM) in MS media to analyze growth responses, Cd accumulation in the shoots and roots of plantlets, proline contents, chlorophylls content and MDA levels of both plantlets. As well as RAPD analysis and leaves ultrastructure were detected. Results The results showed that there was a significant decrease in root and shoot lengths, Chl a, Chl b, total Chl and carotenoids contents for both Egyptian clover and Sudan grass. However, there was a significant increase in Cd accumulation, proline and malondialdehyde (MDA) levels. The genetic variation between Egyptian clover and Sudan grass were evaluated using random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) markers to establish specific DNA markers associated with Cd stress. The results of transimssion electron microscopy (TEM) showed a clear disorder in the Cd treated Egyptian clover and Sudan grass seedlings. Conclusion In conclusion, biochemical, molecular and ultrastructure changes in Egyptian clover and Sudan grass could be used as a useful biomarker assay for the detection of genotoxic effects of Cd stress on plants. However, it is necessary to be further confirmed and optimized in the future research.
Biomarker, cadmium stress, dna (rapd), genomic template stability, ultrastructure
Короткий адрес: https://sciup.org/14323592
IDR: 14323592
Текст научной статьи Application of DNA (RAPD) and ultrastructure to detect the effect of cadmium stress in Egyptian clover and Sudan grass plantlets
Cadmium is a non essential heavy metal element and is a major environmental pollutant that is toxic to organisms including plants. In plants, Cd disturbs various biochemical and physiological processes, leading to cell death and inhibition of growth (Xu et al., 2009). Although, Cd is not essential for plant growth, its ions are taken up readily by the plant roots and translocated to the above-ground vegetative parts (Shamsi et al., 2008). Reports indicated that environmental heavy metals like Cd are mobilized in the food chain affecting producers and consumers (Veltman et al., 2008). Traditional methods of dealing with metal pollution are either the extremely costly process of removal and burial or simply isolation of the contaminated sites (Nanda-Kumar et al., 1995). Thus, new methods based on environmentally friendly and low-cost technology are needed. Phytoextraction by using hyperaccumulator plants has been proposed for decreasing the toxic-metal concentrations of contaminated soils (McGrath et al., 2002). There are several plants, which have the ability to absorb high amounts of heavy metals, while their growth remains unaffected. Crop species and cultivars differ widely in their ability to absorb, accumulate and tolerate Cd (Miller et al., 2006). The technology of using plants for removing heavy metals from soil is called ״phytoremediation״ (Verbruggen et al., 2009). Photosynthesis is usually suppressed by high concentrations of heavy metals but the effect of individual heavy metal can be specific for a given plant species and even cultivar (Küpper et al., 2002). Consequently the metal can inactivate many important enzymes resulting in inhibition of photosynthesis, respiratory rate and other metabolic processes in plants (Torres et al., 2000). Proline levels increased with cadmium stress and osmotic adjustment was associated with increase in proline and polyamine contents of plant cells (Dinakar et al., 2008). Reactive oxygen species (ROS) are main part of free radicals, which can lead to oxidative stress. ROS can react with lipids, proteins, pigments, and nucleic acid and cause lipid peroxidation, membrane damage and inactivation of enzymes, thus affecting cell viability (Dixit et al., 2001). Cd was known to induce the burst of active oxygen species (AOS) in plant tissues, leading to oxidative stress (Artetxe et al., 2002). There have been numerous reports on increasing malondialdehyde content induced by cadmium stress (Shah et al., 2001). Moreover, DNA based techniques (RAPD, AFLP and SSR) are used to evaluate the variation at the DNA sequence level. Random amplified polymorphic DNA (RAPD) of these techniques can be used to detect genotoxicity and the differences in RAPD profiles can clearly be shown when comparing DNA fingerprints from untreated and treated individuals to genotoxic agents (Theodorakis et al., 2006; Liu et al., 2009). Additionally, ultrastructural analyses of tissues stress to Cd are limited. Heavy metals induce anatomic and structural changes as well as physiological tolerance and/or adaptation in plant tissues (Gzyl et al., 2009). Cadmium induced loss of plasma membrane integrity, as well as ultrastructural changes of root cells of Sedum alfredii H (Tian et al., 2011). The current study was designed to detect the impact of cadmium stress on some biochemical parameters such as Cd accumulation in the shoots and roots of plantlets, proline content, chlorophylls content and MDA levels together with RAPD profiles and ultrastructure in both Egyptian clover and Sudan grass seedlings.
MATERIALS AND METHODS
Plant materials, growth conditions, and treatments: Mature dry seeds of Egyptian clover ( Trifolium alexandrinum L.) and Sudan grass ( Sorghum bicolor vulgare sudanense Piper.) were obtained from Forage Department, Agriculture Research Center, Giza-Egypt. Seeds were surface sterilized for 1 min in ethanol 70%, followed by 20 min in sodium hypochlorite 5.25% (Clorox) containing one drop of tween 20, then rinsed 5
times with sterilized distilled water. Seeds were placed on one-half-strength MS agar medium Murashige and Skoog (1962) containing 0.0, 25.0, 50.0 and 100.0 µM of cadmium chloride (CdCl 2 . H 2 O) under aseptic condition in 300 ml jars. These jars were maintained in a growth chamber at 23°C with a 12-hrs photoperiod provided by cool-white fluorescent tubes at a light intensity of approximately 80 m-2 s-1. After 21 days of growth, the biochemical, molecular and ultrastructure parameters were analyzed. The experiments were repeated three times.
Growth parameters : Ten plantlets were chosen randomly after 21-days of growth to record length of both the roots and shoots per plantlet.
Cadmium accumulation: Roots and shoots tissue were dried at 65°C for 24 hrs, and then ashed in Muffle furnace at 550°C for 20 hrs. The ash residue was incubated with 31 % HNO 3 and 17.5% H 2 O 2 at 72°C for about 2 hrs, and then dissolved in distilled water. Cadmium concentration in the digest was determined using an atomic absorption spectrophotometer (AA-6800, Shimadzu, Japan). The result was expressed as µg g-1 d.w. (Hsu and Kao., 2003).
Determination of chlorophylls and carotenoids content: The photosynthetic pigments (chlorophyll a, b, total chlorolophylls and total carotenoids) were determined according to the method of Lichtenthaler (1987). Shoots (0.5 g) were homogenized in chilled 80% acetone in a mortar and pestle in the dark at 4°C and the homogenates were centrifuged at 8800 rpm for 10 min. The chlorophylls a and b was measured using UV-Visible spectrophotometer at 662 nm and 644 nm, respectively. As well as, total carotenoids was measured at 470 nm. A solution of 80% acetone was used as a blank. The concentration of chlorophyll a, chlorophyll b and total carotenoids were expressed as mg g-1 f.w.
Proline determination: The proline content in the fresh shoots was estimated according to the method of Bates et al. (1973). Proline concentration was computed from a standard curve of proline and the results were expressed as mg g-1 f.w.
Lipid peroxidation (TBA test): The level of lipid peroxidation products in the shoots tissue were assayed using 2-thiobarbituric acid (TBA) reagent according to Buege and Aust (1978). The absorbance of the end product of lipid peroxidation (mainly malondialdehyde, MDA) was measured at 535 nm and corrected for non-specific turbidity by subtracting the absorbance at 600 nm. The level of lipid peroxidation products was expressed as nmol MDA g-1 f.w. by using an extinction coefficient of 155 mM-1cm-1 .
DNA extraction and RAPD-PCR technique: After 21 days of growth shoots were collected, grinded in liquid nitrogen and total genomic DNA was extracted according to DNeasy Plant Mini Kit (QIAGEN, Chatsworth, CA). The concentration of DNA was determined at a wavelength of 260/280 nm using spectrophotometer and the quality verified by electrophoresis on 1.4 % agarose gel. Twenty primers (Operon Technologies, Alameda, California, USA) were used for RAPD analysis; only eight Op-10 mer primers that produced clear and reproducible fragments were selected for the amplification of the DNA samples as shown in Table (1). PCR conditions were optimized according to Williams et al. (1990). PCR was performed in a reaction mixture of 25µl containing approximately 20 ng of genomic DNA, 0.2 μM primer, 20 μM dNTPs, 2.5 mM MgCl2, 0.5U of Taq DNA polymerase (Promega) and 1× reaction buffer. The PCR program comprised an initial denaturation step of 2 min at 94°C, followed by 35 cycles of 94°C for 1 min (denaturation), 36°C for 1 min (annealing) and 72°C for 1.5 min (extension) followed by a final extension period of 8 min at 72°C. The amplifications were carried out in triplicate. PCR products and a 100-bp DNA ladder (Fermentas) were resolved electrophoretically in 1.6% agarose gels containing 0.5 μl ml-1 ethidium bromide, and run at 60V for about 3 hrs. The molecular weights of the amplification products were calculated using 100-bp DNA ladder standards (Gibco BRL/Life Technology; Rockville, Maryland). RAPD banding patterns were visualized using a UV transilluminator and photo documentation was performed under UV light using a photo imaging system.
Table 1. List of primers name (ID) and their nucleotide sequences used in the RAPD analysis
|
Primer number |
Primer ID |
Primer sequence 'ــــــــــــــــ3'5 |
|
1 |
OP-B01 |
GTTTCGCTCC |
|
2 |
OP-B03 |
GTAGACCCGT |
|
3 |
OP-B11 |
GTAGACCCGT |
|
4 |
OP-C05 |
GATGACCGCC |
|
5 |
OP-C07 |
GTCCCGACGA |
|
6 |
OP-D01 |
ACCGCGAAGG |
|
7 |
OP-F01 |
ACGGATCCTG |
|
8 |
OP-F05 |
CCGAATTCCC |
Estimation of genomic template stability: Genomic template stability (GST) was calculated as follows: GST% = (1- a/n) X 100. Where (a) was RAPD polymorphic profiles detected in each samples treated and (n) the number of total bands in the control. Polymorphism observed in RAPD profiles included disappearance of a normal band and appearance of a new band in comparison to the control RAPD profiles (Atienzar et al., 2002) and the average was then calculated for each experimental group exposed to different Cd treatments.
RAPD data analysis: Amplicons (bands) were scored as 1 (presence) or 0 (absence). Only strong bands were scored for analysis. The size of each amplification product was automatically estimated using the Bio Image Analyzer System (Vendor, Italy). Images were captured using a high resolution scan and digitalized images were counted directly for RAPD analysis.
Transmission electron microscopy: Fresh leaves (about 1–3 mm in length) with different treatments were selected and fixed in 4 % glutaraldehyde (v/v) in 0.2 M sodium phosphate buffer (pH 7.2) for 2 hrs, post-fixed in 2 % osmium tetroxide (OsO4) for 1 hr and washed in 0.2 M sodium phosphate buffer (pH 7.2) for 1–2 hrs. Dehydration was carried out in a graded ethanol series (50%, 60%, 70%, 80%, 90%, 95%, and 100%) followed by acetone (100%), then samples were filtrated and embedded in Semith’s resin (Barcelo et al., 1988). Ultra-thin sections (80 nm) were prepared, stained with uranyl acetate and lead citrate and examined under transmission electron microscope (JEOL TEM-1200EX, Japan) at the National Centre for Radiation Research and Technology, Nasr City, Cairo-Egypt..
Statistical analysis: All data are reported as mean ± standard deviation (SD) for the three independent samples (n = 3). Analysis of variance and significant differences among means were tested by two-way ANOVA using the COSTAT computer package (Snedecor and Cochran, 1980). The least significant difference (LSD) at P≤0.05 level was calculated.
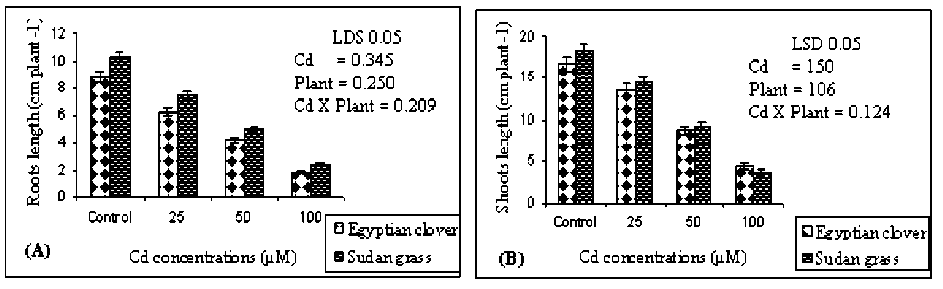
Figure 1 (A and B). Roots length and shoots length of the Egyptian clover and Sudan grass, respectively grown on MS media treated with different concentrations of Cd for 21 days. Means are significantly differed (p≤0.05). Bars indicate standard division (n=3).
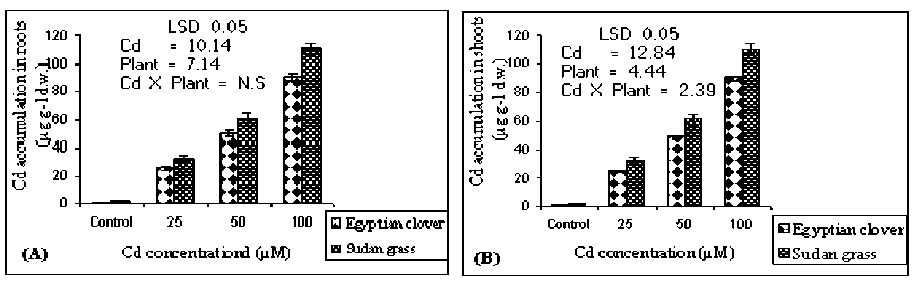
Figure 2 (A and B). Cd accumulation in roots and shoots of the Egyptian clover and Sudan grass, respectively grown on MS media treated with different concentrations of Cd for 21 days. Means are significantly differed (p≤0.05). Bars indicate standard division (n=3). N.S: Not significant.
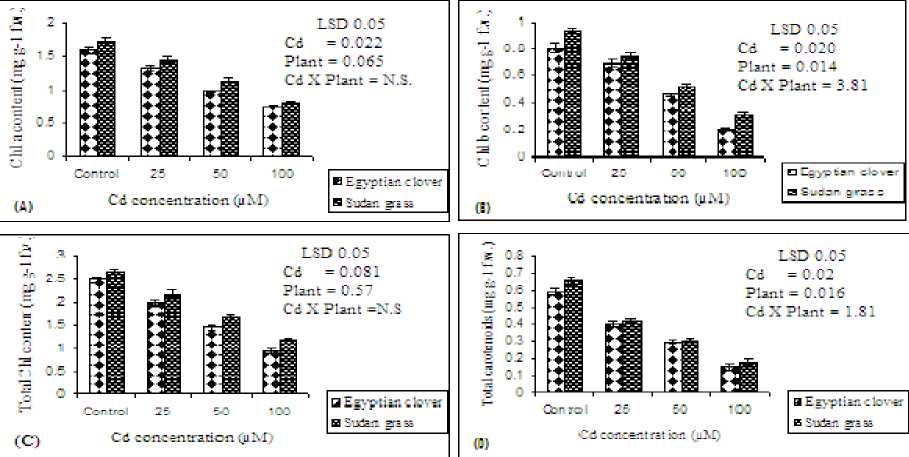
Figure 3 (A, B, C and D). Chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll and total carotenoids contents (mg g-1 f.w.), respectivily of Egyptian clover and Sudan grass grown on MS media treated with different concentrations of Cd for 21 days. Means are significantly differed (p≤0.05). Bars indicate standard division (n=3). N.S: Not significant.
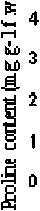
LSD 0.05
Cd = 0.094
Plant = 0.067
Cd X Plant = 2.22

Control 25 50
ClconjcaatntiiiE (цМ)
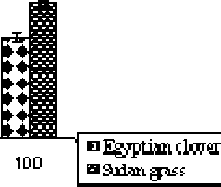
Figure 4. Proline content in the shoots of the Egyptian clover and Sudan grass grown on MS media treated with different concentrations of Cd for 21 days. Means are significantly differed (p≤0.05). Bars indicate standard division (n=3).
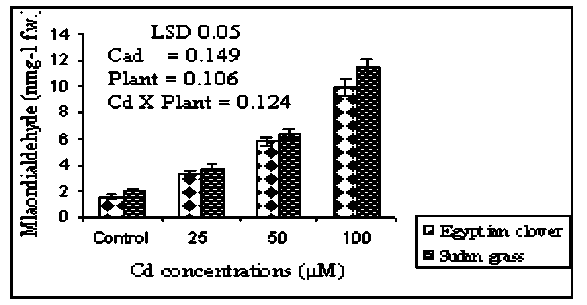
Figure 5. Malondialdehyde content of the Egyptian clover and Sudan grass grown on MS media treated with different concentrations of Cd for 21 days. Means are significantly differed (p≤0.05). Bars indicate standard division (n=3).
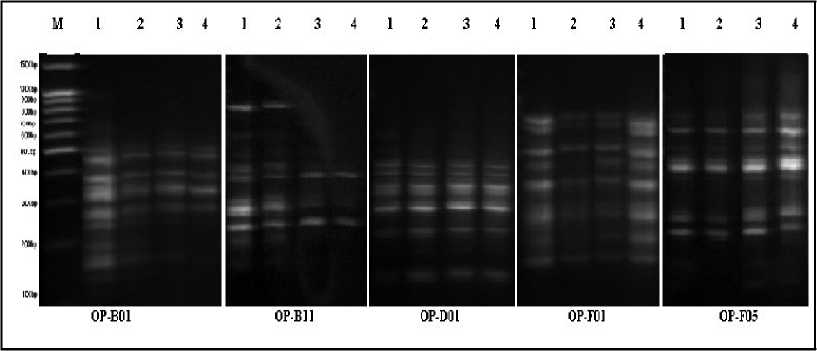
Figure 6. RAPD profiles in the shoots of Egyptian clover seedlings exposed to Cd different levels, whereas; 1,2,3 and 4 refers to 0.0, 25, 50 and 100 µM of Cd respectively. RAPD profiles were generated using primer OP-B-1, OP-B11, OP-D01, OP-F01 and OP-F05. M: 100-bp DNA ladder standards (100 - 1500 bp).
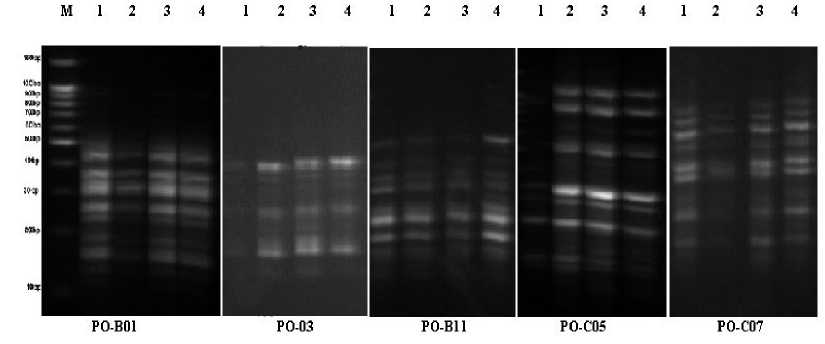
Figure 7. RAPD profiles in the shoots of Sudan grass seedlings exposed to Cd different levels, whereas; 1,2,3 and 4 refers to 0.0, 25, 50 and 100 µM of Cd respectively. RAPD profiles were generated using primer OP-B-1, OP-B03, OP-B11, OP-C05 and OP-C07. M: 100-bp DNA ladder standards (100 -1500 bp).
Table 2: The number of bands in control (0.0) and molecular sizes (base pair, bp) of appearance and/or disappearance of DNA bands for the used primers in the Cd-treated Egyptian clover seedlings using UVI soft image analyzer software
|
Primer ID |
Cd concentration (µM) |
|||
|
0.0 (Total bands) |
25.0 |
50.0 |
100.0 |
|
|
OP-B01 + |
12 |
161 1408; 1357; 535; 286; 257; 182 |
ND 1408; 1357; 535; 286; 257; 227; 182; 147 |
155 1408;1357; 535; 286; 257; 227, 182; 1 47 |
|
OP-B11 + |
8 |
210; 207 938; 392; 203 |
ND 938; 823; 392; 243; 226; 203 |
200 938; 823; 392; 243; 226; 203 |
|
OP-D01 + |
8 |
159; 113 203 |
159; 113 203; 197 |
159; 113 203; 197 |
|
OP-F01 + |
10 |
ND 793; 713; 464; 335 |
643; 193 713; 366; 335 |
ND 793; 713; 464; 366; 335; 238 |
|
OP-F05 + |
5 |
ND 766; 668; 256 |
766; 571; 476; 302 668; 258 |
766; 476; 302; 262 668; 256: 258 |
|
Total |
43 |
5 (+);17 (-) |
8 (+); 21 (-) |
8 (+); 25 (-) |
Table 3. The number of bands in control (0.0) and molecular sizes (base pair, bp) of appearance and/or disappearance of DNA bands for the used primers in the Cd-treated Sudan grass seedlings using UVI soft image analyzer software.
|
Primer ID |
Cd concentration (µM) |
|||
|
0.0 (Total bands) |
25.0 |
50.0 |
100.0 |
|
|
OP-B01 + |
12 |
ND 510; 242; 193; 178 |
173; 147 510; 193; 178; 154 |
173; 147 510; 242; 193; 178; 154 |
|
OP-B03 + |
9 |
412; 294; 268 551 |
412; 294; 268; 235 551 |
412; 294; 268; 235 551; 329 |
|
OP-B11 + |
10 |
ND 526; 380 |
ND 526; 380; 329 |
625; 365; 396 526; 380; 329 |
|
OP-C05 + |
10 |
224 1003; 480; 323; 231; 209 |
224 1003; 480; 510; 323; 297; 209 |
224 1003; 480; 510; 323; 297; 209; 147 |
|
OP-C07 + |
12 |
ND 622; 374; 291; 225 |
227 622; 374; 225; 179 |
334; 227 622; 374; 225; 179 |
|
Total |
53 |
4 (+); 16 (-) |
8 (+); 18 (-) |
12 (+); 21 (-) |
Table 4. Genomic template stability (GTS, %) in shoots of Egyptian clover exposed to different Cd concentrations (0.0, 25.0, 50.0 and 100.0 µM) for 21 days.
|
Primer ID |
Cd concentration (µM) |
|||
|
0.0 |
25.0 |
50.0 |
100.0 |
|
|
OP-B01 |
100 |
41.67 |
33.4 |
25.0 |
|
OP-B11 |
100 |
37.5 |
25.0 |
12.5 |
|
OP-DO1 |
100 |
62.5 |
50.0 |
37.5 |
|
OP-F01 |
100 |
60.0 |
50.0 |
40.0 |
|
OP-F05 |
100 |
40 |
20.0 |
12.5 |
|
Average |
100 |
48.33 |
35.68 |
25.5 |
Table 5. Genomic template stability (GTS%) in shoots of Sudan grass seedlings exposed to different Cd concentrations (0.0, 25.0, 50.0 and 100.0 µM) for 21 days.
|
Primer ID |
Cd concentration (µM) |
|||
|
0.0 |
25.0 |
50.0 |
100.0 |
|
|
OP-B01 |
100 |
66.67 |
58.30 |
41.67 |
|
OP-B03 |
100 |
55.56 |
44.44 |
33.33 |
|
OP-B11 |
100 |
90.00 |
70.0 |
40.00 |
|
OP-C05 |
100 |
40.00 |
30.00 |
20.00 |
|
OP-C07 |
100 |
66.67 |
58.33 |
50.0 |
|
Average |
100 |
52.23 |
50.23 |
48.25 |
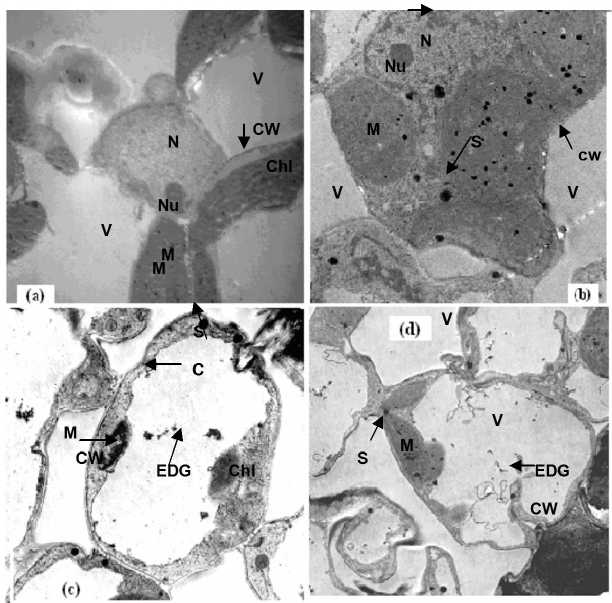
Figure 8. Electron micrographs of leaf mesophyll cells of 21-days old seedlings of Egyptian clover treated with Cd. Whereas, a,b,c and d refers to 0.0, 25, 50 and 100 µM of Cd, respectively. C = cytoplasm, Chl = chloroplast, CW = cell wall, EDG = electron dense granules, M = mitochondria, N = nucleus, Nu = nucleoli, S = Starsh granules, V = vacuole.
Chi cw
Chi
EDO
CW
Nu
/ V
CW
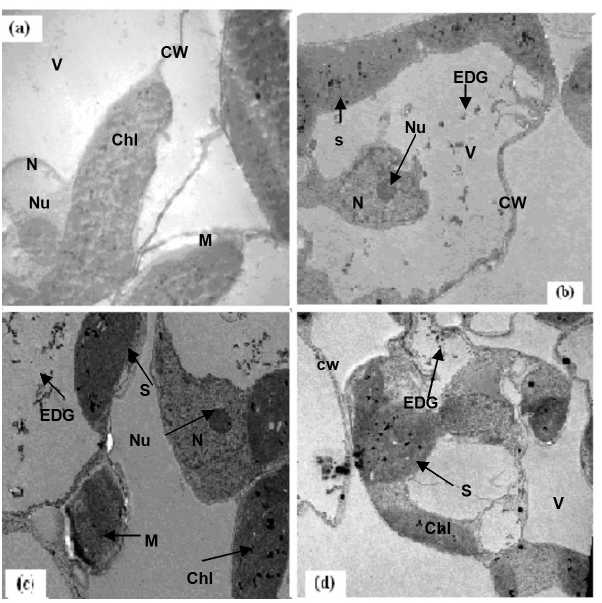
Figure 9. Electron micrographs of leaf mesophyll cells of 21-days old seedlings of Sudan grass treated with Cd Whereas, a,b,c and d refers to 0.0, 25, 50 and 100 µM of Cd, respectively. C = cytoplasm, Chl = chloroplast, CW = cell wall, EDG = electron dense granules, M = mitochondria, N = nucleus, S = Starsh granules, V = vacuole.
RESULTS
Cadmium has been shown to cause many physiological, biochemical and molecular changes in plants. The exposure of Egyptian clover and Sudan grass to different concentrations of cadmium (0.0, 25, 50 and 100 µM) for 21-days showed an exponential negative relationship between plantlets growth and the concentration of cadmium supplied to the medium. After 21 days of exposure to 0.0, 25.0 and 50.0 µM Cd, Egyptian clover and Sudan grass did not show any visual symptoms. But, when the concentration of Cd was up to 100.0 µM the chlorosis on the leaves was observed (data not shown). Such symptoms were observed mainly at higher Cd concentration (100.0 µM). Meanwhile, roots length of Egyptian clover was significantly (P≤0.05) reduced and this reduction increased by increasing Cd level up to 100.0 µM (1.86 cm plant-1) compared to control (8.84 cm plant-1) as shown in Figure (1-A). With the increase of Cd concentration in the medium, the shoots length of the plantlets was significantly (P≤0.05) decreased gradually for both, Egyptian clover and Sudan grass Figure (1-B). Cd significantly affects plant growth and morphology in a variety of species (Tamás et al., 2008; Daud et al., 2009). Additionally, Cd stress leads to protein degradation through amino acid metabolism resulting in decreased plant growth (Dinakar et al., 2008).
Cadmium accumulation: Generally, Cd increased in Egyptian clover and Sudan grass plantlets with increasing Cd levels in the culture media. Cadmium concentration in roots was generally greater than in shoots, the maximum Cd accumulation was found to be 308.17 and 110.32 µg g-1 d.w. in the roots and shoots of Sudan grass, respectively when the media were treated with 100 µM Cd in comparison to the control (Figure 2 A and
B). The accumulation of Cd in the roots and shoots of Sudan grass is more pronounced than in Egyptian clover. The present results are compatible to those reported in other (Lozano-Rodriguez et al; 1997; de la Rosa et al., 2004; Chen et al., 2007). Moreover, Mohamed et al. (2009) obtained similar observations that, Cd content in Radish is increased with the increasing application of Cd in the growth media. Cadmium can be easily absorbed as free ion, and roots of most plants have been found to be a major site of Cd accumulation (Di Cagno et al., 1999).
Chlorophylls and carotenoids contents: In the present study, exposure to Cd stress significantly (P≤0.05) decreased photosynthetic pigments (Chl a, Chl b, total Chl and carotenoids) concentrations in comparison with control seedlings (Figure 3 A, B, C and D). It was noted that maximum significant (P≤0.05) reduction occurred with 100 µM Cd. The decrease in Chl a, Chl b, total Chl and carotenoids contents of Egyptian clover was 51.02%, 34.37%, 45.16% and 27.41% at 100 µM Cd, respectively, compared to control (100%). The reduction in Chl a content was higher than Chl b. The same trend was observed for Sudan grass. The reduction in photosynthetic pigments in Sudan grass is greater than in Egyptian clover. Similar observations have been reported by Hayat et al. (2007) in Brassica juncea and (Gill et al., 2012) in garden cress (Lepidium sativum L.). Decrease in the chlorophyll is the primary bioindicator of Cd phytotoxicity (Zhao, 2011). Overall, the destruction of photosynthetic pigments by metals could be due to impairment of the electron transport chain, replacement of Mg2+ ions associated with the tetrapyrrole ring of chlorophyll molecules, inhibition of important enzymes associated with chlorophyll biosynthesis i.e; 5-aminolaevulinic acid dehydrates and protochlorophyllide reductase or peroxidation processes in chloroplast membrane lipids by the reactive oxygen species (Ylmaz and Parlak, 2011).
Proline content : Proline can play an important protective role against heavy metal stress. Proline increased significantly (P≤0.05) in both the Egyptian clover and Sudan grass under all applied Cd concentrations. Proline levels are greater in Sudan grass than in Egyptian clover (Figure 4). In comparison with control, proline content of Sudan grass shoots increased by 2.45, 3.69 and 5.01 folds at 25.0, 50.0 and 100.0 µM Cd, respectively. The same trend was observed for Egyptian clover, but proline accumulation was more pronounced in Sudan grass than in. Egyptian clover Therefore, this suggests that free proline might play an important protective role against Cd stress and that the Sudan grass has stronger self-protection. This is in accordance with Sun et al. (2009) who demonstrated that with the increasing Cd concentration in the soils, the enhanced concentrations of free proline were observed in both the leaves and the roots of ( Solanum nigrum L.). Also, Zhao (2011) has reported that proline levels increased in corn and wheat leaves with cadmium stress. Shoots increased their proline pool in response to mild Cd stress as a form of osmotic adjustment and protection of enzymes, membranes and ribosomes. Worth mentions that, proline plays a role in ROS scavenging (Torres et al., 2008), additionally, proline accumulation has also been proposed as a mechanism of storage of excess nitrogen. A detailed account has been studied about the production of the stress proline whose synthesis and degradation is a very guarded phenomenon, regulated by a set of enzymes.
Lipid peroxidation: In this study, the elevation in MDA content in shoots compared with the control under different Cd-induced oxidative stress shown in Figure 5. Levels of MDA in Sudan grass shoots were 1.95, 3.27 and 5.88 times increased at 25.0, 50.0 and 100.0 µM Cd, respectively compared with control. The same trend was observed with the Egyptian clover shoots. MDA level was more pronounced in Sudan grass than in Egyptian clover. Cd has been reported to enhance lipid peroxidation in many plant species, e.g., Triticum aestivum, Oryza sativa and Allium. Sativum (Sun et al., 2009). Additionally, the accumulation of Cd in Lonicera japonica contributed to the formation of ROS, confirmed by elevated MDA content (Liu et al., 2011). Thus, increased MDA indicates the prevalence of oxidative stress and this may be one of the possible mechanisms by which toxicity due to Cd is manifested in plant tissues (Gupta et al., 2009).
Effect of Cd stress on RAPD-PCR profile: A random amplified polymorphic DNA (RAPD) technique was applied to detect genotoxin-induced DNA damage of plants in Cd contaminated media. This study compared the effects occurring at molecular level in Egyptian clover and Sudan grass seedlings exposed to Cd stress (0.0, 25, 50 and 100 µM). Twenty 10-mer oligonucleotide primers were utilized used for screening Egyptian clover and Sudan grass or alterations, however only eight primers generated specific and stable results. RAPD profiles showed substantial differences between control and Cd-treated seedlings with apparent changes in the number and size of amplified DNA fragments with different primers. In the case of Egyptian clover (Figure 6 and Table 2) presented a summary of all RAPD profile modification and RAPD products of selected 5 primers. The used primers generated total number of 43 bands, the molecular size of amplified bands ranged from 147 to 1408 pb (OP-B01). Each primer generated between 5 (OP-F05) and 12 (OP-B01) bands with an average of 8.6 bands per primer. The disappearing of a normal band and/or appearing of a new band are the obvious changes in the RAPD patterns generated by Cd treatments. The number of disappearing RAPDs bands for (OP-B01) was the highest (8) in Cd treated seedlings (50 and 100 µM of Cd treated samples) as compared with (OP-D01) which gave the lowest number (2) of disappearing RAPDs bands. Moreover, in the case of Sudan grass (Figure7 and Table 3) which exposed to Cd different levels, RAPD profils varied in loss of normal bands and appearance of new bands, compared with the control seedlings. The samples grown at 100 µM displayed the highest variation in the number of bands in RAPD analysis (33), in the form of band appearance (12) and disappearance (21) with molecular sizes of 147–1003 pb. In comparison between seedlings of Egyptian clover and Sudan grass the variation in RAPD profiles was more in Egyptian clover than in Sudan grass and these variations were increased by increasing Cd level in the growth media. The genomic template stability (GTS, %) values, a qualitative measure reflecting changes in RAPD profiles was calculated for each 5 primers and presented in Tables (4 and 5). GTS values decreased obviously with an increase in Cd concentration.
Cadmium is one of the most toxic environmental and industrial pollutants causing DNA damage, elevating lipid peroxidation with a long biological half-life time and represents a serious environmental pollutant for animal and plants. It influences many plants, animal and human communities. Therefore, a deeper understanding of the mechanism of Cd toxicity is important. Cd could induce DNA damage such as single- and double-strand breaks, modified bases, abasic sites, DNA-protein crosslink, and oxidized bases and even bulky adducts in organisms (Liu et al., 2005, 2009; Cenkci et al., 2009). In earlier studies, the RAPD profiles generated from the barley and rice seedling treatment cadmium revealed different patterns of the appearance and disappearance of bands to the control RAPD pattern (Liu et al., 2007; Liu et al., 2009). The present finding support this claim that DNA polymorphisms detected by RAPD can be considered as a powerful biomarker assay for detection of the genotoxic effects of environmental pollutants like Cd stress. Modifications observed in RAPD profiles are likely to be due to the changes in oligonucleotide primingsites and/or interactions of DNA polymerase with damaged DNA. These events could block or reduce polymerization of DNA in the PCR reaction (Nelson et al., 1996). Genomic template stability is related to the level of DNA damage, the efficiency of DNA repair and replication. Therefore, a high level of DNA damage does not necessarily decrease the genomic template stability (in comparison with a low level of DNA alterations), because DNA repair and replication are inhibited by the high frequency of DNA damage (Atienzar and Jha, 2006). These markers would be widely applicable to study the effect of contaminants on population genetics and its adaptation to different stresses. DNA polymorphism detected using RAPD analysis due to the presence or absence of bands in treatments than control could be used as an investigation tool for environmenat toxicology and as a useful biomarker assay.
Electron microscopy leaves of Egyptian clover and Sudan grass: In the present study, one of the main objectives was to explore the cellular mechanism being involved against Cd detoxification in two plants; Egyptian clover and Sudan grass. According to the microscopic investigation, the ultra-morphological changes in the mesophyll cells were dose-dependent. With the increase in Cd levels, changes in the fine structures of leaf mesophyll cells also became evident. In sections of non-Cd, leaves tissue (Figure 8-a and 9-a), among the many cellular components evident is vacuole. The vacuole of control cells is empty and did not have any particles in it. The cell wall of these cells does not have any grains in it. On the other hand, at 100µM Cd level, damage to the fine structure of leaf mesophyll cells of the both plantlets of Egyptian clover and Sudan grass were more scattered as compared with control (Figures 8 and 9 a-d). Also, the number and size of the starch grains increased. The toxic effects of Cd were more evident in the symplastic organelles, namely, nucleoli and chloroplast. Size and the numbers of nucleoli decreased progressively and internal structure of chloroplast was roughly smashed along with the increase in the size of starch grains. Also electron dense granules, although insignificant in number, inside the vacuoles and attached to the cell walls in both plants could be seen in these electron micrographs, these dense granules might be Cd. Nucleus, which has a genetic organization and is responsible to control the action of cell through selective expression by genes, became irregular and number of nucleoli increased with the increase in Cd concentration (Jiang et al., 2007). The presence of Cd particles may be due to the possible functions of Cd binding peptides synthesis (CdBPs) in detoxification of Cd. Exposure to Cd induces the synthesis of CdBPs, which presumably takes place in the cytoplasm or in chloroplast (Daud et al., 2009). The accumulation of starch in leaves either in case of the present study or that of Sandalio et al. (2001) might be due to Cd stress (Daud et al., 2009). The ultrastructural studies on leaf mesophyll cells of Egyptian clover and Sudan grass reveal that as a whole Cd did not drastically alter the ultramorphology of different cell organelles, which might be due to the differences in the genetic make up of these materials and it needs further investigation.
CONCLUSION
In conclusion, results of the current study suggested that Cd induced physiological, biochemical and molecular changes in Egyptian clover and Sudan grass seedlings. Also, Egyptian clover and Sudan grass showed impaired in chlorophylls and carotenoids contents. Under elevated Cd concentrations, cadmium content (roots and shoots), proline, and lipid peroxidation increased. The free proline accumulation in Egyptian clover and Sudan grass shoots played an important role in Cd tolerance. Based on the abovemention results, it could be concluded that Sudan grass seedlings was superior to Egyptian clover in most of the measured parameters, it could be suggested that Sudan grass is more tolerance to Cd stress than Egyptian clover. It is worth to mention that, DNA polymorphism detected using RAPD analysis could be used as an investigation tool for environmental toxicology and as a useful biomarker assay that can be used as an early warning system. Furthermore, the present results suggested that RAPD analysis in conjunction with other biomarkers such as growth parameter, chlorophylls and proline, etc. would prove a powerful ecotoxicological tool. However, more information is needed at the molecular and subceluar levels in order to insights to the mechanistic explanation of cadmium toxicity in Egyptian clover and Sudan grass.
Список литературы Application of DNA (RAPD) and ultrastructure to detect the effect of cadmium stress in Egyptian clover and Sudan grass plantlets
- Artetxe, U., Garcia-Plazaola, J.I., Hernandez, A., and Becerril, J.M. (2002) Low light grown duckweed plants are more protected against the toxicity induced by Zn and Cd. Plant Physiol. Biochem., 40, 859-863.
- Atienzar, F.A. and Jha, A.N., (2006) The random amplified polymorphic DNA (RAPD) assay and related techniques applied to genotoxicity and carcinogenesis studies: a critical review. Mutat. Res., 613, 76-102.
- Atienzar, F.A., Venier, P. and Jha, A.N. (2002) Evaluation of the random amplified polymorphic DNA (RAPD) assay for the detection of DNA damage and mutations. Mutat. Res., 521, 151-163.
- Barcelo, J., Vazquez, M.D. and Poschenrieder, C. (1988) Structural and ultrastructural disorders in cadmium-treated bush bean plants (Phaseolus vulgaris L.). New Phytologist, 108, 37-49.
- Bates, L.S., Waldren, R.P. and Teare, I.D. (1973) Rapid determination of free proline for water-stress studies. Plant Soil, 39, 205-207.
- Buege, J.A. and Aust, S.D. (1978) Microsomal lipid peroxidation. Methods Enzymol., 52, 302-310.
- Cenkci, S., Yildiz, M., Cigerci, I., Konuk, M. and Bozdag, A. (2009) Toxic chemicals-induced gentoxicity detected by random amplified polymorphic DNA (RAPD) in bean (Phaseolus vulgaris L.) seedlings. Chemosphere, 76, 900-906.
- Chen, F., Wu, F.B., Dong, J., Vinczx, E., Zhang, G.P., Wang, F., Huang, Y.Z. and Kang, W. (2007) Cadmium translocation and accumulation in developing barley grains. Planta, 227, 223-232.
- Daud, M.K., Variath, M.T., Ali, S., Najeeb, U., Jamil, M., Hayat, Y., Dawood, M., Khand, M.I., Zaffar, M., Cheemad, S.A., Tonga X.H., and Zhu, S. (2009) Cadmium-induced ultramorphological and physiological changes in leaves of two transgenic cotton cultivars and their wild relative. J. Hazard. Mater., 168, 614-625.
- de la Rosa, G., Peralta-Videa, J.R., Montes, M., Parsons, J.G., Cano-Aguilera, I. and Gardea-Torresdey, J.L. (2004) Cadmium uptake and translocation tumbleweed (Salsola kali), a potential Cd-hyperaccumulator desert plant species: ICP/OES and XAS studies. Chemosphere, 55, 1159-1168.
- Dinakar, N., Nagajyothi, P.C., Suresh, S., Udaykiran, Y. and Damodharam, T. (2008) Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J. Environ. Sci., 20, 199-206.
- Di Cagno, R., Guidi, L., Stefani, A. and Soldatini, G.F. (1999) Effects of cadmium on growth of Helianthus annus seedlings: physiological aspects. New Phytol., 144, 65-71.
- Dixit, V., Pandey, V. and Shyam, R. (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot., 52, 1101-1109.
- Gzyl, J., Przymusinski, R. and Gwozdz, E.A. (2009) Ultrastructure analysis of cadmium-tolerant and -sensitive cell lines of cucumber (Cucumis sativus L.). Plant Cell Tiss Organ Cult., 99, 227-232.
- Gill, S.S., Khana, N.A. and Tutejab, N. (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci., 182, 112-120.
- Gupta, M., Sharma, P., Sarin, N.B. and Sinha, A.K. (2009) Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere, 74, 1201-1208.
- Hayat, S., Ali, B., Aiman, S. and Ahmad, H.A. (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ. Exp. Bot., 60, 33-41.
- Hsu, Y.T. and Kao, C.H. (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ., 26, 867-874.
- Jiang, H.M., Yang, J.C. and Zhang, J.F. (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ. Pollut., 147, 750-756.
- Kupper, H.I., Spiller, M., Kupper, F.C. and Ondrej, P. (2002) Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J. Phycol., 38, 429-441.
- Lichtenthaler, H.K. (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol., 148, 350-382.
- Liu, W., Li, P., Qi, X., Zhou, Q., Zheng, L., Sun, T.H. and Yang, Y.S. (2005) DNA changes in barely (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD. Chemosphere, 61, 158-167.
- Liu, W., Yang, Y.S., Li, P.J., Zhou, Q.X., Xie, L.J. and Han, Y.P. (2009) Risk assessment of cadmium contaminated soil on plant DNA damage using RAPD and physiological indices. J. Hazard. Mater., 161, 878-883.
- Liu, Z., Chen, W. and He, X. (2011) Cadmium-induced changes in growth and antioxidative mechanisms of a medicine plant (Lonicera japonica Thunb.) J. Medicinal Plants Res., 5, 1411-1417.
- Lozano-Rodriguez, E., Hernandez, L. E., Bonay, P. and. Carpena-Ruiz, R. O. (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J. Exp. Bot., 48, 123-128.
- McGrath, S.P., Zhao, F.J. and Lombi, E. (2002) Phytoremediation of metals, metalloids, and radionuclides. Adv. Agron., 75, 1-56.
- Miller, J.F., Green, C.E., Li, Y.M. and Chaney, R.L. (2006) Registration of three low cadmium (HA 448, HA 449, and RHA 450) confection sunflower genetic stocks. Crop Sci., 46, 489-490.
- Mohamed, A.A, El-Beltagi, H.S. and Rashed, M.M. (2009) Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. EJEAF Chem., 8, 969-983.
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant., 15, 473-497.
- Nanda-Kumar, P.B.A., Dushenkov, V., Motto, H. and Raskin, I. (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ. Sci. Technol., 29, 1232-1238.
- Nelson, J.R., Lawrence, C.W. and Hinkle, D.C. (1996) Thymine-thymine dimer bypass by yeast DNA-polymerase-zeta. Science, 272, 1646-1649.
- Sandalio, L.M., Dalurzo, H.C., G?mez, M., Romero-Puertas, M.C., and del Rio, L.A. (2001) Cadmium-induces changes in the growth and oxidative metabolism of pea Plants. J. Exp. Bot., 52, 2115-2126.
- Shah, K., Kumar, R.G., Verma, S. and Dubey, R.S. (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci., 161, 1135-1141.
- Shamsi, I.H., Wei, K., Zhang, G.P., Jilani, G. and Hassan, M.J. (2008) Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biol. Plant, 52, 165-169.
- Snedecor, G.W. and Cochran, G.W. (1980) Statistical Methods, 7th edition. The Iowa State, University Press, Ames, Iowa.
- Sun, Y.B. Zhou, Q.X. Wang, L. and Liu, W.T. (2009) Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator, J. Hazard. Mater., 161, 808-814.
- Tamas, L., Dudikova, J., Durcekova, K., Haluskov, L.u., Huttova, J., Mistrik, I. and Olle, M. (2008) Alterations of the gene expression, lipid peroxidation, proline and thiol content along the barley root exposed to cadmium. J. Plant Physiol., 165, 1193-1203.
- Theodorakis, C.W., Lee, K.L., Adams, S.M. and Law, C.B. (2006) Evidence of altered gene flow, mutation rate, and genetic diversity in redbreast sunfish from a pulp-mill-contaminated river. Environ. Sci. Technol., 40, 377-386.
- Tian, S.K., Lu, L.L., Zhang, J., Wang, K., Brown, P.H., He, Z.L., Liang, J. and Yang, X.E. (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere, 84, 63-69.
- Torres, M.A., Barros, M.P., Campos, S.C.G., Pinto, E., Rajamani, S., Sayre, R.T. and Colepicolo, P. (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol. Environ. Saf., 71, 1-15.
- Torres, E., Cid, A., Herrero, C. and Abalde, J. (2000) Effect of cadmium on growth, ATP content, carbon fixation and ultrastructure in the marine diatom Phaeodactylum tricornutum Bohlin. Water Air Soil Poll., 117,1-14.
- Veltman, K., Huijbregts, M.A.J. and Hendriks, A.J. (2008) Cadmium bioaccumulation factors for terrestrial species: application of mechanistic bioaccumulation model OMEGA to explain field data. Sci. Total Environ., 406, 413-418.
- Verbruggen, N., Hermans, C. and Schat, H. (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol., 181, 759-776.
- Williams, J., Kubelik, A.R. and Livak, K.J. (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acid Res., 18, 6531-6535.
- Xu, J., Yin, H.X. and Li, X. (2009) Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep., 28, 325-333.
- Y?lmaz, D.D. and Parlak, K.U. (2011) Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecological Indicators, 11, 417-423.
- Zhao, Y. (2011) Cadmium accumulation and antioxidative defenses in leaves of Triticum aestivum L. and Zea mays L. Afr. J. Biotech., 10, 2936-2943.


