Application of fluxes for melting and processing of aluminum alloys
Автор: Turakhujaeva Sh.N., Turakhodjaeva F.N., Abdurakhmanov Kh.Z., Karimov K.A., Turakhodjaev N.J., Obidov Z.R., Mirmuhamedov M.M., Sharipov Ja.H., Komolov Kh., Abduvaliev A.M.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Рубрика: Исследования. Проектирование. Опыт эксплуатации
Статья в выпуске: 1 т.18, 2025 года.
Бесплатный доступ
This article explores of the effect of the composition of the protective flux on the melting process of aluminum alloys and their mechanical properties. Experimental studies have been carried out with various flux compositions in order to optimize the melting process and improve the quality of the alloys obtained. The results showed that the change in the composition of the flux has a significant effect on the structure of the alloy and its mechanical characteristics. Optimization of the flux composition can lead to improved strength, corrosion resistance and other important properties of the alloy. The results obtained can be used in the metallurgical industry to optimize the melting process of aluminum alloys and improve the quality of products.
Аluminum alloys, melting process, composition of the flux, conducting of the flux, alloys structure, mechanical properties
Короткий адрес: https://sciup.org/146283038
IDR: 146283038 | УДК: 621.882
Текст научной статьи Application of fluxes for melting and processing of aluminum alloys
Melting of aluminum alloys is an important process in the metallurgical industry. One of the key aspects of this process is the use of a protective flux, which prevents the oxidation of the molten metal and ensures its purity. In this paper, we investigate the effect of the flux composition on the melting process and the properties of the obtained alloys.
Currently, there is a need to find and develop new methods and flux compositions that could optimize the melting process and improve the properties of the resulting alloys. This is important to increase production efficiency, reduce costs and improve the quality of the final product.
In this paper, we will focus on studying the influence of the composition of the protective flux on the melting process of aluminum alloys and the mechanical properties of the resulting materials. We will perform experimental studies with various flux compositions to determine their effectiveness and potential for improving the melting process and alloy quality. The results obtained will have important practical application in the metallurgical industry and can become the basis for the development of new technologies and methods for the production of aluminum products.
Resource saving in the melting of aluminum alloys is of particular importance in view of the peculiarities of heat exchange processes in which alloys are saturated with gas and non-metallic inclusions. In this regard, research is being conducted worldwide to optimize the melting processes of aluminum alloys and improve existing melting process technologies [1–3]. The protective flux plays a major role in the melting of aluminum alloys, where it reacts actively with the alloy, and at the same time protects the alloy from the environment [1–3]. All over the world, research is actively underway to develop new compositions of protective fluxes that prevent aluminum oxidation with oxygen and saturation of the melt with gas inclusions [4–6].
As is known, in order to remove undesirable impurities from aluminum alloys, various types of fluxes are used, such as solid and gaseous [17]. To extract gas inclusion from an aluminum alloy, fluxes based on a mixture of KCl-NaCl [18] are used, which form a low-temperature eutectic (665 °C). Another commonly used component of the flux is NaF, which forms a triple eutectic with KCl and NaCl with a melting point of 607 °C. At the same time, the melting point of technically pure aluminum is about 655–660 °C. Coating fluxes for aluminum melt are in great demand in production area. The usual coating flux contains about 47.5 % NaCl, 47.5 % KCl and 5 % fluoride salt. The low melting point increases the fluidity of the flux. Other coating fluxes are based on a mixture of MgCl 2 -KCl, which forms a low-melting eutectic at 424 °C, or on carnallite (MgCl2×KCl), which melts at 485 °C. These coating fluxes have high fluidity and can form a thin layer on the surface of the melt. However, MgCl 2 is quite expensive, so it is mainly used in sodium-free fluxes for aluminum alloys with a magnesium content of more than 2 % [19–21].
To explore the formation of aluminum oxides and the diffusion of gases into the melt, it is necessary to understand the interaction of aluminum and moisture in the furnace atmosphere. Since the combustion products of natural gas have water vapor in their composition, this reaction is inevitable in all gas furnaces [8–10]. Atmospheric moisture reacts with molten aluminum, followed by the formation of an oxide film and atomic hydrogen between aluminum atoms. As a result, the oxides not only remain on the surface, but also some of them enter the melt [22–24].
The main non-metallic inclusions in aluminum alloys are gas and oxides. Where oxygen will react with aluminum and create film on the surface of the alloy and gasses will diffuse to the alloy which in further provide the porosity of the product [25].
Experimental part
The main non-metallic inclusions in aluminum alloys are aluminum oxide Al 2 O 3 and hydrogen H 2 . The gas impurities present in the metal negatively affect the physical and mechanical characteristics of aluminum and its alloys. To obtain a high-quality structure of aluminum alloys, it is necessary to protect the melt from the effects of the furnace atmosphere. To determine the effect of the integrity of the oxide film on the melt surface, as well as the composition of the protective flux on the diffusion of non-metallic inclusions into the melt, 4 flux compositions with different carbon content were used. The composition of the flux was developed taking into depend on the furnace atmosphere, which has an excessive content of hydrogen and oxygen. Table1 and table 2 show the composition of fluxes used in research work in the melting of aluminum alloys under the conditions of the joint venture Uzavtoinzi llc. Table 1 provides a comparative analysis of the use of flux for melting aluminum alloys in various gas furnaces.
Free carbon located in the melting zone can restore aluminum and take away oxygen from it. To determine the effect of the free carbon content, carbon of various amounts was introduced into the composition of the flux. Table 2 shows the composition of the developed flux using free carbon.
Table 1. Resultative analysis of the use of flux for melting aluminum alloys in various gas furnaces
|
The purpose of the flux |
The components of the flux in weight percentages |
||||||
|
NaCl |
KCl |
C |
MgCl 2 |
CaCl 2 |
NaF |
BaCl 2 |
|
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
30 |
10 |
10 |
– |
– |
– |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
20 |
20 |
– |
10 |
– |
– |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
20 |
25 |
– |
– |
15 |
– |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
30 |
30 |
30 |
– |
– |
– |
10 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
20 |
45 |
35 |
– |
– |
– |
– |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
40 |
10 |
– |
– |
10 |
– |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
– |
20 |
10 |
30 |
– |
– |
Table 2. A composition of the developed flux using free carbon
|
The purpose of the flux |
The components of the flux in weight percentages |
||||||
|
NaCl |
KCl |
CaF 2 |
MgCl 2 |
CaCl 2 |
NaF |
С |
|
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
20 |
20 |
– |
– |
– |
10 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
20 |
20 |
– |
– |
– |
20 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
20 |
5 |
– |
– |
5 |
30 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
30 |
10 |
– |
– |
– |
10 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
25 |
5 |
– |
– |
– |
20 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
10 |
10 |
– |
– |
10 |
30 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
40 |
– |
20 |
10 |
10 |
– |
20 |
|
Heat of melting |
|||||||
|
Specific heat of melting, cal/g |
123.5 |
74.1 |
– |
70 |
54.2 |
27,8 |
– |
|
Melting point, оС |
804.3 |
790 |
1330 |
712 |
772 |
962 |
2800 |
|
Density, g/sm3 |
2.163 |
189 |
3.18 |
2.32 |
2.152 |
– |
2.33 |
Table 3. Resultative analysis of the use of flux for melting aluminum alloys in various gas furnaces
|
The purpose of the flux |
The components of the flux in weight percentages |
||
|
NaCl,% |
KCl,% |
C,% |
|
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
40 |
10 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
30 |
20 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
50 |
20 |
30 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
30 |
30 |
40 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
20 |
30 |
50 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
20 |
20 |
60 |
|
As a protective layer for remelting aluminum alloys in gas furnaces |
30 |
– |
70 |
Table 3 provides a comparative analysis of the use of flux for melting aluminum alloys in various gas furnaces.
Results and discussion
The analysis of the results showed that a change in the composition of the protective flux leads to changes in the structure of the obtained alloys. For example, the use of fluxes with a high carbohydrate content helps to reduce the amount of oxides in the melt, which has a positive effect on the quality of the alloy. It has also been found that optimizing the composition of the flux can improve the mechanical properties of alloys, such as strength and corrosion resistance.
To determine the consequence of the flux on the quality of the resulting melt, laboratory studies were carried out. Experimental melting of aluminum to determine the effect of melting modes on the quality of the alloy was carried out in the laboratory conditions of the enterprise (Table 4).
Table 5 shows changes in the content of gas inclusions in the melt depending on the temperature of overheating of the melt in the refining flux. The results of studies to determine the optimal temperature of overheating of aluminum melt under a protective layer of flux.
At the same time, the composition of the flux is of primary importance, since at high temperatures the chemical elements of the flux can lose their basic properties. Graphic shows a graph of changes in
Table 4. The developed fluxes for melting aluminum alloys
|
The purpose of the flux |
The components of the flux in weight percentages |
||||||
|
NaCl |
KCl |
С |
MgCl 2 |
CaCl 2 |
NaF |
CaF 2 |
|
|
For remelting process of the waste |
50 |
30 |
20 |
– |
– |
– |
– |
|
For remelting process of the waste |
50 |
20 |
20 |
– |
10 |
– |
– |
|
For remelting process of the waste |
40 |
20 |
8 |
– |
2 |
30 |
– |
|
For remelting process of the waste |
50 |
30 |
10 |
– |
– |
– |
10 |
|
For remelting process of the waste |
50 |
45 |
15 |
– |
– |
– |
– |
|
For remelting process of the waste |
40 |
40 |
18 |
– |
– |
10 |
– |
|
For remelting process of the waste |
40 |
– |
20 |
10 |
30 |
– |
– |
Table 5. The changes in the melt from temperature of overheating of the melt and refining flux
Fig. 2 and 3 show the structures of the resulting alloy after melting in an electroslag furnace with and without the use of a protective layer of refining flux.
The results of our study are an important contribution to understanding the effect of the protective flux composition on the melting process of aluminum alloys and their mechanical properties. In the course of the work, it was found that a change in the composition of the flux has a significant effect on the structure of the alloy and its properties, which corresponds to the results of previous studies in this area.
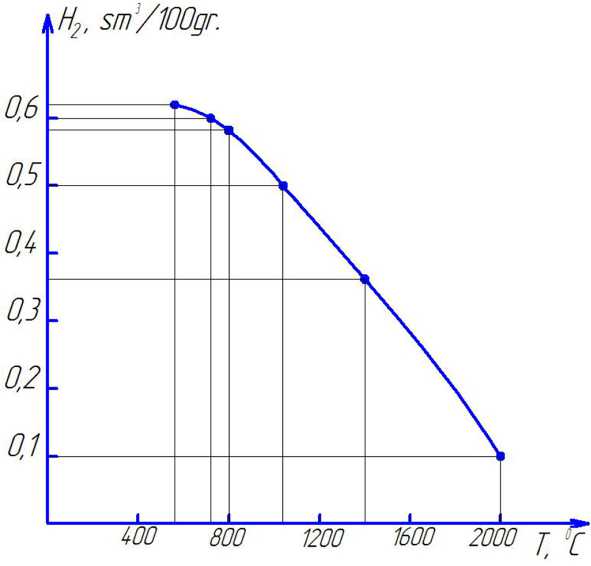
Fig. 1. The amount of hydrogen depend on the temperature
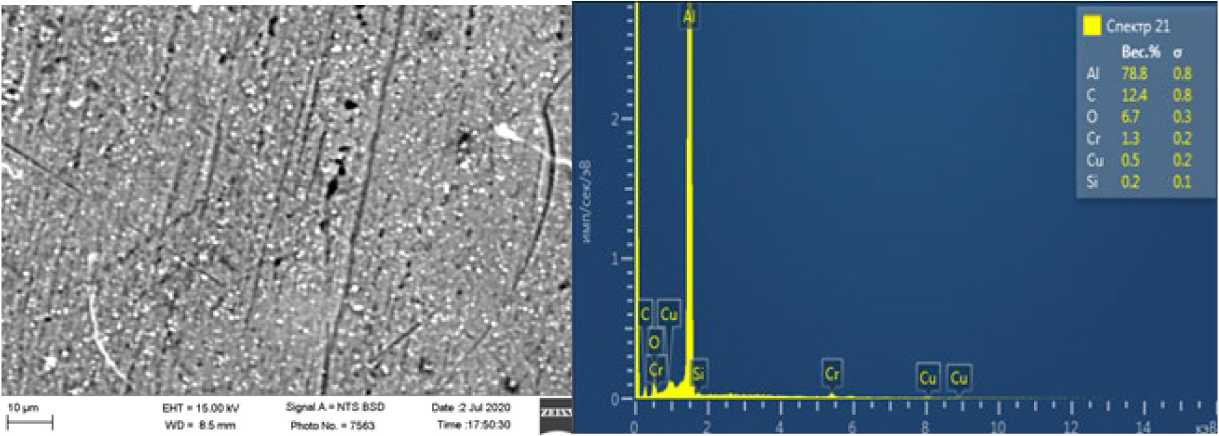
Fig. 2. The structure of the resulting alloy without use of a protective layer of flux.
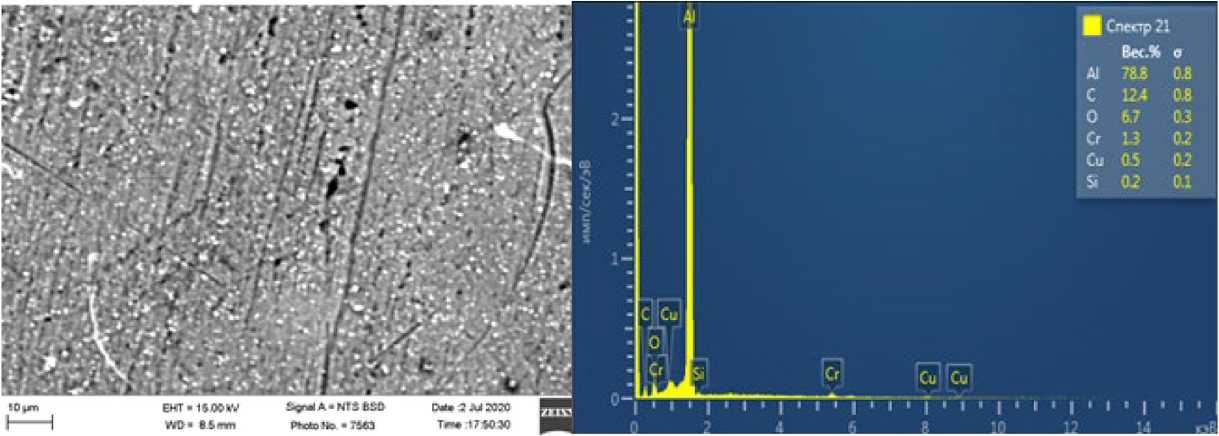
Fig. 3. The structure of the resulting alloy is obtained after using a protective layer of refining flux.
However, it should be noted that there are a number of factors that can affect the effectiveness of the use of optimized flux formulations in industrial conditions. One of these factors is the interaction of the flux with other components of the production process, such as equipment and additives. Further research can be directed to studying these relationships in order to develop more complete and accurate models of the melting process of aluminum alloys.
Despite the above data, these results are of practical importance for the metallurgical industry, as they offer new approaches to optimizing the melting process of aluminum alloys and improving the quality of the materials obtained. This can lead to lower costs and increase the competitiveness of enterprises engaged in the production of aluminum products.
Conclusion
The results of the study confirm that the composition of the protective flux plays a key role in the melting of aluminum alloys. The optimized composition of the flux contributes to the effective removal of oxides from the melt and prevents their formation, which affects the quality of the resulting alloys. The change in the composition of the flux affects not only the structure of the alloy, but also its mechanical properties. Optimization of the flux composition can lead to improved strength, corrosion resistance and other important characteristics of the alloy:
-
– the composition of the flux for melting aluminum alloys has been developed, which reduces gas inclusions by 6–8 %;
-
– a temperature regime for the introduction of flux into the aluminum melt has been developed, which provides accelerated removal of hydrogen from the aluminum melt by 10–12 %;
-
– the technology of conduction flux for melting aluminum alloys has been developed, which ensures an increase in the efficiency of using flux in melting aluminum alloys.
The results obtained in this research can be used in the metallurgical industry to optimize the melting process of aluminum alloys and improve the quality of manufactured products. In the process of applying optimized flux formulations, it is necessary to ensure their standardization and quality control. This will help to maintain the stability of the production process and ensure high quality of products.
Declaration of interest
The authors declare no conflict of interest.
Список литературы Application of fluxes for melting and processing of aluminum alloys
- Daniel B., Hussam J. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. International Journal of Thermo fluids. 2020.V. 1–2. 100007. doi.org/10.1016/j.ijft.2019.100007.
- Obidov Z.R., Ganiev I. N. Anodic behavior and oxidation of the thallium alloyed Al+2.18 % Fe alloy. Russian Journal of Applied Chemistry. 2012. 85(11). 1691–1694.
- Turakhodjaev N., Shukhrat C. Ways to increase the strength of the surface of the parts. Journal of Critical Reviews. 2020. 7(11). 380–386.
- Obidov Z. R. Thermophysical properties and thermodynamic functions of the beryllium, magnesium and praseodymium alloyed Zn‑55Al alloy. High Temperature. 2017. 55(1). 150–153. doi: 10.1134/S 0018151X17010163.
- Turakhodjaev N.D., Yakubov L. E., Tursunov S. Mathematical model of heat treatment to improve TX mechanical properties. Composite materials. 2018. 43–52.
- Obidov Z. R. Effect of pH on the anodic behavior of beryllium and magnesium doped alloy Zn55Al. Russian Journal of Applied Chemistry. 2015. 88(9). 1451–1457.
- Turakhodjaeva F.N., Turakhodjaev N. D. The process of developing a technology for extracting copper and other nonferrous metals from industrial slags. Corporate Governance: Theory and Practice. Collection of scientific papers on. 2019. 23. 363–364.
- Obidov Z.R., Amonova A. V., Ganiev I. N. Influence of the pH of the medium on the anodic behavior of scandium — doped Zn55Al alloy. Russian Journal of Non-Ferrous Metals. 2013. 54(3). 234–238. doi:10.3103/S 1067821213030115.
- Turakhodjaev N., Tashbulatov Sh., Zokirov R., Tursunbaev S., Baydullaev A. Studying the scientific and technological bases for the processing of dumping copper and aluminum slags. Journal of Critical Reviews. 2020. 7(11). doi.org/10.31838/jcr.07.11.79
- Obidov Z. R. Anodic behavior and oxidation of strontium — doped Zn5Al and Zn55Al alloys. Protection of Metals and Physical Chemistry of Surfaces. 2012. 48(3). 352–355.
- Tursunbaev S., Turakhodjaev N., Turakhujaeva Sh., Ozodova Sh. Reduction of gas porosity when alloying A000 grade aluminum with lithium fluoride. IOP Conference Series Earth and Environmental Science. 2022. 1076(1). 012076. doi:10.1088/1755–1315/1076/1/012076.
- Firuzi H., Jobirov U. R., Obidov Z. R. Effect of neodymium and erbium on the kinetics oxidation of Zn0.5Al zinc alloy, in solid state. Journal of Siberian Federal University. Engineering & Technologies. 2022. 15(5). 561–568. doi:10.17516/1999–494X‑0417.
- Sharipov J. Kh., Aliev F. A., Ganiev I. N., Obidov Z. R. Anodic behavior and oxidation of thallium–containing alloy Zn22Al. Inorganic Materials 2023. 59. 475–480.
- Obidov Z.R., Amonova A. V., Ganiev I. N. Effect of scandium doping on the oxidation resistance of Zn5Al and Zn55Al alloys. Russian Journal of Physical Chemistry A. 2013. 87(4). 702–703. doi:10.1134/S 0036024413040201.
- Mirmuhamedov M.M., Jobirov U. R., Obidov Z. R. Anodic behavior of Zn22Al alloy, doped with erbium. Journal of Siberian Federal University. Engineering & technologies. 2023. 16. 354–362.
- Sharipov J.H., Hakimov I. B., Obidov Z. R. The influence of thallium additives on the kinetics of oxidation of the Zn22Al alloy. Journal of Siberian Federal University. Engineering & Technologies. 2023. 16. 369–370.
- Utigard T.A., Roy R. R., Friesen K. Properties of fluxes used in molten aluminium processing. High Temperature Materials and Processes. 2001. 20(3–4). doi:10.1515/HTMP.2001.20.3–4.303.
- Omid M., Shabestari, S.G., Mohamad R. Aboutalebi study of fluxing temperature in molten aluminum refining process. Journal of Materials Processing Technology. 2007. 182(1). 450–455. doi:10.1016/j. jmatprotec. 2006.09.003.
- Silny A.H., Utigard T. A. Light Metals. Warrendale, PA: TMS. 1997. 871–878.
- Peterson R. D. Recycling of metals and engineering materials. Warrendale, PA: TMS. 1990. 69–84.
- Utigard T.A., Togur, J.M., Friesen K. Grjotheim. Canadian Metall. 1987. 26. 129–135.
- Utigard T.A., Toguri J. M. Metall. Trans. B. 1987. 18. 695–702.
- Davies B.R., Thompson W. T. Proceedings of the International Symposium on Extraction Refining, and Fabrication of Light Metals. Ottawa, Canada: CIM. 1991. 191–201.
- Karakaya I., Thompson W. T., J. Electrochem Soc. 1986. 133. 702–706.
- Turakhujaeva S., Karimov K., Turakhodjaev N., Akhmedov A. Mathematical modeling of quantitative changes in hydrogen and oxide inclusions in aluminum alloy. E 3S Web of Conferences. doi:10.1051/e3sconf/202336505016.


