Application of modified sorption material for efficient wastewater treatment of galvanic production
Автор: Dubrovskay Olga G., Kulagin Vladimir A., Kurilina Tatiana A., Li Feng-Chen
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 5 т.10, 2017 года.
Бесплатный доступ
The galvanic production is one of the source of environmental contamination by harmful substances and especially heavy metals. The questions of water pollution prevention by wastewater containing heavy metal ions, are closely linked to the development of event to reduce fresh water consumption for technological needs of production and to reduce the amount of wastewater. One solution to this problem is the creation of low-waste and waste-free environmentally safe production processes of sewage treatment with the use of treated wastewater in the working cycle, which leads to a reduction of negative impacts on the environment.
Sorption detoxification, heavy metal ions, modified sorbent, dolomite rocks
Короткий адрес: https://sciup.org/146115230
IDR: 146115230 | УДК: 628.336 | DOI: 10.17516/1999-494X-2017-10-5-621-630
Текст научной статьи Application of modified sorption material for efficient wastewater treatment of galvanic production
The sorbent material passes the thermal modification by heat treatment of a mineral. A calcination promotes loosening the rocks to form a structure with greater porosity and specific surface [10, 11]. The approximate chemical composition of Akdolit-Gran are calcium carbonate, CaCO3 – 68,9 %; calcium oxide CaO – 1,4 %; magnesium oxide MgO – 25,4 %; magnesium carbonate MgCO 3 – 0,6 %; iron oxide Fe2O3 – 0,6 %; alumina Al2O3 – 2,7 %; silicon oxide SiO2 – 0,3 %; water H2O – 2,7 %. The presented values are average for several years of regular testing.
Research results
The aim of research was to study the physicochemical and sorptive properties of Akdolit-Gran sorbent.
For carrying out the sorption process in the laboratory environment was used a method of alternating batches of sorbent and constant volume of the initial concentration of the solution: Cu (II) = 60 mg/dm3; Ni (II) = 15 mg/dm3; Zn (II) = 20 mg/dm3. These concentrations are most common in wastewater of the electroplating. The residual concentration was determined by an atomic emission spectrometer with inductively coupled plasma, ICAP-6500. Mineralogical sorbent composition was determined based on X-ray diffraction analysis performed on DRON-3, in a Cu-Ka radiation, Fig. 1.
Analysis of diffraction patterns shows that the main phase in the sorbent is calcite CaCo3 ( d = 0.38; 0.30; 0.23; 0.19; 0.18 Å), in addition, there is a considerable amount of magnesium oxide MgO ( d = 0.21; 0.15 Å). Diffraction peaks with the low intensity correspond to magnesium hydroxide Mg (OH)2 ( d = 0.21; 0.15 Å) and calcium hydroxide Ca (OH) 2 ( d = 0.49; 0.26 Å), formed by hydrolysis of magnesium and calcium oxides contained in the sorbent.
The rest of the substances listed in the technical documentation for the sorbent (MgCO 3 , Fe 2 O 3 , Al2O3, and SiO3) have been identified because of their low concentration. The thermal analysis was conducted for a more detailed study of the sorbent sample with the help of STA 449 F1 instrument (simultaneous thermal analyzer), NETZSCH company (Germany) in an inert argon gas environment.
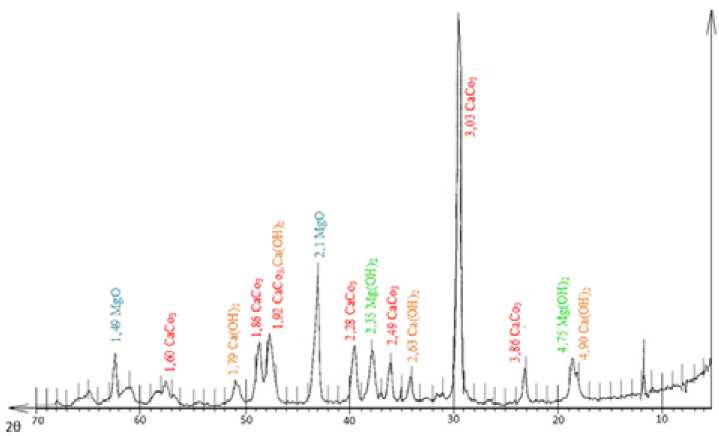
Fig. 1. The diffraction pattern of Akdolit-Gran sorbent
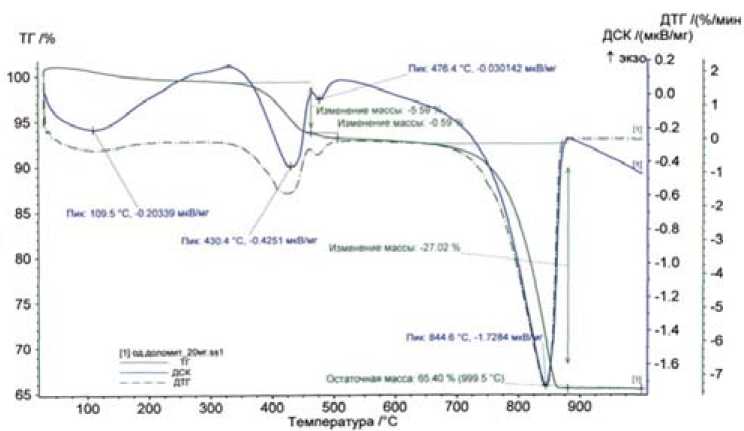
Fig. 2. The thermogram of sorbent Akdolit-Gran: DTС – differential thermogravimetric curve (showing a mass rate of change, this is the first derivative of the TG), TG – thermogravimetric curve, which shows the change-of mass during heating (mass increases or decreases), DSC – differential scanning curve (DSC and DTA show taking place at the on-heating the endo-and exothermic peaks, DTA – analysis from a single point, DSC – analysis with all on-surface)
A thermogram of sorbent sample Akdolit-Gran is shown in Fig. 2. A qualitative identification of the sample was produced according to the number, shape and position of various exo and endo-thermal peaks which are relative to the temperature scale.
Data of the sample thermal analysis show that the DSC curve are observed 4 endoeffect. Slight endoeffect at 109 ᴼC relates to the removal of adsorbed water, endoeffect at 430 ᴼC is caused by dehydration of Mg (OH) 2 :
Mg(OН)2 → MgO+Н2О wherein the sample weight decreases, as the TG curve shows ~ 5.59 % at these temperatures, the compound Mg(OH)2 in a sample of ~ 18 % then is followed by endoeffect at 476 ᴼC, which is caused by dehydration of Ca (OH)2:
Са(ОН)2 → СаО+Н2О the weight decreases by 0.59 %, there is a large endoeffect at t = 8440S relating to the decarbonisation of calcite, ie the decomposition of calcite CaCO3 and CO2 formation:
СаСО3 → СаО+СО2↑ the sample weight is reduced approximately 27 %, CaCO3 content of the sample according to the thermogram 61.4 %.
According to the differential thermal and X-ray Akdolit-Gran analysis of sorbent, we can conclude in the process of heat treatment, the chemical transformation also occurs as a result calcium carbonate and magnesium oxide are formed.
The standard techniques of sorbent were determined according to the standard procedures (Table 1). The dose values of proposed sorption material, which were found by experiment, are presented in Table 2.
The experiment (Table 2) showed that the cleaning effect using the sorbent Akdolit – Gran drastically reduced in an acidic medium. The reason of that could be a change of colloid-chemical properties of the sorbent, which the isoelectric point corresponds to approximately pH = 5.4, therefore if the pH values are below this value, the grain of sorbent loses the bimolecular gravitation of binary layer, which causes electrostatic repulsion of metal ions from the sorbent surface by contrast the typical gravitation of the alkaline medium. Furthermore, in the alkaline environment the metal ions are delivered to the reaction centers in the larger mass of sorbent metals.
The amount of copper ions in the sorbent phase (adsorption value) was calculated from A known equation [12].
Table 1. Specifications of the sorbent
|
Total void content, V ∑ (cm3/g) |
0.103 |
|
Bulkdensity, ρ н (g/cm3) |
1.15 |
|
Real density ρ (g/cm3) |
2.37 |
|
Average density ρ 0 (g/сm3) |
2.26 |
|
Porosity П (%) |
4.64 |
|
Water absorption W (%) |
10.3 |
Table 2. Results of experiment
|
№ |
Reagent dose mg/dm3 |
Values pH |
Residual concentration Cu2+, mg/dm3 |
Residual concentration Ni2+, mg/dm3 |
Residual concentration Zn2+, mg/dm3 |
|
1 |
1.0 |
3.0 |
9.736 |
6.128 |
4.902 |
|
2 |
1.0 |
7.0 |
0.123 |
1.495 |
1.295 |
|
3 |
1.0 |
9.0 |
1.131 |
0.183 |
0.0152 |
|
4 |
1.0 |
11.0 |
1.268 |
0.199 |
0.0098 |
|
5 |
1.2 |
3.0 |
8.131 |
6.103 |
3.663 |
|
6 |
1.2 |
7.0 |
0.305 |
0.923 |
1.306 |
|
7 |
1.2 |
9.0 |
0.192 |
0.163 |
0.0081 |
|
8 |
1.2 |
11.0 |
0.193 |
0.198 |
0.0093 |
|
9 |
1.6 |
3.0 |
7.961 |
5.932 |
3.569 |
|
10 |
1.6 |
7.0 |
0.129 |
0.138 |
1.0061 |
|
11 |
1.6 |
9.0 |
0.109 |
0.162 |
0.0062 |
|
12 |
1.6 |
11.0 |
0.203 |
0.204 |
0.0161 |
|
13 |
2.0 |
3.0 |
7.805 |
5.862 |
4.998 |
|
14 |
2.0 |
7.0 |
0.905 |
0.132 |
1.0092 |
|
15 |
2.0 |
9.0 |
0.129 |
0.193 |
0.0103 |
|
16 |
2.0 |
11.0 |
0.151 |
0.235 |
0.0198 |
The absorbtion and concentration of substances from the solution at the surface and in the pores of the sorbent occur at the sorption. A metal distribution coefficient between the solution and carbonate K d and also the degree of metal recovery from solution were determined. The calculation results are shown in Tables 3, 4.
Sorption ability of Akdolit – Gran differs in relation to the studied materials. The evaluation of the sorbent effectiveness for the metals extraction from aqueous solutions with the help of specific may lead to erroneous conclusions. Thus, evaluation of the effectiveness of heavy metals immobilization with the help of adsorptive capacity values gives the following series of sorption: Cu2+ > Zn2+ > Ni2+, but with the help of a metal distribution coefficient and the degree of metals recovery, we can see the following sequence: Zn2+ > Cu2+ > Ni2+. It is connected with the parameter of adsorptive capacity which depends on mass of the taken sample.
Ionic potential, i.e. the surface charge of the ion can be used to assess the degree of “surface dissociation”. The ionic potential is determined by the formula
n • e
IP =---
r
where n – number of electrons; e – the electron charge.
For Cu2+, Zn2+ и Ni2+ the number of electrons are 2, and the electron charge is 1.602. There is a relationship the greater ion radius, the lower ionization potential. For Cu2+ the atoms radius is 1.278 Å ,̇ for Zn2+ the atoms radius is 1.333 Å ̇ and Ni2+ the atom radius is 1.246 Å , on that basis, the investigated
Table 3. The sorption capacity of absorption Akdolit – Gran (mg/g)
|
Number |
Dose Akdolit – Gran, g/dm3 |
Cu2+ |
Ni2+ |
Zn2+ |
|
1 |
1.0 |
57.74 |
13.01 |
19.87 |
|
2 |
1.2 |
48.16 |
11.71 |
16.37 |
|
3 |
1.4 |
41.28 |
10.27 |
14.14 |
|
4 |
1.6 |
36.76 |
8.63 |
12.35 |
|
5 |
1.8 |
32.26 |
7.26 |
10.54 |
|
6 |
2.0 |
28.15 |
6.37 |
9.19 |
Table 4. The metal distribution coefficient between the solution and sorbent (g/dm3)
Table 5 is shown the kinetic dependence of sorption process of copper ion (II), nickel (II) and zinc (II).
It also gives the following sequence of metals distribution under extraction rate
Zn 2+ > Cu 2+ > Ni 2+
It is known that the sorption process is exothermic, as the temperature increases, the sorbent capacity reduces in relation to the metals [14, 15], which is confirmed by the results of Table 6.
The phenomena of the physical and chemical sorption are clearly distinguished in rare cases. Usually, the intermediate options are carried out, when the mass of the adsorbed substance links relatively weakly, and only a small part is firmly [16–18]. The chemical adsorption occurs, as the temperature increases, which begins to overlap the downfall of physical sorption at definite temperature. (Table 7).
These experimental studies were used to develop the project on reconstruction of treatment facilities with the proposed sorption material.
Conclusion
The study’s results of sorption properties of natural modified mineral Akdolit – Gran show that it is highly effective sorbent, being relatively cheap natural mineral, which can provide the treatment
Table 5. The degree of metal recovery from solution ( %)
|
Number |
Dose Akdolit – Gran, g/dm3 |
Cu2+ |
Ni2+ |
Zn2+ |
|
1 |
1.0 |
96.24 |
86.76 |
99.36 |
|
2 |
1.2 |
96.32 |
93.75 |
98.22 |
|
3 |
1.4 |
96.32 |
95.85 |
98.96 |
|
4 |
1.6 |
98.03 |
92.11 |
98.92 |
|
5 |
1.8 |
96.78 |
87.16 |
94.87 |
|
6 |
2.0 |
93.83 |
84.94 |
91.86 |
Table 5. The results of calculation of the rate constant, depending on the reagent dose
|
Doses mg/dm3 |
K , sек-1 |
||
|
Cu2+ |
Ni2+ |
Zn2+ |
|
|
1.0 |
3.28 |
2.02 |
5.05 |
|
1.2 |
3.3 |
2.77 |
4.02 |
|
1.4 |
3.3 |
3.18 |
4.56 |
|
1.6 |
3.9 |
2.54 |
4.44 |
|
1.8 |
3.4 |
2.05 |
2.97 |
|
2.0 |
2.78 |
1.89 |
2.51 |
Table 6. The dependence of the adsorptive capacity of the solution temperature, mg/g
|
Number |
Temperature ºС |
Cu(II) |
Ni(II) |
Zn(II) |
|
1 |
11.5 |
41.78 |
10.22 |
14.06 |
|
2 |
17.0 |
42.75 |
10.59 |
14.28 |
|
3 |
25.0 |
42.76 |
10.63 |
14.26 |
|
4 |
33.0 |
42.76 |
10.67 |
14.28 |
|
5 |
38.5 |
42.77 |
10.70 |
14.27 |
|
6 |
60.0 |
42.76 |
10.62 |
14.22 |
|
7 |
70.5 |
42.71 |
10.61 |
14.20 |
|
8 |
80.0 |
42.71 |
10.57 |
14.14 |
Table 7. The calculation results of the residual concentration depending on the environmental temperature
We can draw the following conclusions from the results:
-
1. It is appropriate to use the sorbent for wastewater electroplating purification by way of a potential sorbention exchanger because the ion exchanger is calcium and magnesium ions.
-
2. The sorption of cations occurs as the mechanism of ion exchange (the exchange with the cations which are situated in the interstices spaces) and by the formation of complex connections.
-
3. The optimum dose of sorption material Akdolit – Gran is approximately 1,4–1,6 gr/dm3 for solutions with initial concentration: Cu (II) = 60 mg/dm3; Ni(II) = 15 mg/dm3; Zn(II) = 20 mg/dm3, temperature conditions are in the range 33,0–38,0 ᴼС.
-
4. A treatment effect of using sorbent Akdolit – Gran is drastically reduced in an acidic media.
The reported study was funded by Russian Foundation for Basic Research, Government of Krasnoyarsk Territory, Krasnoyarsk Region Science and Technology Support Fund to the research projects № № 17-48-240386 р_а and 16-41-242156 р_офи_м.
-
1 These authors contributed equally to this work.
Список литературы Application of modified sorption material for efficient wastewater treatment of galvanic production
- Бек Р.Ю. Воздействие гальванотехнических производств на окружающую среду и способы снижения наносимого ущерба, Аналитический обзор АН СССР. Сиб. отд-ние. Ин-т химии твердого тела и переработки минерального сырья; Новосибирск: ГПНТБ, 1991. 96 с
- Тарасевич Ю.И. Природные сорбенты в процессах очистки воды. Киев: Наукова думка, 1981. 206 с
- Алыков Н.М., Павлова А.В., Нгуэн К.Х. и др. Новый сорбент для очистки воды от ионов токсичных металлов, Естественные науки. Журнал фундаментальных и прикладных исследований, 2009, 4(29), 150-158
- Джигола Л.А., Симакова Ю.М., Рублева А.В. и др. Изучение сорбции на опоках и диффузии в глинах ионов тяжелых металлов, Естественные науки. Журнал фундаментальных и прикладных исследований, 2009, 4(29), 175-180
- Рогалева Е.В., Воронцова Н.В., Пилипенко А.И., Ганяев В.П. Очистка природных вод от ионов тяжелых металлов методом сорбции и озонирования, Известия высших учебных заведений. Нефть и газ, 2008, 2, 102-104
- Хурамшина И.З., Никифоров А.Ф., Мигалатий Е.В., Баранов О.Ю. Взаимодействие меди (II) с природным минеральным сорбентом в процессах очистки водных растворов, Водоочистка. Водоподготовка. Водоснабжение, 2008, 2(74), 22-26
- Никифоров А.Ю. Использование природного минерала доломита и его термомодифицированных форм для очистки сточных вод от катионов тяжелых металлов, Изв. вузов. Химия и химическая технология, 1999, 4
- Nadeem R., Hanif M., Shaheen F. et al. Physical and chemical modification of distillery sludge for Pb(II) biosorption, J. Hazard. Matter, 2008, 150, 335-342
- Padmavathy V. Biosorption of nickel (II) ions by baker’s yeast: Kinetic, thermodynamic and desorption studies, Bioresource. Technol, 2008, 99, 3100-3109
- Годымчук А.Ю., Решетова А.А. Исследование процессов извлечения тяжелых металлов на природных минералах, Вестник Отделения наук о Земле РАН , 2003, 1(21), URL: http://www.scgis.ru/russian/cp1251/h_dgggms/1-2003/informbul-1/hydroterm-17.pdf
- Ниязи Ф.Ф., Мальцева В.С., Сазонова А.В. Кинетические закономерности сорбции ионов железа (II, III) модифицированными карбонатными породами, Известия ЮЗГУ. Серия Физика и химия, 2012, 1, 40-47
- Домрачева В.А. Извлечение металлов сточных вод и техногенных образований. Иркутск: Из-во ИрГТУ, 2006. 151 с
- Левченко С.И. Физическая и коллоидная химия. Конспект лекций для студентов биофака ЮФУ http://www.physchem.chimfak.rsu.ru/. Ростов на Дону, 2004. 77 с
- Бикулова В.Ж., Латыпова Ф.М., Мухаметдинова Л.Х. Адсорбционная очистка промышленных сточных вод от ионов цинка, Вода, химия и экология. 2013, 3
- Оразова С.С., Белов В.М., Евстигнеев В.В. Эффективность использования природных сорбентов восточного Казахстана в очистке воды от ионов тяжелых металлов (Сu2+), Известия Томского политехнического университета, 2007, № 2, 311
- Андрышев А.К., Колпаков В.П., Лопухов Ю.И., Даумова Г.К. Об эффективной технологии очистки хромсодержащих сточных вод с применением модифицированных сорбентов, Водоочистка. Водоподготовка. Водоснабжение, 2014, 9
- Щербаков А.В. Очистка стоков от солей тяжелых металлов, Энергосбережение и водоподготовка, 2013, 3
- Баталова Ш.Б. Физико-химические основы получения и применения катализаторов и адсорбентов из бентонитов, Алма-Ата: Наука, 1986, 168 с


