Arsenic induced oxidative stress and role of scavenging enzymes in phytoremediation by Pteris vittata and Eichhornia crassipes
Автор: Sen S.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.19, 2023 года.
Бесплатный доступ
Arsenic pollution is a growing menace in major parts of West Bengal, India and Bangladesh. Arsenic phytoremediation abilities of two common plants Pteris vittata and Eichhornia crassipes growing abundantly under natural tropical conditions of India were biochemically analyzed. Reactive oxygen species, taking total peroxide and malondialdehyde contents as the parameters, indicate the extent of arsenic induced oxidative stress, while the activities of the scavenging enzymes catalase, peroxidase and superoxide dismutase indicate the comparative effectiveness of the two plants in arsenic detoxification. Total peroxide and MDA contents were significantly higher in all samples of Eichhornia as compared to Pteris throughout the experimental period while the three scavenging enzymes viz. catalase, peroxidase and superoxide dismutase exhibited higher activities in Pteris with increasing arsenic concentration while Eichhornia showed a reverse trend. The comparative study reveals that Pteris vittata is the more efficient plant in combating and tolerating arsenic stress, as revealed by the results obtained of biochemical constituents and enzymatic profile. Of the two selected plant species, Pteris is found to be more effective in arsenic removal can serve as a cheap and easily available green source for arsenic detoxification.
Arsenic toxicity, oxidative stress, reactive oxygen species, plant antioxidant defense, stress amelioration
Короткий адрес: https://sciup.org/143180989
IDR: 143180989
Текст научной статьи Arsenic induced oxidative stress and role of scavenging enzymes in phytoremediation by Pteris vittata and Eichhornia crassipes
Arsenic (As) pollution is on the rise globally and is a looming environmental threat. Arsenic is found chiefly as arsenate (AsV) and arsenite (AsIII) showing a broad range of solubility controlled by the ionic environment and pH. Arsenate behaves as a phosphate chemical analog, taking the help of phosphate transporters to enter the plant system, resulting in disparity of phosphate supply (Finnegan and Chen, 2012). Within the cell, arsenate disrupts the phosphate-controlled metabolism including ATP synthesis causing toxicity. Arsenite toxicity is mainly due to its affinity to react with thiol (-SH) groups of enzymes and proteins with cysteine residues, disturbing their structure and function (Hasanuzzaman et al ., 2015).
Phytoremediation, an important eco-friendly technology, uses different techniques, such as uptake, transport, translocation, and detoxification, to remediate such harmful metals and metalloids. Hyperaccumulator plants take up metals and metalloids from soils, translocate and store them in their above ground biomass (Reeves, 2006). Similarly, arsenic hyperaccumulator plants have also developed different approaches to accumulate and withstand high concentrations of As. Arsenic toxicity induces oxidative stress due to abundant production of reactive oxygen species (ROS). The hyperaccumulator plants by their strong antioxidative defenses could constitute an important arsenic detoxification strategy.
The objective of this work was to compare the arsenic phytoremediation abilities of two common plants Pteris vittata and Eichhornia crassipes both growing abundantly under natural tropical conditions of India. Arsenic pollution is a growing menace in major parts of West Bengal, India and Bangladesh. The two selected plant species, if found effective in arsenic removal could serve as cheap and easily available green sources for detoxification. An analyses of their reactive oxygen species, taking total peroxide and malondialdehyde contents as the parameters, could indicate the extent of arsenic induced oxidative stress, while the activities of the scavenging enzymes catalase, peroxidase and superoxide dismutase could indicate the comparative effectiveness of the two plants in arsenic detoxification.
MATERIALS AND METHODS
Pteris vittata and Eichhornia crassipes used for the experimental work were ensured to be of identical age. Pteris plants were germinated from spores and 4 month old plants were acclimatized in a hydroponic system to promote root growth. After acclimatization in 0.2 strength Hoagland nutrient solution for 2 weeks, the plants were transferred into 0.2-strength Hoagland nutrient solution containing different concentrations of arsenic as As (V), as sodium arsenate (Na 2 HAsO 4. 7H 2 O). The finally selected concentrations of arsenic are 0, 130-133 and 267-270 µM. The plants were harvested at three intervals - 1, 5 and 10 days post arsenic treatment. Eichhornia was collected from local ponds and thereby grown, acclimatized and allowed to reproduce under laboratory conditions. The daughter plants obtained were of the same age and were taken as test materials. Eichhornia was also subjected to the same treatment with arsenic in the form of sodium arsenate. The treated plants were collected at three intervals, i.e. 1, 5 and 10 days post arsenic treatment. Control sets were maintained throughout for comparison.
The test materials were subjected to biochemical and enzymatic analyses using standard methods and tests. Estimation of total peroxide was done by ferrithiocyanate method of Thurman et al. (1972) and Malondialdehyde (MDA) was estimated by the method of Heath and Packer (1968). Catalase enzyme activity was assayed by the method of Gasper and Lacoppe (1968), peroxidase enzyme activity was assayed spectrophotometrically according to Chance and Maehly (1955) and superoxide dismutase (SOD) was assayed by the method of Marshall and Worsfold (1978). All the experiments were carried out in triplicates and then subjected to statistical analyses using analysis of variance (ANOVA) table.
RESULTS
The results showed that total peroxide content (Fig.1) and MDA content (Fig.2) was significantly higher in all samples of Eichhornia as compared to Pteris throughout the experimental period. The results of the enzymatic analyses revealed an identical trend in all the three assayed enzymes viz. catalase (Fig.3), peroxidase (Fig.4) and SOD (Fig.5). The three scavenging enzymes exhibited higher activities in Pteris with increasing arsenic concentration while Eichhornia showed a reverse trend.
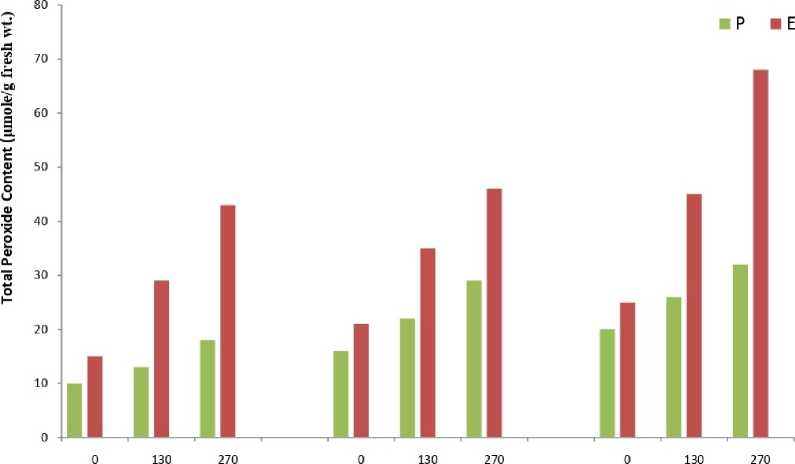
1 Day 5 Day 10 Day
Arsenic Concentration |1M
Figure. 1. Total Peroxide Content
P – Pteris , E - Eichhornia
S.E.= 0.74
C.D. =0.78 (5%)
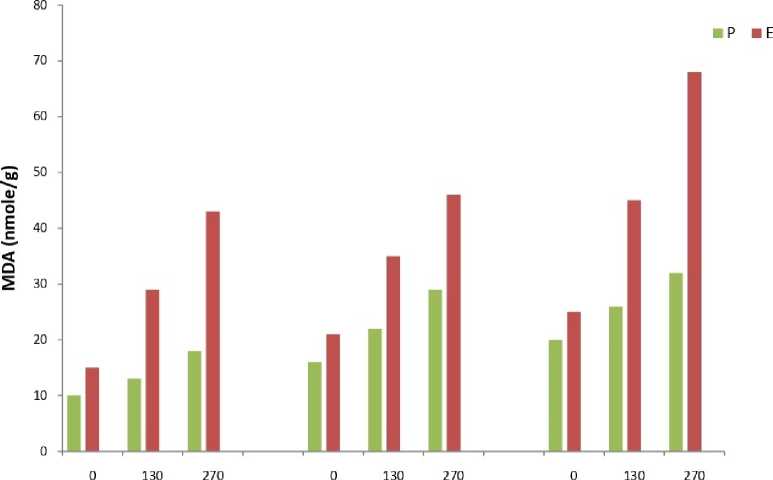
1 Day 5Day 10 Day
Arsenic Concentration цМ
Figure 2. Malondialdehyde Content
P – Pteris , E - Eichhornia
S.E.= 0.73
C.D. =0.75 (5%)
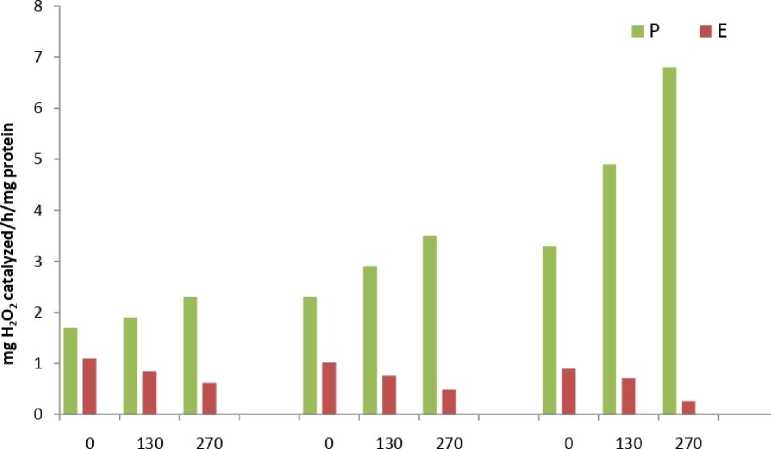
1 Day 5 Day 10 Day
Arsenic Concentration цМ
Figure 3. Catalase enzyme activity P – Pteris , E - Eichhornia
S.E.= 0.26
C.D. =0.27 (5%)
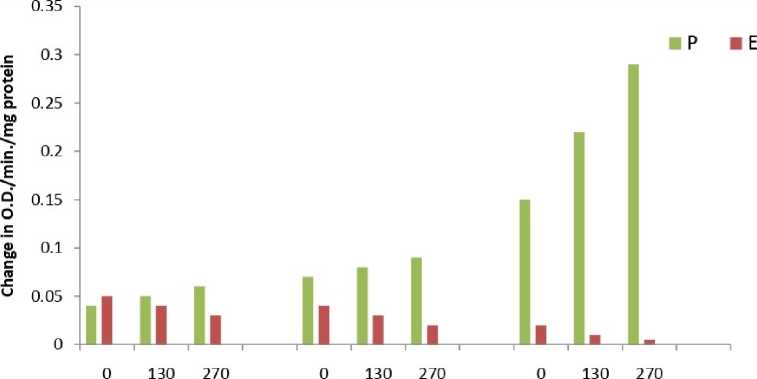
1 Day 5 Day 10 Day
Arsenic Concentration цМ
Figure 4. Peroxidase enzyme activity
P – Pteris , E - Eichhornia
S.E.= 0.07
C.D. =0.07 (5%)
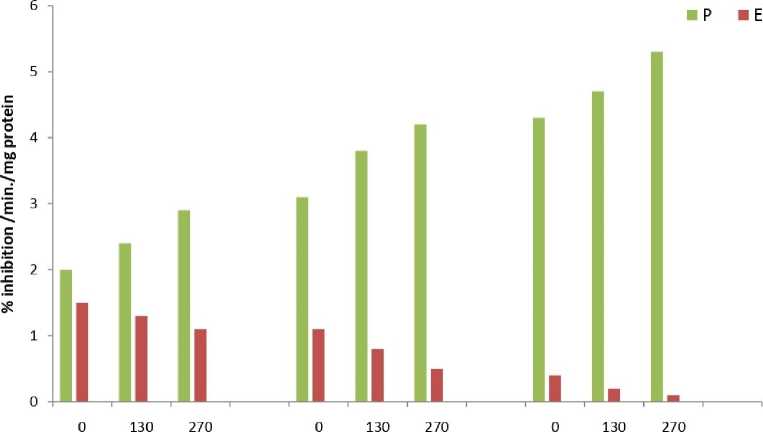
1 Day 5 Day ю Day
Arsenic Concentration цМ
Figure 5. Superoxide dismutase (SOD) enzyme activity
P – Pteris , E - Eichhornia
S.E.= 0.07
C.D. =0.07 (5%)
DISCUSSION
Список литературы Arsenic induced oxidative stress and role of scavenging enzymes in phytoremediation by Pteris vittata and Eichhornia crassipes
- Chance B. and Maehly A.C. (1955) Assay of catalases and peroxidases. In : Methods in Enzymology, (Ed. Colowick, S.P. and Kaplan, N.O.), Academic Press, New York. 2, 764-775.
- Ciurli, L. L., Alpi A. and Pardossi A. (2014) Arsenic uptake and translocation by plants in pot and field experiments. Int J. Phytoremediat., 16, 804-823.
- da Silva, E.B., Lessl J.T., Wilkie A.C., Liu X., Liu Y., Ma L.Q. (2018) Arsenic removal by As-hyperaccumulator Pteris vittata from two contaminated soils: A 5-year study. Chemosphere, 206, 736-741
- Finnegan Patrick and Weihua C. (2012) Arsenic Toxicity: The Effects on Plant Metabolism. Frontiers in Physiology. 3 D0I=10.3389/fphys.2012.00182 https://www.frontiersin.org/articles/10.3389/fphys.201 2.00182
- Gasper T. and Lacoppe J. (1968) The effect of CCC and AMO-1618 on growth, catalase, peroxidase, 1M -oxidase activity of young barley seedlings. Physiol. Plant., 2, 1104-1109.
- Hartley-Whitaker J., Ainsworth G. and Meharg A.A. (2001) Copper- and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ., 24, 713.
- Hasanuzzaman M., Nahar K., Rehman K., Hakeem, Munir Ozturk, Masayuki Fujita,Chapter 16 - Arsenic Toxicity in Plants and Possible Remediation,Editor(s): Khalid Rehman Hakeem, Muhammad Sabir, Munir Ozturk, Ahmet Ruhi Mermut, Soil Remediation and Plants,Academic Press,2015, Pages 433-501, ISBN 9780127999371,https://doi.org/10.1016/B978-0-12-799937-1.00016-4.(https://www.sciencedirect.com/ science/article/pii/B9780127999371000164)
- Heath R.L. and Packer L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125, 189- 198
- Karabal E., Yucel M. and Oktem H.V. (2003) Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci., 164, 925.
- Karimi N. and Souri Z. (2016) Antioxidant enzymes and compounds complement each other during arsenic detoxification in shoots of Isatis cappadocica Desv. Chemistry and Ecology, 32, 1-15. 10.1080/02757540.2016.1236087.
- Ma L.Q., Komar, K.M., Tu C., Zhang W., Cai Y. and Kennelley E.D. (2001) A fern that hyperaccumulates arsenic. Nature, 409, 579.
- Marshall M.J. and Worsfold M. (1978) Superoxide dismutase: a direct, continuous linear assay using the oxygen electrode. Anal. Biochem., 86, 561-573.
- Nguyen T.N., Mohapatra P.K. and Fujita K. (2003) Leaf necrosis is a visual symptom of the shift from growth stimulation to inhibition effect of Al in Eucalyptus camaldulensis. Plant Sci., 165, 147.
- Reeves R. (2006) Hyperaccumulation of trace elements by plants. In: Morel, JL., Echevarria, G., Goncharova, N. (eds) Phytoremediation of Metal-Contaminated Soils. NATO Science Series, vol 68. Springer, Dordrecht. https://doi.org/10.1007/1-4020-4688-X_2
- Sen S. (2014a) Changes in Biochemical Constituents in response to Arsenic-induced Stress in Pteris vittata and Eichhornia crassipes to determine Stress Tolerance - A Review. Beats of Natural Sciences, 2, 1-5.
- Sen S. (2014b) A comparative study on Arsenic-induced biochemical changes in Eichhornia crassipes (Mart.) Solms (Water Hyacinth) and Pteris vittata L. (Brake Fern). Seminar Proceedings of Impact of Pollution: Assessment and Awareness Dept. of Zoology, Hooghly Women's College, pp.185-192. ISBN 97881-921083-8-4
- Sen S. (2014c) Restoring the Environment: Microbes in Phytoremediation in Sen, S. (ed.) Good Earth: Exploring Possibilities, pp. 31-36. ISBN 978-81922305-9-7
- Sen S. (2016a) Phytoremediation of Arsenic by Pteris vittata (Brake Fern) and Eichhornia crassipes (Water Hyacinth): A Comparative Study. Centurion, 1(1), 161-166.
- Sen S. (2016b) One Environment, Myriad dimensions: Exploring the Indian Perspective. Lambert Academic Publishing ISBN 978-3-659-87031-6
- Sen S. (2016c) Eco-Physiology of two Indian Crop Plants: Impact of Seasonal Stress. Lambert Academic Publishing ISBN 978-3-659-91778-3
- Sen S. (2019) Blueprint for Sustainability: Reviewing the Gupta, D. K., Vandenhove H., and Inouhe M. (2013) Role Indian Scenario. Lambert Academic Publishing ISBN 978-620-0-23781-1
- Tack F. and Meers E. (2010) Assisted phytoextraction: helping plants to help us. Elements, 6(6), 383-388. https://doi.org/10.2113/gselements.6.6.383
- Thao NP, Khan MIR, Binh N, Thu A, Lan X, Hoang T et al. (2015) Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Cell Environ., 169, 7384. https://doi.org/10.1104/pp.15.00663
- Thurman R.G., Ley H.G. and Scholz R. (1972) Hepatic microsomal ethanol oxidation, hydrogen peroxide formation and the role of catalase. Eur. J. Biochem., 25, 420-430.
- Tu C. and Ma L.Q. (2002) Effects of arsenic concentrations and forms on arsenic uptake by the hyperaccumulator ladder brake. J. Environ. Qual., 31, 641.
- Tu S. and Ma L.Q. (2003) Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyperaccumulator Pteris vittata L. under hydroponic conditions. Environ. Exp. Bot., 50, 243.
- Wan X., Lei M., Chen T. (2016) Cost-benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Science of The Total Environment, 563-564, 796-802. ISSN 0048-9697 https://doi.org/10.1016/j.scitotenv.2015.12.080. (https://www.sciencedirect.com/science/article/pii/S00 48969715312377)


