Assessment of Nanotoxicity of Silver Nanoparticles on Pea (Pisum sativum) grown under ex situ conditions
Автор: Poonam Rani, Shailendra Singh Gaurav, Lily Trivedi, Amardeep Singh, Gyanika Shukla
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.17, 2021 года.
Бесплатный доступ
There has been an expanding interest for eco friendly synthesis of silver nanoparticles that don't have so much toxic impacts on crops. Silver nanoparticles have a wide scope of utilizations, for example, catalysis, hardware, photonics, optoelectronics, detecting, agriculture and drugs. In this study, the biologically synthesized and characterization of silver nanoparticles have become the prime areas. Green synthesis of nanoparticles using plant extracts is being explored globally owing to the absence of disadvantages associated with conventional methods. This study reports the synthesis of silver nanoparticles using the extract of Bambusa vulgaris (Bamboo), Azadirachta indica (Neem) leaves, characterization of the synthesized by using techniques such as Ultraviolet-Visible (UV-Vis) spectroscopy confirmed the synthesis of nanoparticles, Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM) and EDX studies revealed the characteristics of the nanoparticles synthesized. Also under this, we examined the effects of silver nanoparticles (AgNPs) on pea plants in the terms of silver accumulation, production of reactive oxygen species (ROS), Quantification of Cell Death under ex situ conditions.
Silver nanoparticles, reactive oxygen species, Bambusa vulgaris, accumulation
Короткий адрес: https://sciup.org/143173886
IDR: 143173886
Текст научной статьи Assessment of Nanotoxicity of Silver Nanoparticles on Pea (Pisum sativum) grown under ex situ conditions
Recently, nanotechnology has become a seriously investigated region and nanoproducts are broadly picking up employments, particularly in gadgets, medical care, makeup and medication( el et al., 2006). Ps have pulled in much consideration for their likely uses in plant development improvement, controlled arrival of agrochemicals, and yield insurance ( air et al., 2010; Khot et al., 2012). The positive or negative impacts of Ag Ps on plants relies upon the size, shape, surface covering, term of introduction, plant species, and formative stage (Gupta et al., 2020; air et al., 2016;
Remédios et al., 2012). Inhibition of plant development, decrease in seed germination rate, expanded chromosomal abnormalities and oxidative harm, have been accounted for in numerous plants when presented to Ag Ps (Verma et al., 2018 Tripathi et al., 2017; Oukarroum et al., 2013;Patlolla et al., 2012). onetheless, it is hazy whether the harmfulness of Ag Ps is identified with explicit impacts brought about by the nanoparticles, Ag particles, or both (Cui et al., 2014).
Plants, as a basic segment in the climate, are basic to biological system capacity and food gracefully trustworthiness. In view of the discoveries of late research center examinations, abiotic and oxidative stresses brought about by Ps presentation in plants were depicted at both physiological and biochemical levels(Khan et al., 2020 Rossi et al., 2017;Asli et al., 2009; Dimkpa et al., 2012’).A typical finding from plant nanotoxicity contemplates is that abundance measures of ROS are delivered upon P (CuO Ps, Ag Ps, CeO 2 Ps) presentation to earthbound plant species, for example, wheat ( Triticum aestivum L .), rice ( Oryza sativa L .), onion ( Allium cepa L .), and ( Zea mays ) corn (Panda, et al., 2011; irzajani et al., 2013.) ROS could actuate harm of basic natural atoms, for example, lipids, proteins, and D A (Gill et al., 2010)
By and large, obviously metal-based Ps can make harmfulness biota in the climate. Despite the pathway of metal based Ps are delivered or released in the climate, potential dangers should be completely described to keep away from negative effect on ecological and human wellbeing. In the last 10 years, a lot of examination zeroed in on the vehicle and destiny of Ps in plants, creatures, and microorganisms has been done. In this investigation of the pertinent writing, we try to audit ( el et al., 2006).the impacts of Ps on higher plants at physiological, biochemical, and subatomic levels; (Ali et al.,2020).
Collection of different types of plant sources from different locations of Meerut, UP
Plants resources such as Bambusa vulgaris (Bamboo), Azadirachta indica ( eem), were used for the synthesis of silver nanoparticles.
Silver nanoparticles synthesis and characterization
For the arrangement of extract, effectively reviled new green leaves of each plant were utilized with measure of 25 gms. What's more, these leaves blended in with 200 ml of distil water after that combination was heated for 30 minutes at that point separated with Whatman o.1 filter paper (25 μm). Fluid extract was put away at 4°C for additional utilization. This technique for silver nanoparticles arrangement previously utilized by V. Parashar, et al 2009.The strategy for biosynthesis of silver nanoparticles through plants extract was at that point utilized by Kalaiarasi, et al., (2013). As per the Kalaiarasi, et al., (2013), aqueous solution of silver nitrate two milimolar (2m ) was ready for the amalgamation of Ag Ps. Boiled leaves concentrate of plants (10 ml) added to the silver nanoparticles (90 ml) answer for decrease on Ag particles saved for the 280C temperature at shaker (150 rpm) for overnight in dark conditions. After brooding period, the test shading change from light to dim earthy colored which is demonstrates the unions of silver nanoparticles. The bio-change was regularly checked outwardly after time spans (0 hr, 4 hrs, 12 hrs, 24 hrs, 48 hrs, 72 hrs, 96 hrs and 120 hrs). further characterization was done by FESE (facilitated from Indian Institute of Technology, Kanpur), EDX (facilitated from Indian Institute of Technology, Kanpur) ,FTIR(facilitated from Indian Institute of Technology, Bombay).
Plant material and treatments
Pea plants ( Pisum sativum ) were used as host plants and leaves of pea plants were utilized for experiments. All tests (Ag Ps (T1 and T2) and
(C)control plants) were led in triplicate. After a month, ex situ developed plants were collected for the estimation of different parameters.
Nano-toxicity evaluation in pea plants
For the determination of level of accumulation of nanoparticles and evaluation of toxicity caused in plants; following methods were used:
Silver content measurement in pea plant
The Pea ( Pisum sativum ) plants treated with Ag Ps (T1 and T2) and control plants (C) were eliminated from the soil at 90 DAT and washed with deionized water to eliminate any Ag holding fast to the plant surface. The edible segments of plants (food or feed) for example leaf and natural product tests were dried at 70oC for 48 hrs and ground.A combination of 30% nitric acid (H O 3 ), 30% hydrogen peroxide (H 2 O 2 ) was set up in 3:1(v/v) proportion and 50 mg of each ground tests were processed in the blend by warming at 120oC for a hr.After cooling, the reviews were sifted however channel paper and the volume of blends were made up to 10 ml with deionized water.These tests were dissected by Atomic Absorption Spectrophotometer (AAS) to set up the µg/g grouping of Ag in the samples (figure.1). The office was benefited from Advance Research and Analytical Services, Ghaziabad. (Homaee and Ehsanpour ,2016 were likewise utilized this strategy for estimation of Ag in the patato plants in his exploration).
Quantification of Cell Death
To assess cell viability, the strategy proposed by Baker and ock (1994) was utilized. ew leaves of C, T1 and T2 plants were gathered, and lowered in 0.25% (w/v) Evans blue for 5 min.Leaves were washed with refined water and hatched in 1 mL of half (v/v) ethanol containing 1% (w/v) sodium dodecyl sulfate (SDS). The test was conveyed in triplicate. The absorbance of the concentrates was estimated by spectrophotometer at 600 nm against Evans blue standard arrangements. Cell suitability was communicated as Evans blue take-up (figure:2).The information were examined by A OVA including LSD utilizing SPSS programming.
Biosynthesis of silver nanoparticles
The progressive extension in shade of the plants separate after the extension of 2m Ag O 3 showed the improvement of Ag Ps. The shade of the reaction mixes turned earthy colored dark because of the arrangement of Ag Ps. Figure 3&4 shows the association of silvernanoparticles from Bambusa vulgaris (Bamboo), A. indica ( eem) (figure: 3&4) .
UV Vis analysis
FESEM analysis
In this assessment study, dried instances of Ag Ps were set up by putting two drops (20µl) of the nanoparticle plan on aluminum foil and after that dry these models setting in a hot air oven at 50oC for 24 hrs. The FESE office was profited by Advance Imaging Center, Indian Institute of Technology (IIT), Kanpur (UP, India). The photos were taken at Everhart-Thornley Detector (ETD) mode at 15 kV electron high strains (EHT). Scanning electron microscopy images of the lyophilized silver nanoparticles showed mostly spherical particles of a size below 100 nm as shown in (figure:7&8).The particles were vigilant, non-smooth, round in nature, and polydispersed. Examinations of SE micrograph furthermore revealed nanoparticles with a few monoclinic non-round structures. The nanoparticles size in went from 20 to 75 nm joined from Bambusa vulgaris (Bamboo) and A. indica ( eem).
EDX analysis
FTIR analysis
FTIR technique was used for characterization of silver nanoparticles. The FTIR facility was availed from Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology (IIT), Bombay to recognize the organic, inorganic, biomolecule residues along with nanoparticle formation, which may come along via reducing agent on to the surface of Ag Ps. Absorption bands for B. vulgaris Ag Ps were found to be at 3368.49 cm-12932.99 cm-1,1614.73 cm-1, 1517.26 cm-1, 1450.12cm-1, 1368.06 cm-1, 1228.62 cm-1, 1173.70 cm-1,1059.81 cm-1, 981.37 cm-1, 912.12 cm-1, 835.59 cm-1, 764.43 cm-1, 565.69 cm-1, 517.52 cm-1s (Fig.11).
Absorption bands for A. indica Ag Ps were found to be at 3388.75 cm-1, 2931.79 cm-1, 1613.03 cm-1,1516.92 cm-1,1449.90 cm-1,1384.07 cm-1, 1239.47 cm-1,1173.54 cm-1, 1058.93 cm-1, 980.60 cm-1,912.06 cm-1,836.03 cm-1, 565.99 cm-1, 517.81cm-1 (Fig.12).
Quantification of silver content
The reports of nanoparticle collections in leaves during both the preliminaries are given in figure no.13&14.The qualities spoke to here are determined after duplication with weakening element. Despite the fact that, the amassing of both the nanoparticles in particular T1 (Ag Ps 1: B. vulgaris ), T2 (Ag Ps2: A. indica ) are in traceable amount but, T1 (Ag Ps 1: B. vulgaris), demonstrated overall greater accumulation in treated plants. The greatest accumulation recorded in the T1 plants of trial 2. The outcomes recommend that there is extremely slight aggregation of nanoparticles in the medicines examined by Atomic Absorption Spectrophotometer (AAS).
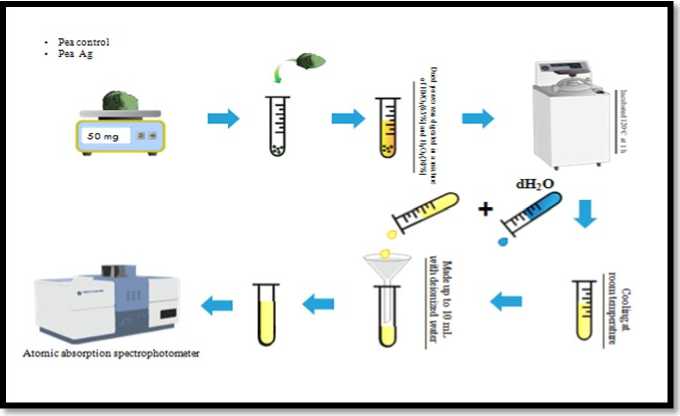
Figure 1. Diagrammatic representation of method for the measurement of Ag content in Pea ( Pisum sativum ) plant.
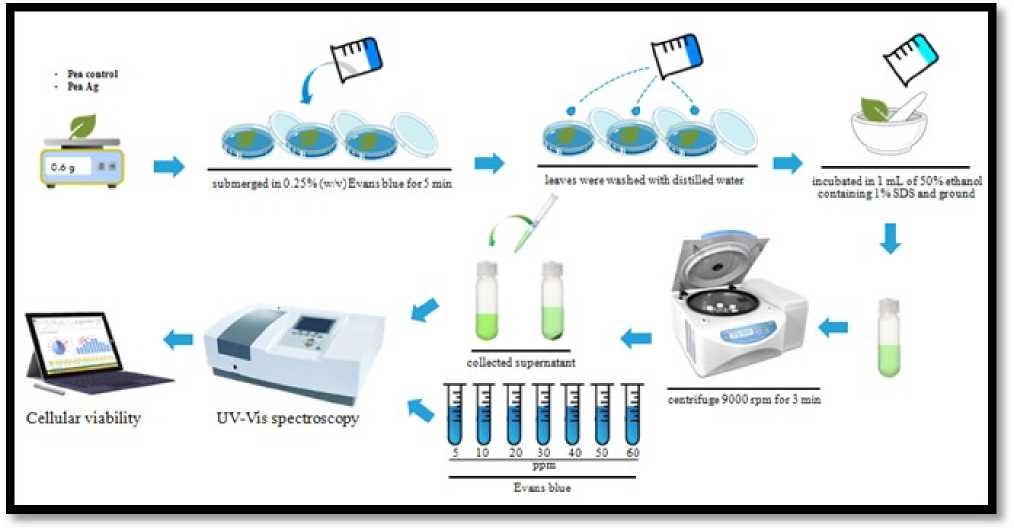
Figure 2. Evans blue tests for quantification of Cell Death in Pea ( Pisum sativum ) leaves.

Figure 3 & 4 : Shows the development of Ag Ps in leaves concentrates and difference observed in the color of control and test flasks of B. vulgaris (Bamboo) and control and test flasks of Azadirachta indica ( eem) were utilized for unions of silver nanoparticles after 96 hrs.
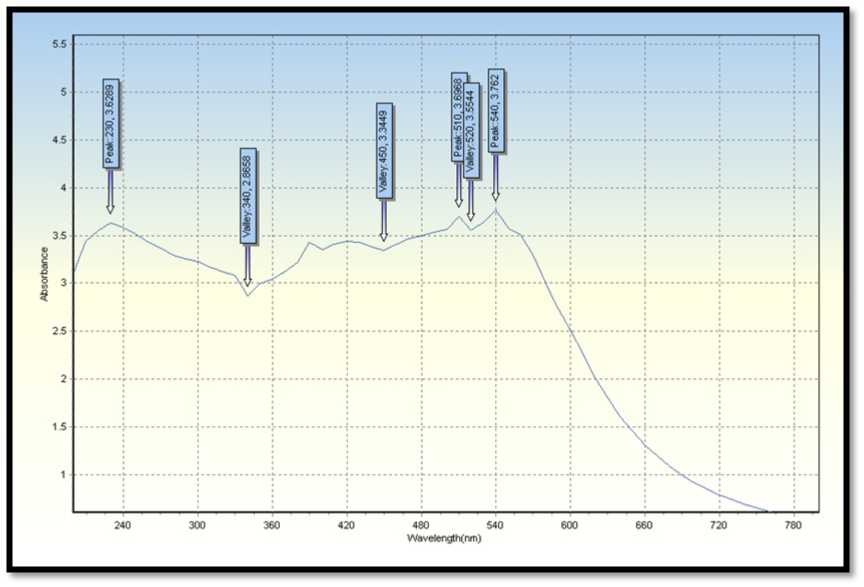
Figure 5. UV-Vis spectral reports of leaves extract B. vulgaris (Bamboo) were used for syntheses of silver nanoparticles.
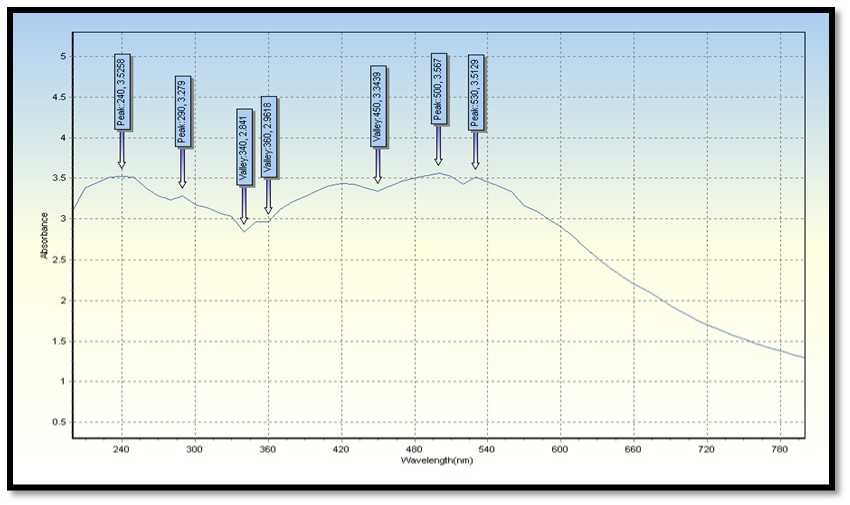
Figure 6. UV-Vis spectral reports of leaves extract A. indica ( eem),were used for syntheses of silver nanoparticles.

Figure 7. Shows the FESE image of Ag Ps synthesized from B. vulgaris leaves extract.

Figure 8. Shows the FESE image of Ag Ps synthesized from A. indica ( eem),leaves extract.
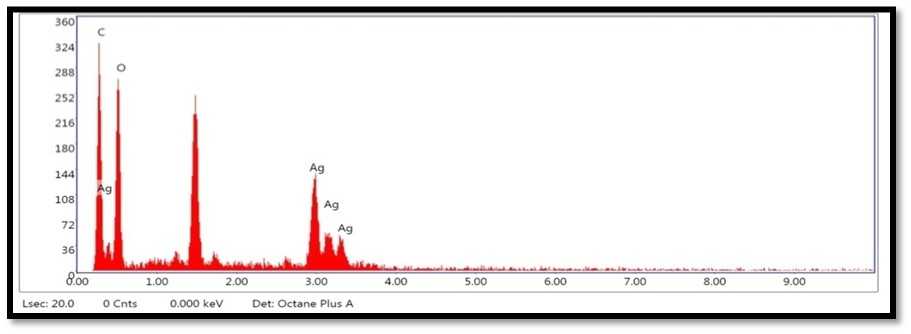
Figure 9. Shows the EDX analysis graph ofAg Ps synthesized from B. vulgaris leaves extract.
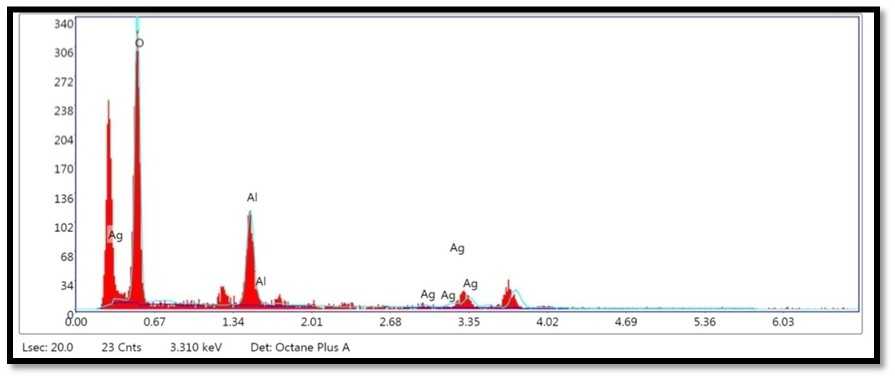
Figure 10. Shows the EDX analysis graph ofAg Ps synthesized from A. indica ( eem), leaves.
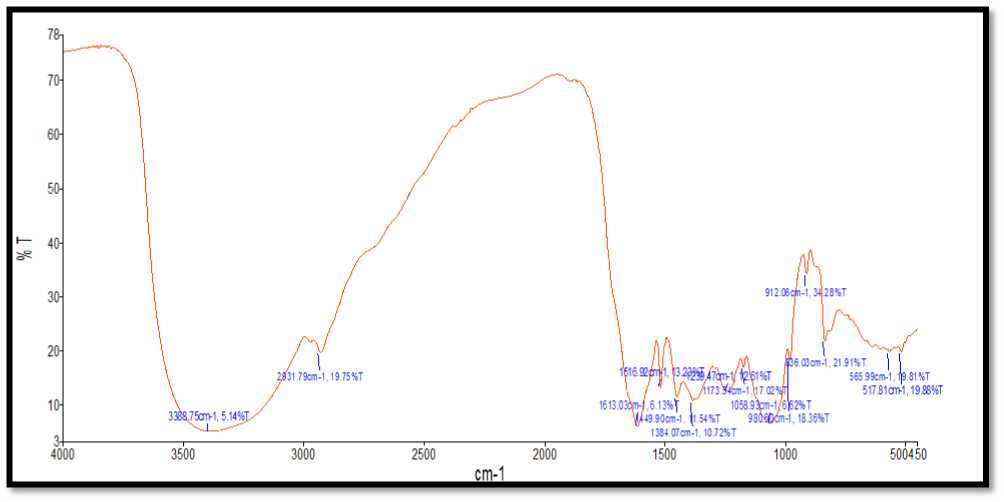
Figure 11. Shows the FTIR spectra of Ag Ps synthesized from B. vulgaris leaves extract.
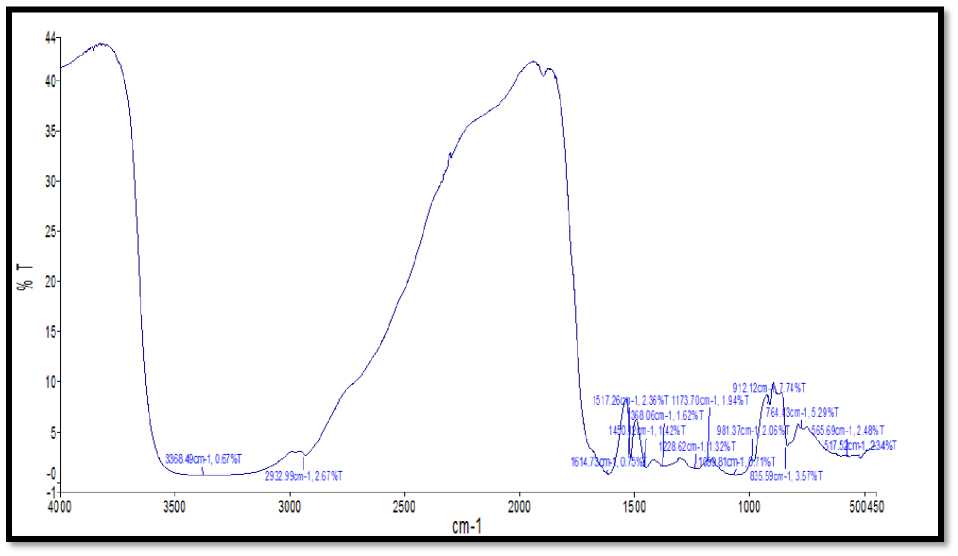
Figure 12. Shows the FTIR spectra of Ag Ps synthesized from A. indica leaves extract.
Concentration of Ag content accumulation inPea plants (pg/g)
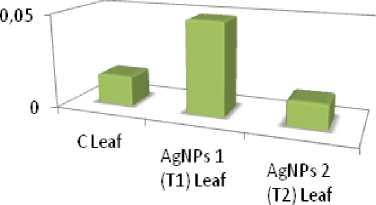
Concentration ofAg (ug/g)
Figure 13. Accumulation quantity of silver content in C, T1 and T2 Pea ( Pisum sativum ) plant study groups during Trial 1.
Concentration of Ag content accumulation in Pea plants (pg/g)
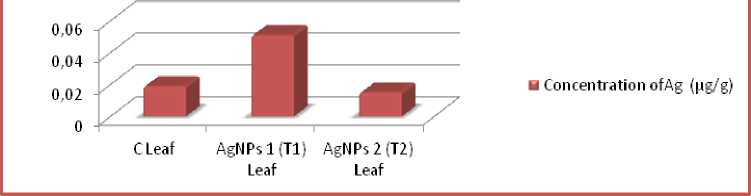
Figure 14. Accumulation quantity of silver content in C, T1 and T2 Pea plant study groups during Trial 2.
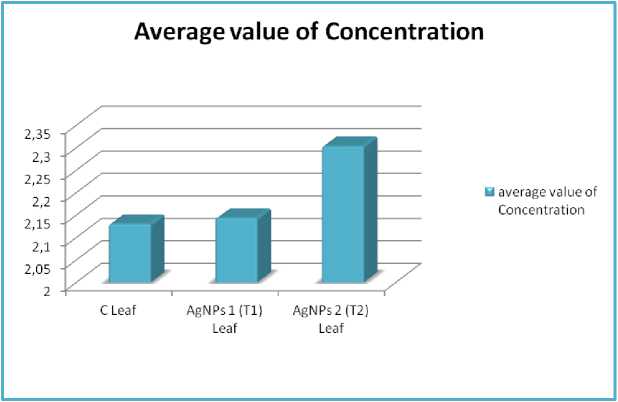
Figure 15. Amount of Evans blue dye trapped in different samples in trial 1 plants at 90 DAT, where, average value of concentration of three replicates.
Average of Concentration
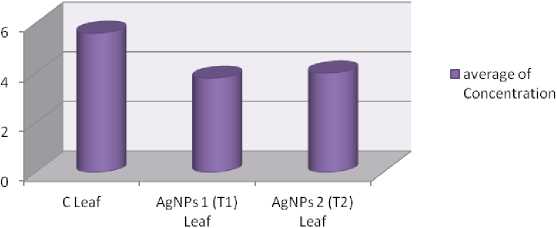
Figure 16. Amount of Evans blue dye trapped in different samples in trial 2 plants at 90 DAT, where, average value of concentration of three replicates.
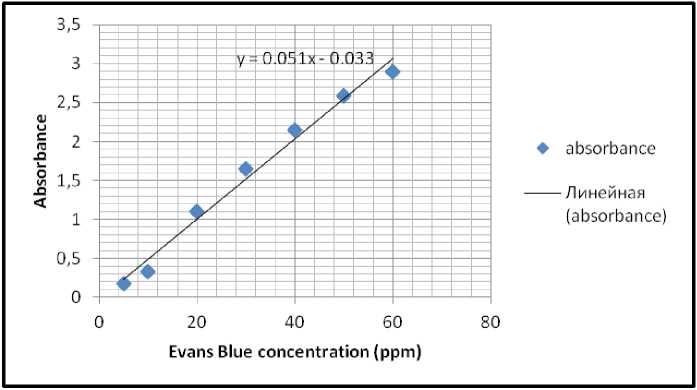
Figure 17. Evans Blue standard linear graph recorded during testing samples of trial 1 at RT.
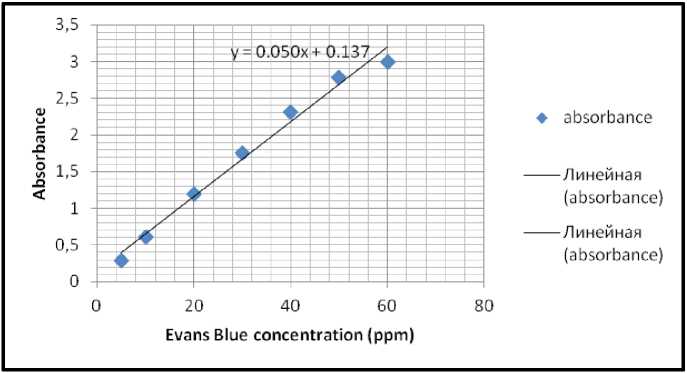
Figure 18. Evans Blue standard linear graph recorded during testing samples of trial 2 at RT.
Result of quantification of cell death
This study investigated the biosynthesis of Ag Ps and characterization of silver nanoparticles using the aqueous leaf extract Bambusa vulgaris and A. indica . Biosynthesis of silver nanoparticles from fluid leaf concentrates of Cucumis prophetarum (Hemlata et al., 2020), Cucumis sativus (Senthil et al., 2010) and Cucumis elo (Babulreddy et al., 2013) has been accounted earlier. Furthermore the Synthesis of metal nanoparticles utilizing different plant separates were accounted for previously (Udayasoorian et al 2011, Rai et al., 2009; Ingle et al., 2008; Kim et al., 2007).
UV-visible spectroscopy is one of the most broadly utilized procedures for auxiliary portrayal of nanoparticles (Harmsen et al., 2017). The presence of an absorbance top at around 420 nm obviously demonstrates the development of Ag Ps in the arrangement because of surface plasmon reverberation (SPR) electrons present on the nanoparticle surface (Bilal et al., 2017).
SE and FESE picture shows agglomerates of little grains and some scattered nanoparticles (Guzmán et al., 2009). EDX instrument generally remains fixed with FESE and it is used to research the components present inside an example. It affirms the component of nanoparticle test with its volume rate and besides gives bits of knowledge concerning some other contaminant part that might be connected with the perfect metal nanoparticles (Rao et al., 2013; Bakar et al., 2012; Goudarzi et al., 2016; Zhang, et al., 2017 Hemmati et al., 2019). The pinnacle comparing to aluminum is clear as the example smear was set up on the aluminum foil base. For the most part metallic silver nanocrystals show run of the mill optical assimilation top roughly at 3 keV because of surface plasmon reverberation (Gomathi et al., 2020).
FTIR spectroscopic examination affirmed that carbonyl gathering structure amino corrosive buildups and proteins has the more grounded capacity to tie metal, might shape a layer covering the metal nanoparticles (covering of silver nanoparticles) to forestall agglomeration and accordingly settle the medium (Khan et al., 2018; Chokshi et al.,2016; Paul et al., 2015; Saware et al., 2014; Balaji et al., 2009; andal et al., 2005).
Plant toxicities studies were carried out to see the impacts Ag Ps on pea plant.The results of toxicity on pea ( Pisum sativum ) plant were found similar in both control and treated plants and the concentration was very low.Plant toxicities studies were carried out to see the impacts Ag Ps on pea plant.The results of toxicity on pea ( Pisum sativum ) plant were found similar in both control and treated plants and the concentration was very low. Ex situ approaches are advantageous in nanotoxicology examination since they can create a similar exploration results rapidly and financially without utilizing costly creature models (Sharifi et al., 2012). Ex situ moves toward that make unambiguous and quantitative consequences of toxicity are esteemed for fundamentally surveying the evaluated biocompatibility of nanoformulations The dependability of ex situ screening examinations to give an in vivo harmfulness forecast of nanoformulations in the lungs of rodents (Sayes et al., 2007). Ex situ strategies are commonly important to recognize properties of Ps that can feature their toxicity as well as create a grouping of P harmfulness for unthinking investigations .
anoparticles are being widely studied for their potential use as , nanofertilizers, nanopesticides and nanofungicides etc (Kah et al, 2018; Shukla et al, 2020a; Shukla et al, 2020b; Singh et al, 2020) and this is raising concern about the possible nano-toxicity that might be caused on plants and environment due to the use of nanoparticles. The present study concludes that application of nanoparticles on plants does not cause any noticeable toxicity to the plant; hence, it is safer to be used as crop growth enhancer or protecting agent.
All authors have declared that they do not have any conflict of interest for publishing this research.
Ali, K., Cherian, T., Fatima, S., Saquib, Q., Faisal, ., Alatar, A.A., usarrat, J. and Al-Khedhairy, A.A., 2020. Surface Engineering Techniques Associated with Stability, Biocompatibility, and Toxicity of anoparticles. In Green Synthesis of Nanoparticles: Applications and Prospects (pp. 75101). Springer, Singapore.
Asli, S. and eumann, P. ., 2009. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport Plant, cell & environment , 32 (5), pp.577584.
Babulreddy, ., Sahoo, S.P., Ramachandran, S. and Dhanaraju, .D., 2013. Anti-hyperglycemic activity of Cucumis elo Leaf extracts in Streptozotocin induced Hyperglycemia in Rats. Inter J , 2 (4), pp.22-27.
Bilal, ., Rasheed, T., Iqbal, H. ., Li, C., Hu, H. and Zhang, X., 2017. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities International journal of biological macromolecules , 105 , pp.393-400.
Blackwell, J. ., Barton, C.H., White, J.K., Roach, T.I., Shaw, .A., Whitehead, S.H., ock, B.A., Searle, S., Williams, H. and Baker, A. ., 1994. Genetic regulation of leishmanial and mycobacterial infections: the Lsh/Ity/Bcg gene story continues Immunology letters, 43(1-2), pp.99-107.
Cui, D., Zhang, P., a, Y.H., He, X., Li, Y.Y., Zhao, Y.C. and Zhang, Z.Y., 2014. Phytotoxicity of silver nanoparticles to cucumber (Cucumis sativus) and wheat (Triticum aestivum) Journal of Zhejiang University SCIENCE A , 15 (8), pp.662-670.
Dimkpa, C.O., cLean, J.E., Latta, D.E., anangón, E., Britt, D.W., Johnson, W.P., Boyanov, .I. and Anderson, A.J., 2012. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. Journal of Nanoparticle Research , 14 (9), p.1125.
Gill, S.S. and Tuteja, ., 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology and biochemistry , 48 (12), pp.909-930.
Gomathi, ., Prakasam, A., Rajkumar, P.V., Rajeshkumar, S., Chandrasekaran, R. and
Anbarasan, P. ., 2020. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity South African Journal of Chemical Engineering , 32 , pp.1-4.
Goudarzi, ., ir, ., ousavi-Kamazani, ., Bagheri, S. and Salavati- iasari, ., 2016. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Scientific reports, 6, p.32539.
Gupta, S.D., Saha, ., Agarwal, A. and Venkatesh, V., 2020. Silver nanoparticles (Ag Ps) induced impairment of in vitro pollen performance of Peltophorum pterocarpum (DC.) K. Heyne Ecotoxicology , 29 (1), pp.75-85.
Guzmán-Dávalos, L., Ortega, A., Contu, ., Vizzini, A., Rodríguez, A., Villalobos-Arámbula, A.R. and Santerre, A., 2009. Gymnopilus maritimus (Basidiomycota, Agaricales), a new species from coastal psammophilous plant communities of northern Sardinia, Italy, and notes on G. arenophilus Mycological progress , 8 (3), pp.195205.
Harmsen, S., Wall, .A., Huang, R. and Kircher, .F., 2017. Cancer imaging using surface-enhanced resonance Raman scattering nanoparticles. Nature protocols , 12 (7), p.1400.
Hemlata, eena, P.R., Singh, A.P. and Tejavath, K.K., 2020. Biosynthesis of Silver anoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS omega , 5 (10), pp.5520-5528.
Hemmati, S., Rashtiani, A., Zangeneh, . ., ohammadi, P., Zangeneh, A. and Veisi, H., 2019. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens Polyhedron , 158 , pp.8-14.
Homaee, .B. and Ehsanpour, A.A., 2016. Silver nanoparticles and silver ions: oxidative stress responses and toxicity in potato (Solanum tuberosum L) grown in vitro Horticulture, Environment, and Biotechnology, 57(6), pp.544553.
Ingle, A., Gade, A., Pierrat, S., Sonnichsen, C. and Rai, ., 2008. ycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria Current Nanoscience , 4 (2), pp.141-144.
Kah, ., Kookana, R.S., Gogos, A. and Bucheli, T.D., 2018. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues Nature nanotechnology , 13 (8), pp.677684.
Kalaiarasi, R., Prasannaraj, G. and Venkatachalam, P., 2013. A rapid biological synthesis of silver nanoparticles using leaf broth of Rauvolfia tetraphylla and their promising antibacterial activity.
Khan, Z.S., Rizwan, ., Hafeez, ., Ali, S., Adrees, ., Qayyum, .F., Khalid, S., Ur Rehman, .Z. and Sarwar, .A., 2020. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels Environmental Science and Pollution Research , 27 (5), pp.4958-4968.
Khot, L.R., Sankaran, S., aja, J. ., Ehsani, R. and Schuster, E.W., 2012. Applications of nanomaterials in agricultural production and crop protection: a review Crop protection, 35, pp.64-70.
Kim, J.S., Kuk, E., Yu, K. ., Kim, J.H., Park, S.J., Lee, H.J., Kim, S.H., Park, Y.K., Park, Y.H., Hwang, C.Y. and Kim, Y.K., 2007. Antimicrobial effects of silver nanoparticles Nanomedicine:
Nanotechnology, Biology and Medicine , 3 (1), pp.95-101.
irzajani, F., Askari, H., Hamzelou, S., Farzaneh, . and Ghassempour, A., 2013. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria Ecotoxicology and environmental safety , 88 , pp.48-54.
air, R., 2016. Effects of nanoparticles on plant growth and development. In Plant Nanotechnology (pp. 95-118). Springer, Cham.
air, R., Varghese, S.H., air, B.G., aekawa, T.,
Yoshida, Y. and Kumar, D.S., 2010.
anoparticulate material delivery to plants. Plant science , 179 (3), pp.154-163.
el, A., Xia, T., ädler, L. and Li, ., 2006. Toxic potential of materials at the nanolevel science , 311 (5761), pp.622-627.
Oukarroum, A., Barhoumi, L., Pirastru, L. and Dewez, D., 2013. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba Environmental Toxicology and Chemistry , 32 (4), pp.902-907.
Panda, K.K., Achary, V. . ., Krishnaveni, R., Padhi, B.K., Sarangi, S. ., Sahu, S. . and Panda, B.B., 2011. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants Toxicology in vitro , 25 (5), pp.1097-1105.
Parashar, V., Parashar, R., Sharma, B. and Pandey, A.C., 2009. Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Digest Journal of Nanomaterials & Biostructures (DJNB) , 4 (1).
Patlolla, A.K., Berry, A., ay, L. and Tchounwou, P.B., 2012. Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. International journal of environmental research and public health , 9 (5), pp.1649-1662.
Pokhrel, L.R. and Dubey, B., 2013. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles Science of the Total Environment , 452 , pp.321-332.
Rai, ., Yadav, A. and Gade, A., 2009. Silver nanoparticles as a new generation of antimicrobials Biotechnology advances, 27(1), pp.76-83. f Cassia auriculata Dig J Nanomater Biostruct, 6(1), pp.279-283.
Rao, Y.S., Kotakadi, V.S., Prasad, T. .V.K.V., Reddy, A.V. and Gopal, D.S., 2013. Green synthesis and spectral characterization of silver nanoparticles from Lakshmi tulasi (Ocimum sanctum) leaf extract Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy , 103 , pp.156-159.
Remédios, C., Rosário, F. and Bastos, V., 2012. Environmental nanoparticles interactions with plants: orphological, physiological, and genotoxic aspects Journal of Botany .
Rossi, L., Zhang, W., Schwab, A.P. and a, X., 2017. Uptake, Accumulation, and in Planta Distribution of Coexisting Cerium Oxide anoparticles and Cadmium in Glycine max (L.) err. Environmental Science & Technology , 51 (21), pp.12815-12824.
Senthil, V., Ramasamy, P., Elaiyaraja, C. and Elizabeth, A.R., 2010. Some phytochemical prosperities affected by the infection of leaf spot disease of Cucumis sativus (Linnaeus) caused by Penicillium notatum African Journal of Basic & Applied Sciences , 2 (3-4), pp.64-70.
Shukla, G., Gaurav, S.S., Rani, V., Singh, A., Rani, P., Verma, P. and Kumar, B., 2020a. Evaluation of larvicidal effect of mycogenic silver nanoparticles against white grubs (Holotrichia sp). Journal of Advanced Scientific Research , 11 (1 Suppl 1).
Shukla, G., Gaurav, S.S., Singh, A., 2020. Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs ( Holotrichia sp. ). Springer – International Nano Letters 10 (2), pp.131-139.
Singh, A., Gaurav, S.S., Shukla, G., Rani, P., Kumar, B. and Kumar, A., 2020b. Evaluation of mycogenic silver and zinc oxide nanoparticles as potential control agent against early blight (Alternaria solani) of potato (Solanum tuberosum L.) Journal of Advanced Scientific Research , 11 (2).
Tripathi, D.K., Singh, S., Singh, S., Pandey, R., Singh, V.P., Sharma, .C., Prasad, S. ., Dubey, .K. and Chauhan, D.K., 2017. An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity Plant Physiology and Biochemistry, 110, pp.2-12.
Udayasoorian, C., Kumar, K.V. and Jayabalakrishnan, ., 2011. Extracellular synthesis of silver nanoparticles using leaf extract o
Verma, S.K., Das, A.K., Patel, .K., Shah, A., Kumar, V. and Gantait, S., 2018. Engineered nanomaterials for plant growth and development: a perspective analysis Science of the Total Environment, 630, pp.1413-1435.
Список литературы Assessment of Nanotoxicity of Silver Nanoparticles on Pea (Pisum sativum) grown under ex situ conditions
- Ali, K., Cherian, T., Fatima, S., Saquib, Q., Faisal, M., Alatar, A.A., Musarrat, J. and Al-Khedhairy, A.A., 2020. Surface Engineering Techniques Associated with Stability, Biocompatibility, and Toxicity of Nanoparticles. In Green Synthesis of Nanoparticles: Applications and Prospects (pp. 75-101). Springer, Singapore.
- Asli, S. and Neumann, P.M., 2009. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant, cell & environment, 32(5), pp.577-584.
- Babulreddy, N., Sahoo, S.P., Ramachandran, S. and Dhanaraju, M.D., 2013. Anti-hyperglycemic activity of Cucumis Melo Leaf extracts in Streptozotocin induced Hyperglycemia in Rats. Inter J, 2(4), pp.22-27.
- Bilal, M., Rasheed, T., Iqbal, H.M., Li, C., Hu, H. and Zhang, X., 2017. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. International journal of biological macromolecules, 105, pp.393-400.
- Blackwell, J.M., Barton, C.H., White, J.K., Roach, T.I., Shaw, M.A., Whitehead, S.H., Mock, B.A., Searle, S., Williams, H. and Baker, A.M., 1994. Genetic regulation of leishmanial and mycobacterial infections: the Lsh/Ity/Bcg gene story continues. Immunology letters, 43(1-2), pp.99-107.
- Cui, D., Zhang, P., Ma, Y.H., He, X., Li, Y.Y., Zhao, Y.C. and Zhang, Z.Y., 2014. Phytotoxicity of silver nanoparticles to cucumber (Cucumis sativus) and wheat (Triticum aestivum). Journal of Zhejiang University SCIENCE A, 15(8), pp.662-670.
- Dimkpa, C.O., McLean, J.E., Latta, D.E., Manangón, E., Britt, D.W., Johnson, W.P., Boyanov, M.I. and Anderson, A.J., 2012. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. Journal of Nanoparticle Research, 14(9), p.1125.
- Gill, S.S. and Tuteja, N., 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology and biochemistry, 48(12), pp.909-930.
- Gomathi, M., Prakasam, A., Rajkumar, P.V., Rajeshkumar, S., Chandrasekaran, R. and Anbarasan, P.M., 2020. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. South African Journal of Chemical Engineering, 32, pp.1-4.
- Goudarzi, M., Mir, N., Mousavi-Kamazani, M., Bagheri, S. and Salavati-Niasari, M., 2016. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Scientific reports, 6, p.32539.
- Gupta, S.D., Saha, N., Agarwal, A. and Venkatesh, V., 2020. Silver nanoparticles (AgNPs) induced impairment of in vitro pollen performance of Peltophorum pterocarpum (DC.) K. Heyne. Ecotoxicology, 29(1), pp.75-85.
- Guzmán-Dávalos, L., Ortega, A., Contu, M., Vizzini, A., Rodríguez, A., Villalobos-Arámbula, A.R. and Santerre, A., 2009. Gymnopilus maritimus (Basidiomycota, Agaricales), a new species from coastal psammophilous plant communities of northern Sardinia, Italy, and notes on G. arenophilus. Mycological progress, 8(3), pp.195-205.
- Harmsen, S., Wall, M.A., Huang, R. and Kircher, M.F., 2017. Cancer imaging using surface-enhanced resonance Raman scattering nanoparticles. Nature protocols, 12(7), p.1400.
- Hemlata, Meena, P.R., Singh, A.P. and Tejavath, K.K., 2020. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS omega, 5(10), pp.5520-5528.
- Hemmati, S., Rashtiani, A., Zangeneh, M.M., Mohammadi, P., Zangeneh, A. and Veisi, H., 2019. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron, 158, pp.8-14.
- Homaee, M.B. and Ehsanpour, A.A., 2016. Silver nanoparticles and silver ions: oxidative stress responses and toxicity in potato (Solanum tuberosum L) grown in vitro. Horticulture, Environment, and Biotechnology, 57(6), pp.544-553.
- Ingle, A., Gade, A., Pierrat, S., Sonnichsen, C. and Rai, M., 2008. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Current Nanoscience, 4(2), pp.141-144.
- Kah, M., Kookana, R.S., Gogos, A. and Bucheli, T.D., 2018. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature nanotechnology, 13(8), pp.677-684.
- Kalaiarasi, R., Prasannaraj, G. and Venkatachalam, P., 2013. A rapid biological synthesis of silver nanoparticles using leaf broth of Rauvolfia tetraphylla and their promising antibacterial activity.
- Khan, Z.S., Rizwan, M., Hafeez, M., Ali, S., Adrees, M., Qayyum, M.F., Khalid, S., Ur Rehman, M.Z. and Sarwar, M.A., 2020. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environmental Science and Pollution Research, 27(5), pp.4958-4968.
- Khot, L.R., Sankaran, S., Maja, J.M., Ehsani, R. and Schuster, E.W., 2012. Applications of nanomaterials in agricultural production and crop protection: a review. Crop protection, 35, pp.64-70.
- Kim, J.S., Kuk, E., Yu, K.N., Kim, J.H., Park, S.J., Lee, H.J., Kim, S.H., Park, Y.K., Park, Y.H., Hwang, C.Y. and Kim, Y.K., 2007. Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 3(1), pp.95-101.
- Mirzajani, F., Askari, H., Hamzelou, S., Farzaneh, M. and Ghassempour, A., 2013. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicology and environmental safety, 88, pp.48-54.
- Nair, R., 2016. Effects of nanoparticles on plant growth and development. In Plant Nanotechnology (pp. 95-118). Springer, Cham.
- Nair, R., Varghese, S.H., Nair, B.G., Maekawa, T., Yoshida, Y. and Kumar, D.S., 2010. Nanoparticulate material delivery to plants. Plant science, 179(3), pp.154-163.
- Nel, A., Xia, T., Mädler, L. and Li, N., 2006. Toxic potential of materials at the nanolevel. science, 311(5761), pp.622-627.
- Oukarroum, A., Barhoumi, L., Pirastru, L. and Dewez, D., 2013. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environmental Toxicology and Chemistry, 32(4), pp.902-907.
- Panda, K.K., Achary, V.M.M., Krishnaveni, R., Padhi, B.K., Sarangi, S.N., Sahu, S.N. and Panda, B.B., 2011. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicology in vitro, 25(5), pp.1097-1105.
- Parashar, V., Parashar, R., Sharma, B. and Pandey, A.C., 2009. Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Digest Journal of Nanomaterials & Biostructures (DJNB), 4(1).
- Patlolla, A.K., Berry, A., May, L. and Tchounwou, P.B., 2012. Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. International journal of environmental research and public health, 9(5), pp.1649-1662.
- Pokhrel, L.R. and Dubey, B., 2013. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Science of the Total Environment, 452, pp.321-332.
- Rai, M., Yadav, A. and Gade, A., 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnology advances, 27(1), pp.76-83. f Cassia auriculata. Dig J Nanomater Biostruct, 6(1), pp.279-283.
- Rao, Y.S., Kotakadi, V.S., Prasad, T.N.V.K.V., Reddy, A.V. and Gopal, D.S., 2013. Green synthesis and spectral characterization of silver nanoparticles from Lakshmi tulasi (Ocimum sanctum) leaf extract. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 103, pp.156-159.
- Remédios, C., Rosário, F. and Bastos, V., 2012. Environmental nanoparticles interactions with plants: Morphological, physiological, and genotoxic aspects. Journal of Botany.
- Rossi, L., Zhang, W., Schwab, A.P. and Ma, X., 2017. Uptake, Accumulation, and in Planta Distribution of Coexisting Cerium Oxide Nanoparticles and Cadmium in Glycine max (L.) Merr. Environmental Science & Technology, 51(21), pp.12815-12824.
- Senthil, V., Ramasamy, P., Elaiyaraja, C. and Elizabeth, A.R., 2010. Some phytochemical prosperities affected by the infection of leaf spot disease of Cucumis sativus (Linnaeus) caused by Penicillium notatum. African Journal of Basic & Applied Sciences, 2(3-4), pp.64-70.
- Shukla, G., Gaurav, S.S., Rani, V., Singh, A., Rani, P., Verma, P. and Kumar, B., 2020a. Evaluation of larvicidal effect of mycogenic silver nanoparticles against white grubs (Holotrichia sp). Journal of Advanced Scientific Research, 11(1 Suppl 1).
- Shukla, G., Gaurav, S.S., Singh, A., 2020. Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs (Holotrichia sp.). Springer – International Nano Letters, 10(2), pp.131-139.
- Singh, A., Gaurav, S.S., Shukla, G., Rani, P., Kumar, B. and Kumar, A., 2020b. Evaluation of mycogenic silver and zinc oxide nanoparticles as potential control agent against early blight (Alternaria solani) of potato (Solanum tuberosum L.). Journal of Advanced Scientific Research, 11(2).
- Tripathi, D.K., Singh, S., Singh, S., Pandey, R., Singh, V.P., Sharma, N.C., Prasad, S.M., Dubey, N.K. and Chauhan, D.K., 2017. An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiology and Biochemistry, 110, pp.2-12.
- Udayasoorian, C., Kumar, K.V. and Jayabalakrishnan, M., 2011. Extracellular synthesis of silver nanoparticles using leaf extract o Verma, S.K., Das, A.K., Patel, M.K., Shah, A., Kumar, V. and Gantait, S., 2018. Engineered nanomaterials for plant growth and development: a perspective analysis. Science of the Total Environment, 630, pp.1413-1435.


