Assessment of salinity-induced antioxidative defense system in Colubrina asiatica Brongn
Автор: Sonar B.A., Desai Nivas, Gaikwad D.K., Chavan P.D.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.7, 2011 года.
Бесплатный доступ
The present study examined the salinity-induced oxidative damage and differential response of enzymatic and nonenzymatic antioxidants of Colubrina asiatica. Acceleration of catalase and peroxide production in leaves and roots suggested the onset of oxidative damage. The activities of these enzymatic antioxidants were significantly increased by salt stress, with maximum induction occurring with higher regimes of salinity (200 mM and 300 mM NaCl treatment). Interestingly, under severe stress condition (300 mM NaCl), peroxidase seemed to be more crucial than catalase for peroxide scavenging. Among the studied nonenzymatic antioxidants, ascorbic acid was induced maximally at lower dose of salinity; however, polyphenols, flavonoids and DPPH activity glutathione were increased with increasing NaCl treatment as compared with control plants. Therefore, salinity was found to induce the antioxidative defense system of C. asiatica.
Antioxidants, peroxidase, catalase, oxidative damage, reactive oxygen species, salinity, colubrina asiatica.
Короткий адрес: https://sciup.org/14323530
IDR: 14323530
Текст научной статьи Assessment of salinity-induced antioxidative defense system in Colubrina asiatica Brongn
Colubrina asiatica Brong. is a glabrous, scandent or sprawling shrubs belonging to family Rhamhanaceae (Johnston, 1971). this species is reported to occur in Tropical America, Southeastern Asia, Malesia, Tropical Australia and Polynesia (to Hawaii), Coastal east Africa, Mascarene Islands, Colubrina has a vast pan-oceanic distribution, coastal, lowland, scrub of Eastern Africa (Mozambique Kenya), Tanzania, Madagascar, the Seychelles, East and Northeast to Ceylon and extreme Southeastern India, southern Burma, Andaman and Nicobar Islands, Sumatra, Java, Borneo, the Malay Peninsula, Thailand, Taiwan, Cambodia, Vietnam, Hainan, Ryukyu Islands, Fiji Islands, Samoan Islands, New Hebrides (now Vanuatu), Tonga and Society Islands, New
Caledonia, Cook Islands, Marquesas, Tuamotu, Papua New Guinea, Australia (extreme north Queensland) and Hawaii (McCormick, 2007; Brizicky, 1964; Clarke and Thaman, 1993). In India the species is wide spread in littoral scrub forests, tidal forests of Orissa and Ghats of Konkan (Thaman, 1992; Saxsena and Brhaman, 1994). The plant has some economic value As leaf contains saponin like principle it is used as soap substitute, can be used to prepare bowels, used to wash and whiten textile kilts and garments made from Cyphlophus heterophvllus in Samoa (Richardson et al., 2000). It can be used for food, medicine, as a fish poison, used as chewstick, tooth cleaners .
According to Uphoff et al., (2001) the fruit is used to produce a soft drink. Salinity is a harsh environmental factor that has the major effect on plant quantity and quality (Zhu, 2002). In order to survive in salt stress condition, plants develop the network responses of physiological and biochemical defense mechanisms to protect themselves against stress (Sutee et al., 2009). Since C. asiatica occurs in forested areas and brush along the coast grows in coastal sites adjacent to ocean beach dunes, coastal strand tidal marshes and tidal mangrove swamps, it is included in the list of halophytes by Aronson (1989). However, no attention has been paid to the study of mechanism of salt tolerance in this species. In the present investigation an attempt has been made to study antioxidant metabolism in plants of Colubrina asiatica subjected to sodium chloride salinity.
MATERIAL AND METHODS
During the survey of coastal bioresources in Sindhudurga district, the authors collected seeds of C. asiatica plants growing in estuarine regions in Aronda near Sawantwadi. The seeds were sown in earthenware pots containing garden soil and plants of C. asiatica were raised. Five healthy plants were maintained in each pot for three months. After three months the plants were treated twice a week with different concentrations (i. e. 100mM, 200mM and 300mM) of sodium chloride solutions. Every week treatment was continued for 8 weeks, each treatment was followed by a dose of fresh water to avoid excessive salt accumulation. The plants treated with fresh tap water served as control. After 8 weeks the biochemical analysis was carried out using following methods. Ascorbic acid content was determined using the method of Sadasivam and Manickam (1992). Total flavonoid contents were determined by following the method of Lamaison and Carnet (1990). Total polyphenols contents were estimated according to the method of Ragazzi and Veronese (1973). Activity of enzyme Catalase was assayed by following the method of Luck (1974) as described by Sadasivam and Manikam, (1992). To study peroxidase activity the method of Maehly (1951) was followed. Free radical scavenging activity was assessed using DPPH dye by following the method of Koleva et al., (2002). The values are presented in histogram represent average of three determinations.
Statistical Analysis
Results were statistically analyzed using a one-way ANOVA, followed by Duncan’s new multiple range tests (DMRT). Three independent variables were considered for each experiment.
RESULTS
Antioxidative Enzyme Activities
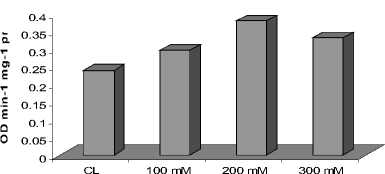
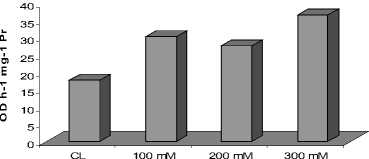
Among the different enzymes studied, Catalase showed increasing activity in leaves as well as roots at 200 mM NaCl treatment as compared with their respective controls, followed by slight decrease in the activity at 300 mM NaCl (Fig. 1 A and B). However, the induction of CAT activity was more prominent as compared with its activity at 200 mM NaCl. While, Peroxidase showed enhanced activities in leaves and roots with increase in NaCl concentration (Fig. 2A and B). Almost two fold higher activity of enzyme peroxidase was noticed in the plants treated with 300 mM NaCl treatment as compared to the control. The studied enzymatic antioxidants, depicted significant positive correlation (P<0.01).
Nonenzymatic Antioxidants
Table 1 presents data of nonenzymatic antioxidants of C. asiatica exposed to different concentrations of NaCl. of the different nonenzymatic antioxidants, Ascorbic acid showed maximum induction at 100 mM NaCl treatment, as compared with control. Likewise, the Polyphenol and falvonoid contents also increased with increasing NaCl treatment. These antioxidants were found to be positively correlated with each other as well as to the parameters of oxidative damage (P<0.01).
There was a significant alteration in the non enzymatic antioxidant potential under salinity stress as compared to the control plants. Ascorbic acid content showed increase in the leaves under salinity conditions and the more significant increase (P<0.01) was recorded under 100 mM NaCl treatment.
Table 1. Nonenzymatic antioxidants contents in the leaves of C. asiatica exposed to different concentrations of NaCl.
|
Sr. No. |
Treatment |
Polyphenol content (mg equiv gallic acid g-1 DW ) |
Flavonoid content (mg equiv Quercetine g-1 DW ) |
Ascorbic acid content (mg 100g-1 DW) |
DPPH activity (% Inhibition) |
|
1 |
Control |
18±0.64d |
8.1±0.33d |
2.7±0.12d |
46±2.2d |
|
2 |
100 mM NaCl |
24±1.0c |
9.3±0.27c |
4.2±0.23a |
58±1.8c |
|
3 |
200 mM NaCl |
32±0.83b |
13.7±1.2b |
3.8±0.18b |
63±2.9b |
|
4 |
300 mM NaCl |
38±1.2a |
14.5±0.93a |
3.1±0.11c |
72±1.6a |
All values are presented as the mean ± SD of three replicates. Values given in parentheses with “+” and “-” signs are percent increase and decrease, respectively, as compared with their respective controls. Values having different alphabets are significantly different (P<0.01; Duncan’s new multiple range test).
A
B
Catalse activity in roots
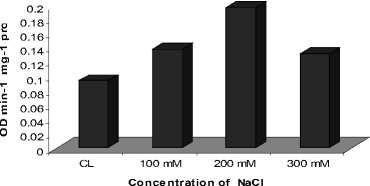
Figure 1. Activity of enzyme Catalase in the roots ( A ) and in the leaves ( B ) of C. asiatica
Catalse activity in Leaves
concentration of NaCl
Peroxidase activity in roots
A
B
Peroxidase activity in Leaves
concentration of NaCl
Figure 2. Activity of enzyme peroxidase in the roots ( A ) and in the leaves ( B ) of C. asiatica
concentration of NaCl
DISCUSSION
As the salinity-induces disturbance in light harvesting and cellular homeostasis, ROS production boosts over their scavenging, leading to oxidative stress as by H 2 O 2 production . Recent works showed that salt tolerance is closely related to the efficiency of antioxidant enzymes (Nawaz and Ashraf, 2010; Joseph and Jini, 2011). Among the different ROS, H 2 O 2 is of great significance owing to its enormous stability (Twiner and Trick. 2000) and role as a secondary messenger to regulate the expressions of defense genes (Yang et al., 2007). The result of salinity promoting H 2 O 2 production in C. asiatica in leaves and roots (Fig. 1A and B) finds support from observations of Yang et al. (2007), who reported its hyper-accumulation in salt-exposed wheat. The other peroxide scavenging enzyme, Peroxidase, was also induced in leaves and roots under salt stress (Fig. 2A and B). This increase may be attributed to a H 2 O 2 -mediated induction of the peroxidase operon as indicated by Vranova et al. , (2002). Therefore, it seems that H 2 O 2 can be detoxified by a combined effort of both CAT and Peroxidase in C. asiatica .
Likewise, all studied nonenzymatic antioxidants were found to be induced after salt stress, with maximum induction in DPPH (Table 1), which is a most effective radial chain-breaking compound. One of the major effects of salinity is the peroxidation of lipids and loss of membrane integrity due to these ROS activity (Joseph and Jini, 2011). Bor et al., (2003) observed higher accumulation of enzyme catalase in salt tolerant Beta maritima than salt sensitive Beta vulgaris they suggested that catalae and ascorbate peroxidase of which are both responsible for detoxification of H 2 O 2 , are probably equally important in the detoxification step in B. maritima .
The other nonenzymatic antioxidants, Polyphenol, flavonoid and Ascorbic acid, work in coordination with other enzymatic antioxidants to detoxify a range of ROS. Halophytes are naturally salt-tolerant plants that may be potentially useful for economical (oilseed, forage, production of metabolites) applications (Single et al., 1996). Plants vary widely in their phenolic composition and content, with both genetics and environment affecting the kind and level of phenolic compounds (Awika and Rooney, 2004). Increase in polyphenol content in different tissues under increasing salinity has also been reported in a number of plants (Agastian et al., 2000). Parida et al., (2002) noticed an accumulation of polyphenols in the leaves of Bruguiera parviflora with increasing levels of salinity. Shetty (1971) and Jamale (1975) reported an increase in the polyphenol content at low NaCl concentration in the leaves of halophytes Achrosticum aureum and Avicennia alba respectively. Navarro et al. (2006) showed increased total phenolics content with moderately saline level in red peppers. Phenols constitute a part of cellular solutes and provide a reducing environment stress (Singh, 2004). Loss of membrane integrity due to these ROS activity by salinity was reported (Joseph and Jini, 2011). Thus, phenol accumulation in salt tolerant plants could be a defense mechanism for scavenging the free radicals of oxygen and preventing cell membrane damage during stress (Singh, 2004). Results of our study showed that as salinity increases, accumulation of phenol increases. These results concur with the findings of Singh (2004) that reported increased contents of phenols in chickpea under salinity stress.
Flavonoids are frequently induced by abiotic stress and promote roles in plant protection (Dixon and Paiva, 1995; Grace and Logan, 2000). These compounds accumulated in plant tissue could help to protect themselves from damaging effects by act as a free radical scavenger because the hydroxyl groups present in their structure. Moreover the modifications of flavonoid structure i.e., glycosylation, prenylation and methylation could affect their antioxidant properties, thus they may help inhibit lipid peroxidation in stressed-plants (Caturla et al., 2003; Potapovich and Kostyuk, 2003). Ascorbic acid has also been shown to play multiple roles in plant growth, such as in cell division, cell wall expansion, and other developmental processes (Conklin, 2001 and Pignocchi and Foyer, 2003). Panda and Upadhyay, (2004) also noticed increase in ascorbate alongwith increase in the NaCl concentration in the roots of Lemna minor. Chen et al., (2005) reported an increase in ascorbic acid level with increasing salinity in salt tolerant species, Spartina alterniflora. Similar types of observations are also found in C. asiatica leaves.
CONCLUSION
C. asiatica plants possess excellent salt tolerance potential as the growth of the plant was found to be only slightly affected by even 300mM NaCl concentrations. The plant is found to possess appreciable antioxidant potential as revealed by various features such as polyphenols and flavonoid level, ascorbic acid content and activities of free radicle scavenging enzymes namely catalase and peroxidase. This aspect can be regarded as a key factor in salt tolerance mechanism in this species.
ACKNOWLEDGEMENTS
Authors are highly thankful to the Head, Department of Botany, Shivaji University, Kolhapur (MS, India ) for laboratory facilities provided to carry out this work and UGC-DRS-I- SAP for the financial assistance.
REFRENCES
Agastian P., Kingsley S.J. and Vivekanandan M. (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes, Photosynthetica, 38 , 287-290.
Aronson, J. (1989) HALOPH a data base of salt tolerant plant of the world. Office of arid land studies, the University of Arizona, Tuscon, Arizona, USA. 75 pp.
Awika J.M. and Rooney L.W. (2004) Sorghum phytochemicals and their potential impact on human health, Phytochemistry 65 , 1199-1221.
Bor M., Ozdemir F. and Turkan I.E. (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci., 164(1), 77-84.
Brizicky. G.K. (1964). The Genera of Anacardiaceae in the Southeastern United States. Journal of American Arboriculture , 43 , 359-375.
Caturla N., Vera-Samper E., Villalain J., Reyes Mateo C. and Micol V. (2003) The relationship between the antioxidant and antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Rad. Biol. Med ., 34 , 648-662.
Chen Y., Zheng H.L., Xio Q., Huang W.B. and Zhu Z. (2005) Effects of salinity on oxidative and antioxidative system of Spartina alterni flora. Xiamen Daxue Xuebao (Ziran Kexue Ban), 44(4) , 576-579.
Clarke, W.C. and Thaman, R.R. (eds.). (1993) Pacific Island agroforestry: Systems for sustainability. United Nations University Press, Tokyo. 297pp.
Conklin P.L. (2001) Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, Cell and Environment, 24, 383–394.
Dixon R.A., and Paiva N. (1995) Stress-induced phenylpropanoid metabolism, Plant Cell 7 , 1085-1097.
Grace S.C. and Logan B.A. (2000) Energy dissipation and radical scavenging by the plant henylpropanoid pathway. Phil. Trans. Royal Soc. B, 355, 1499-1510.
Jamale B.B. (1975) Physiological studies in saline plants. Ph.D. thesis Shivaji University, Kolhapur. India.
Johnston M.C. (1971). Revision of C. asiatica (Rhamnaceae). Brittonia, 23(1), 2-53.
Joseph B. and Jini D. (2011) Development of salt stress-tolerant plants by gene manipulation of antioxidant enzymes. Asian J. Agric. Res. , 5 , 1727.
Koleva I.I., Van Beek T.A., Linssen J.P.H., de Groot A., and Evstatieva L.N. (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemical Analysis , 13 , 8-17.
Lamaison J.L.C. and Carnet A. (1990) Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm. Acta. Helv. , 65 , 315-320.
Luck H. (1974) Methods in Enzymatic Analysis II (ed.) Bergmeyer. (Publ.) Academic Press, New York . 885.
Maehly A.C. (1954) Methods in biochemical analysis. (ed.) Glick, D. (Publ.) Interscience publishers Inc. New York. , 385-386.
McCormick C.M. (2007) Colubrina asiatica (lather leaf) Management Plan. Colubrina Task Force. Florida Exotic Pest Plant Council. South Florida Water Management District West Palm Beach, FL. , 55 pp.
Navarro J.M., Flores P., Garrido C. and Martinez V. (2006) Changes in the contents of antioxidant compounds in pepper fruits at ripening stages, as affected by salinity, Food Chem. 96 , 66-73.
Nawaz K. and Ashraf M. (2010) Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci., 196, 28-37.
Panda S.K. and Upadhyay R.K. (2004) The salt stress injury induces in the roots of Lemna minor . Biologia Plantarum, 48(2) , 249-253.
Parida A., Das A., and Das P. (2002) NaCl Stress Causes Changes in Photosynthetic Pigments, Proteins and other Metabolic Components in the Leaves of a True Mangrove, Bruguira perviflora, in Hydroponic Cultures. J. Plant. Biol ., 45 , 3836.
Pignocchi C. and Foyer C.H. (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Current Opinion in Plant Biology, 6, 379–389.
Potapovich A.I. and Kostyuk V.A. (2003) Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochemistry , 68 , 514-519.
Ragazzi E. and Veronese G. (1973). Quantitative analysis of phenolics compounds after thin-layer chromatographicseparation. J. Chromatogr. , 77 , 369-375.
Richardson J.E., Fay M.F., Cronk Q.C.B., Bowman D. and Chase M.W.. (2000) A phylogenetic analysis of Rhamnaceae using rbcL and trnL-F plastid DNA sequences. American Journal of Botany, 87 , 1309–1324.
Sadasivam S. and Manickam A. (1992) Biochemical method for agricultural science, (Publ.) Willey, Eastern Ltd. 105.
Saxsena H.O. and Brhaman M. (1994) The flora of Orissa. Vol. I
Shetty G.P. (1971) Physiology of growth and salt tolerance of plants. Ph.D. thesis Shivaji University, Kolhapur. India.
Singh A.K. (2004) The physiology of salt tolerance in four genotypes of chickpea during germination. J. Agric. Sci. Technol. , 6 , 87-93.
Single R.S., Glenn E.P. and Squires V. (1996) Growth performance of lambs fed mixed diets containing halophyte ingredients, Anim. Feed Sci. Technol . 63 , 137-148.
Sutee C., Cha-Um S. and Kanokporn S. (2009) Differential accumulations of proline and Flavonoids in indica rice varieties against salinity. Pak. J. Bot ., 41(5), 2497-2506.
Thailand tropical plants: edible plants and medicinal plants of southern Thailand
Thaman R.R. 1992. Batiri kei baravi: the ethnobotany of pacific island coastal plants. Atoll research bulletin, 361 .
Twiner M.J. and Trick C.G. (2000) Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate
Heterosigma akashiwo. J. Plankt. Res . 22 , 19611975.
Uphoff R.A., Van den Maagdenberg A.M., Roon K.I., Ferrari M.D. and Frantus R.R. (2001). The impact of pharmacogenetics for migraine. European Journal of Pharmacology , 9 , 1-10.
Vranova E., Inze D. and Breusegem F.V. (2002) Signal transduction during oxidative stress. J. Exp. Bot. 53, 1227-1236.
Yang Y., Xu S., An L., and Chen N. (2007) NADPH oxidasedependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol . 164 , 1429-1435.
Zhu J.K. (2002) Salt and drought stress signal transduction in plants. Ann. Rev. Plant Biol. , 53 , 247-273.
Список литературы Assessment of salinity-induced antioxidative defense system in Colubrina asiatica Brongn
- Agastian P., Kingsley S.J. and Vivekanandan M. (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes, Photosynthetica, 38, 287-290.
- Aronson, J. (1989) HALOPH a data base of salt tolerant plant of the world. Office of arid land studies, the University of Arizona, Tuscon, Arizona, USA. 75 pp.
- Awika J.M. and Rooney L.W. (2004) Sorghum phytochemicals and their potential impact on human health, Phytochemistry 65, 1199-1221.
- Bor M., Ozdemir F. And Turkan I.E. ()The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris. and wild beet Beta maritima L. Plant Sci., 164(1),77-84.
- Brizicky. G.K. (1964). The Genera of Anacardiaceae in the Southeastern United States. Journal of American Arboriculture, 43, 359-375.
- Caturla N., Vera-Samper E., Villalain J., Reyes Mateo C. and Micol V. (2003) The relationship between the antioxidant and antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Rad. Biol. Med., 34, 648-662.
- Chen Y., Zheng H.L., Xio Q., Huang W.B. and Zhu Z. ()Effects of salinity on oxidative and antioxidative system of Spartina alterni flora. Xiamen Daxue Xuebao (Ziran Kexue Ban), 44(4), 576-579.
- Clarke, W.C. and Thaman, R.R. (eds.). (1993) Pacific Island agroforestry: Systems for sustainability. United Nations University Press, Tokyo. 297pp.
- Conklin P.L. ()Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, Cell and Environment, 24,383-394.
- Dixon R.A., and Paiva N. (1995) Stress-induced phenylpropanoid metabolism, Plant Cell 7, 1085-1097.
- Grace S.C. and Logan B.A. (2000) Energy dissipation and radical scavenging by the plant henylpropanoid pathway. Phil. Trans. Royal Soc. B, 355, 1499-1510.
- Jamale B.B. (1975) Physiological studies in saline plants. Ph.D. thesis Shivaji University, Kolhapur. India.
- Johnston M.C. (1971). Revision of C. asiatica (Rhamnaceae). Brittonia, 23(1), 2-53.
- Joseph B. and Jini D. (2011) Development of salt stress-tolerant plants by gene manipulation of antioxidant enzymes. Asian J. Agric. Res., 5, 17-27.
- Koleva I.I., Van Beek T.A., Linssen J.P.H., de Groot A., and Evstatieva L.N. (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemical Analysis, 13, 8-17.
- Lamaison J.L.C. and Carnet A. (1990) Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm. Acta. Helv., 65, 315-320.
- Luck H. ()Methods in Enzymatic Analysis II (ed.) Bergmeyer. (Publ.) Academic Press, New York. 885.
- Maehly A.C. (1954) Methods in biochemical analysis. (ed.) Glick, D. (Publ.) Interscience publishers Inc. New York., 385-386.
- McCormick C.M. (2007) Colubrina asiatica (lather leaf) Management Plan. Colubrina Task Force. Florida Exotic Pest Plant Council. South Florida Water Management District West Palm Beach, FL., 55 pp.
- Navarro J.M., Flores P., Garrido C. and Martinez V. (2006) Changes in the contents of antioxidant compounds in pepper fruits at ripening stages, as affected by salinity, Food Chem. 96, 66-73.
- Nawaz K. and Ashraf M. (2010) Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci., 196, 28-37.
- Panda S.K. and Upadhyay R.K. ()The salt stress injury induces in the roots of Lemna minor. Biologia Plantarum, 48(2), 249-253.
- Parida A., Das A., and Das P. (2002) NaCl Stress Causes Changes in Photosynthetic Pigments, Proteins and other Metabolic Components in the Leaves of a True Mangrove, Bruguira perviflora, in Hydroponic Cultures. J. Plant. Biol., 45, 38-36.
- Pignocchi C. and Foyer C.H. ()Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Current Opinion in Plant Biology, 6,379-389.
- Potapovich A.I. and Kostyuk V.A. (2003) Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochemistry, 68, 514-519.
- Ragazzi E. and Veronese G. (1973). Quantitative analysis of phenolics compounds after thin-layer chromatographicseparation. J. Chromatogr., 77, 369-375.
- Richardson J.E., Fay M.F., Cronk Q.C.B., Bowman D. and Chase M.W. (2000) A phylogenetic analysis of Rhamnaceae using rbcL and trnL-F plastid DNA sequences. American Journal of Botany, 87, 1309-1324.
- Sadasivam S. and Manickam A. (1992) Biochemical method for agricultural science, (Publ.) Willey, Eastern Ltd. 105.
- Saxsena H.O. and Brhaman M. (1994) The flora of Orissa. Vol. I
- Shetty G.P. (1971) Physiology of growth and salt tolerance of plants. Ph.D. thesis Shivaji University, Kolhapur. India.
- Singh A.K. (2004) The physiology of salt tolerance in four genotypes of chickpea during germination. J. Agric. Sci. Technol., 6, 87-93.
- Single R.S., Glenn E.P. and Squires V. (1996) Growth performance of lambs fed mixed diets containing halophyte ingredients, Anim. Feed Sci. Technol. 63, 137-148.
- Sutee C., Cha-Um S. and Kanokporn S. (2009) accumulations of proline and Flavonoids in indica rice varieties against salinity. Pak. J. Bot., 41(5),2497-2506.
- Thailand tropical plants: edible plants and medicinal plants of southern Thailand http://paddleasia.com/tropical_plant_edible.htm
- Thaman R.R. 1992. Batiri kei baravi: the ethnobotany of pacific island coastal plants. Atoll research bulletin, 361.
- Twiner M.J. and Trick C.G. (2000) Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J. Plankt. Res. 22, 1961-1975.
- Uphoff R.A., Van den Maagdenberg A.M., Roon K.I., Ferrari M.D. and Frantus R.R. (2001). The impact of pharmacogenetics for migraine. European Journal of Pharmacology, 9, 1-10.
- Vranova E., Inze D. and Breusegem F.V. (2002) Signal transduction during oxidative stress. J. Exp. Bot. 53, 1227-1236.
- Yang Y., Xu S., An L., and Chen N. (2007) NADPH oxidasedependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol. 164, 1429-1435.
- Zhu J.K. (2002) Salt and drought stress signal transduction in plants. Ann. Rev. Plant Biol., 53, 247-273.


