Bacopa monnieri (L.) Pennell -a good biomarker of water pollution/contamination
Автор: Hussain K
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.6, 2010 года.
Бесплатный доступ
Effect of water pollution on Bacopa monnieri was studied by culturing their rooted propagules in various polluted water samples and Hoagland nutrient medium artificially contaminated with different micro-level concentrations of HgCl2. Anatomical observations of those plants showed safranin-stained masses deposited in the xylem vessels of stem. The plants treated in chemical solutions which are free from metallic ions, under threshold level of HgCl2, and control plants were devoid of such deposits. Similar deposits were observed in plants cultured in various local water samples. Atomic Absorption Spectrophotometric analyses of these water samples and the bioaccumulation property of the plant detected the presence of Al, As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb and Zn at various levels. The occurrence of the localized stained deposits in the xylem vessels of the stem of the plants cultured in polluted/contaminated aqueous medium, eventhough the growth medium contamination is micro-levels, is indicative of high sensitivity of Bacopa monnieri plants towards water pollution irrespective of the chemical nature of the pollutants. Although these stained deposits are not specific to any individual element that causes pollution, detection of water contamination is possible by observing the safranin-stained masses in the xylem vessels of this medicinal plant.
Bacopa, hgcl2, pollution, xylem, biomarker
Короткий адрес: https://sciup.org/14323494
IDR: 14323494
Текст научной статьи Bacopa monnieri (L.) Pennell -a good biomarker of water pollution/contamination
Most of the symptoms associated with environmental stresses in plants are linked with growth, differentiation and physiological aspects such as photosynthesis, ions uptake and transport (Orcutt and Nilson 2000; Cseh, 2002).
Eventhough root systems are exposed to the presence of heavy metals and/or any other contaminants in growth media, the ions quickly move to the shoot via. apoplastic pathway (Bell et al., 1991) though it depends upon the nature of metal and the plant species (Kabata-Pendias 2001).Investigations on tissue differentiation of plants in response to stresses due to heavy metal toxicity in general and mercury in particular are limited (Setia and Bala
-
1994). Similarly, studies on the localization of heavy metals and their effect on anatomy of plants are very scanty (Shaw 1995; Mor et al ., 2002). While investigating the effect of mercury on growth and development in Bacopa monnieri, the present authors observed localization of some coloured deposits in the xylem vessels of 1st and 2nd internodal stem tissues after a short period of treatment with different micromolar concentrations of HgCl2. So this study was undertaken to test the sensitivity of B. monnieri towards different contaminants inclusive of HgCl2 added to the growth medium. Since B. monnieri is a semi aquatic and vegetatively propagated plant, culture of rooted twigs in nutrient medium and testing of sensitivity of the plant towards contaminants by a simple staining procedure within a period of four days enabled the study rather an easy venture.
In this paper an attempt is made to suggest a plant model for the detection of water pollution in general and an overview of different contaminants and their localization in stem tissues of B. monnieri. Although the identification of the contaminants is not possible, detection of pollutants is highly useful. The paper also reports analytical data of heavy metal contaminants present in the water samples collected from different polluted sources in which the plants were cultivated and the bioaccumulation potential of the plant in order to test the sensitivity of B. monnieri plants towards the heavy metal pollution.
MATERIALS AND METHODS
Healthy cuttings of Baccopa monnieri (L.) Pennell consisting of 6 pairs of leaves (7+1 cm length) was taken from plants grown in pots and properly maintained in green house and rooting was done in distilled water. Rooted propagules were grown in plastic trays containing different growth media and plants were supported by plastic wire nets tied to the trays. Eight rooted cuttings were planted in each tray separately containing 200mls of water samples collected from drinking water supply, well, bore-well, rain water, effluent from Water Treatment Plant of Calicut University Campus, Chaliyar River (an industrial area), paddy-field, marine water, and Hoagland nutrient solution containing 0.01µM, 0.05µM, 0.1µM, 1.0µM and 10µM solutions of HgCl2. Plants were also grown in chemical solutions containing 1Molar concentrations of NH4Cl and NH4PO4. Plants cultivated in Hoagland nutrient solution and distilled water served as controls, while Hoagland medium artificially contaminated with HgCl2 at various micro quantities served as positive controls.
All the experimental trays were maintained under normal condition of green house. Care was taken to dip the root system alone in the growth medium to ensure the translocation of the contaminants from the roots to the shoot. Experiments were repeated a minimum of five times.
Analyses of above mentioned water and digested plant material (hot-block digestion procedure by USEPA 3050) samples were done using Atomic Absorption Spectrophotometry (PERKIN ELMER A Analyst 300) for the detection and estimation of heavy metal contaminants. Bio-accumulation of metals in B. monnieri plants (shoot and root) cultivated and harvested after one week (7 days ) of growth in all media also were estimated by using AAS.
Samples of stem cuttings were taken after 7 days of treatment and free hand sections of first and second internodes from the cut end of the plant were taken and stained in 0.5% safranin (Johansan 1940). Observations and photomicrographs were taken using Nikon microscope (Model ECLIPSE E 400) and Nikon Camera (Model DxM ). Stem sections of plants treated with 10µM HgCl2 was also stained with dithizone which is a specific stain for localizing Hg (Pears 1972) for the confirmation of Hg contamination.
RESULTS
Stem sections of B. monnieri grown in Hoagland solution showed typical anatomy of stem, consisting of vascular tissues of singled raw of xylem vessels and phloem cells (Fig. 1 A & B). Plants grown in distilled water also exhibited similar anatomical features even though the stem girth was slightly reduced (Fig.1 C).
Stem anatomy of plants grown in tap water (drinking water) showed localization of some dark stained deposits filled in the xylem vessels particularly in protoxylem (Fig.1 D). This type of deposits was observed in stem tissue of plants grown in well water (Fig.1 E), and bore-well water (Fig. 1 F). Similarly, plants cultured in rain water (Fig. 2 G), effluent water collected from Water Treatment Plant (Fig. 2 H) also showed stained deposits in almost all xylem vessels of stem tissue.
not show such deposits presumably due to the lack of metallic free ions.
In stem tissues of control plants aerenchyma was present almost uniformly in the cortex. But in plants treated with higher concentrations of HgCl2, the aerenchyma development was much more elaborate and cell lysis and/or disintegration was observed in the cortical region. Other tissues like epidermis, endodermis, phloem and pith did not show much variation due to various treatments. Cell wall thickening was another characteristic of treated plants compared to the control plants.
Quantitative detection of various heavy metals using Atomic Absorption Spectrophotometer revealed that, the tap water contained Al, As, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn. Lead and Fe occurred in higher quantities and As and Ni contents were very low while Hg was absent (Table 1).
Well water showed the presence of all the elements mentioned above except Ni but Hg was present. Bore-well water contained very high quantities of all the elements in general, Cr, Cu, Fe and Pb in particular in comparison with well water or tap water. Large quantities of Pb and Fe were present in rain water. Effluents of Calicut University Water Treatment Plant showed the presence of all the elements, in moderate amounts. Chaliyar River water was contaminated with industrial effluents and exorbitant amounts of Al, Cd, Cr, Hg, Mn, Ni, Pb and Zn were present compared to all other water samples. Comparatively enhanced quantities of Cd, Cu, Hg, Mn, were present in water collected from paddy fields near to Calicut University Campus. Marine water collected from Parappanangadi, the nearest coast of Calicut University contained all elements in which Cd, Cr, Fe, Hg, Mn and Pb contents were the most abundant quantities compared to all other water samples (Table.1)
Table 1. Distributions of different heavy metals in different water samples (mg l-1)
|
Water samples |
Heavy metals detected(mean values of replicates) |
||||||||||
|
Al |
As |
Cd |
Cr |
Cu |
Fe |
Hg |
Mn |
Ni |
Pb |
Zn |
|
|
Hoagland solution |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Double Distilled Water (Control) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Tap water |
3.003 |
0.011 |
0.121 |
0.423 |
0.232 |
6.188 |
0.00 |
0.123 |
0.019 |
8.311 |
3.338 |
|
Well water |
3.009 |
0.007 |
0.001 |
1.702 |
0.299 |
0.808 |
0.198 |
0.816 |
0.00 |
7.697 |
2.003 |
|
Bore well water |
8.010 |
0.012 |
0.101 |
2.313 |
0.823 |
18.188 |
0.00 |
0.418 |
0.423 |
18.168 |
3.889 |
|
Rain water |
1.018 |
0.007 |
0.098 |
1.811 |
0.111 |
6.444 |
0.104 |
0.00 |
0.00 |
8.887 |
0.00 |
|
Calicut University effluent water of Water Treatment Plant |
6.136 |
0.081 |
0.201 |
0.810 |
0.418 |
9.342 |
0.020 |
0.313 |
0.181 |
4.101 |
5.050 |
|
Chaliyar river water (Industrial area) |
16.648 |
0.432 |
0.032 |
7.116 |
0.152 |
2.056 |
1.516 |
2.748 |
3.030 |
28.564 |
16.012 |
|
Paddy field water |
4.120 |
0.008 |
1.018 |
1.001 |
3.434 |
7.469 |
3.243 |
3.243 |
0.096 |
14.326 |
4.001 |
|
Marine water |
0.532 |
0.536 |
4.004 |
8.032 |
0.804 |
27.52 |
3.944 |
3.944 |
2.061 |
40.44 |
12.032 |
|
10 ц М HgCl 2 in Hoagland solution |
- |
- |
- |
- |
- |
- |
2.00 |
- |
- |
- |
- |
Table 2. Bioaccumulations of various heavy metals in Bacopa monnieri cultivated in different water samples (mg g-1 dry tissue)
|
Water Samples |
Heavy metals detected(mean values of replicates) |
||||||||||
|
Al |
As |
Cd |
Cr |
Cu |
Fe |
Hg |
Mn |
Ni |
Pb |
Zn |
|
|
Hoagland solution |
- |
- |
- |
- |
- |
NDR |
NDR |
- |
- |
NDR |
|
|
Double distilled water (Control) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
0.00 (-) |
|
Tap water |
0.428 (35.6) |
0.002 (45.4) |
0.024 (49.5) |
0.084 (49.6) |
0.084 (90.5) |
1.218 (49.2) |
NDR (-) |
0.060 (81.3) |
0.030 (52.6) |
1.618 (48.6) |
0.648 (48.5) |
|
Well water |
0.428 (35.5) |
NDR (-) |
NDR (-) |
0.25 (36.7) |
0.25 (41.8) |
0.104 (32.1) |
0.032 (40.4) |
0.162 (49.6) |
NDR (-) |
1.498 (48.6) |
0.248 (30.9) |
|
Bore well water |
1.478 (46.1) |
0.002 (41.6) |
0.006 (14.8) |
0.262 (28.3) |
0.262 (79.5) |
2.026 (27.8) |
NDR (-) |
0.082 (49.0) |
0.044 (26.0) |
3.034 (41.7) |
0.640 (41.1) |
|
Rain water |
0.200 (49.1) |
NDR (-) |
0.004 (10.2) |
0.142 (19.6) |
0.142 (49.5) |
0.992 (38.4) |
0.016 (38.4) |
NDR (-) |
NDR (-) |
1.444 (40.6) |
NDR (-) |
|
Effluent of Water Treatment Plant of Calicut University |
1.05 (42.7) |
0.016 (49.3) |
0.022 (27.3) |
0.16 (49.3) |
0.16 (95.6) |
1.838 (49.1) |
0.002 (25.0) |
0.060 (47.9) |
0.03 (41.4) |
0.76 (46.3) |
0.846 (41.8) |
|
Chaliyar river water (Industrial area) |
3.2 (48.0) |
0.024 (13.8) |
0.006 (46.8) |
1.156 (40.6) |
1.156 (92.1) |
0.220 (26.7) |
0.106 (17.4) |
0.536 (48.7) |
0.412 (33.9) |
5.148 (45.0) |
1.786 (27.8) |
|
Paddy field water |
0.802 (48.6) |
NDR (-) |
0.200 (49.1) |
1.98 (49.4) |
1.98 (14.4) |
1.446 (48.4) |
0.014 (1.0) |
0.624 (48.1) |
0.018 (46.8) |
2.7 (47.1) |
0.774 (48.3) |
|
Marine water |
0.104 (48.8) |
0.006 (2.79) |
0.616 (38.4) |
0.860 (26.7) |
0.860 (80.8) |
5.41 (49.1) |
0.032 (2.0) |
0.724 (45.8) |
0.402 (48.7) |
5.668 (35.0) |
1.988 (41.3) |
|
10 µ M HgCl2 in Hoagland solution |
- |
- |
- |
66 (16.5) |
- |
||||||
Values in parenthesis are percentage distributions NDR-Non Detectable Range
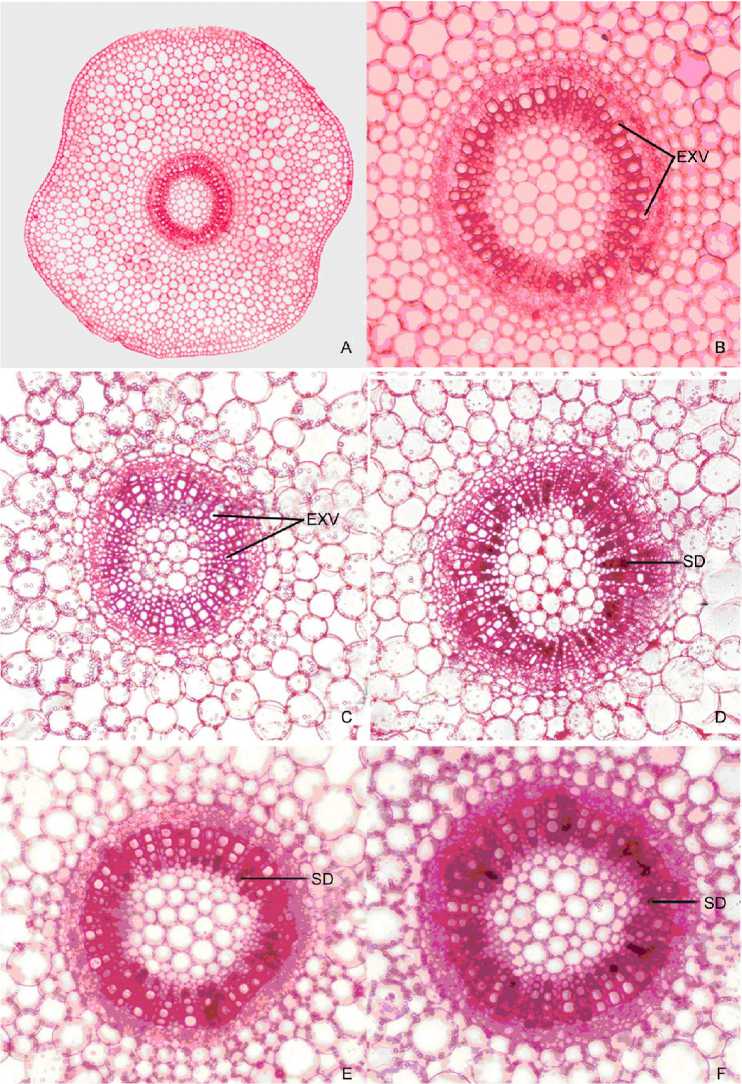
Figure 1. Free-hand crosses sections of stem internodes grown in
A - Entire cross section
B - Stele enlarged
C - Distilled water (control-2)
EXV - Empty Xylem Vessels
D - Tap water
E - Well water
F - Bore well water
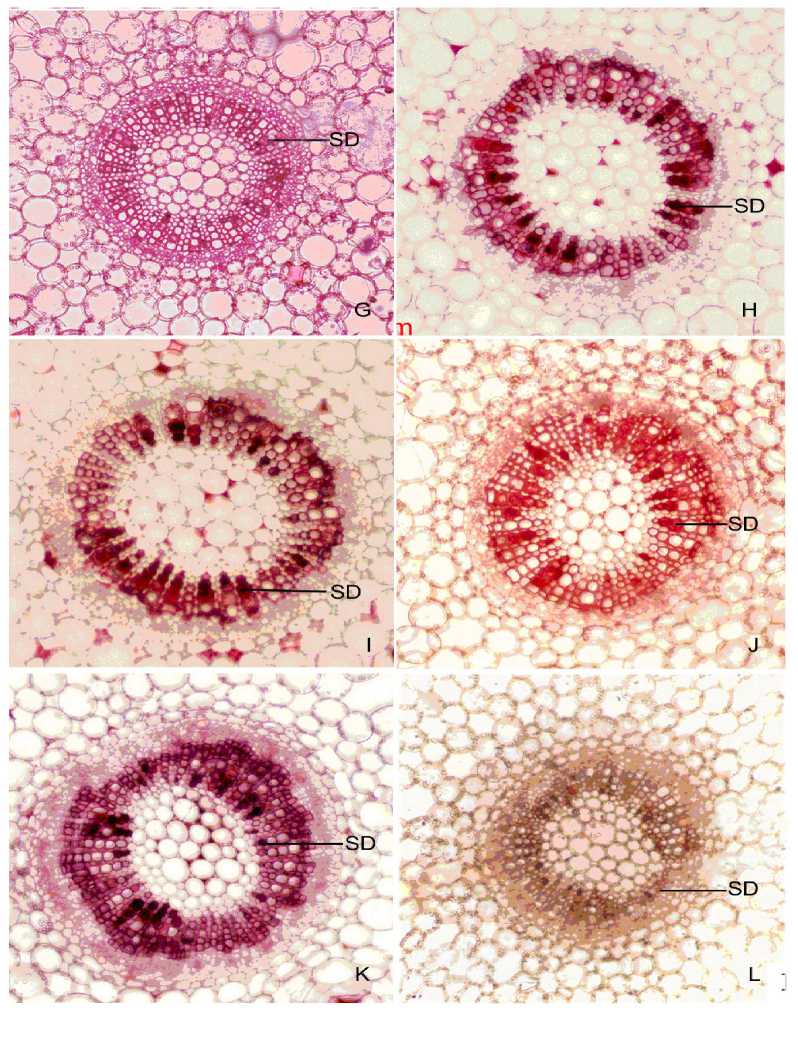
Figure 2. Free-hand crosses sections of stem internodes grown in
|
G - |
Rain water |
J - |
Paddy field water |
|
H - |
Effluent water of Water Treatment Plant |
K - |
Marine water |
|
- |
Chaliyar river water |
L - |
10 µ M HgCl2 treated |
|
SD - |
Stained Deposit |
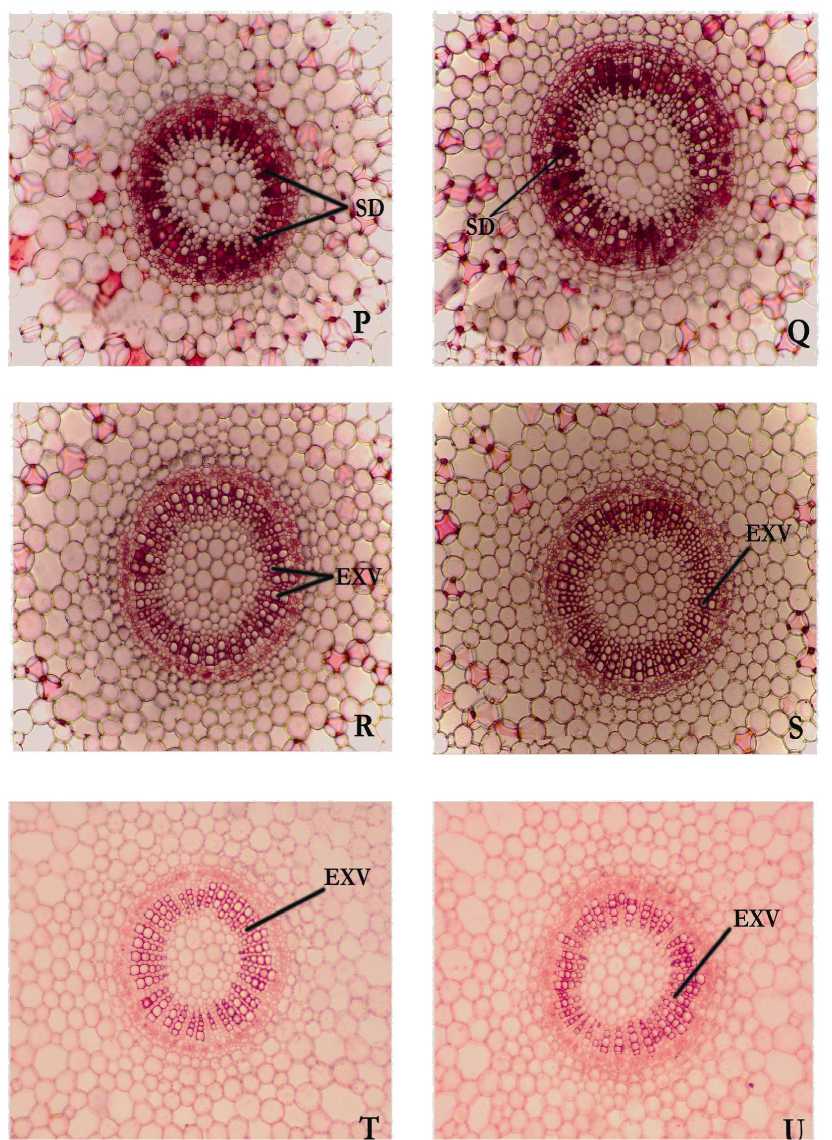
Figure 3. Free-hand crosses sections of stem internodes grown in
P – 1.0 µ M HgCl2 sol.
R – 0. 05 µ M HgCl2 sol
T – 1.0 M. NH4Cl sol.
Q – 0.1 µ M HgCl2 sol.
S – 0.01 µ M HgCl2 sol.
U – 1.0 M.NH4PO4 sol
Bio-accumulation study of plant materials reveals the presence of elements such as Al, As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb and Zn. The quantitative accumulation varied between water samples. When comparison is made between concentration of each metal present in water samples (mg l-1) and that accumulated in Bacopa monnieri shoot tissue (mg g-1 tissue dry weight), the translocation of each element showed more or less uniform pattern i,e., accumulation was proportional to metals available in the water samples. When the accumulation of each element was compared in terms of content and percentage (Tables 2), it was observed that accumulation pattern of each metal varied significantly. For example aluminium (Al) content of all water samples showed about 35-50% accumulation (Table 2) despite, significant variations in the quantities present in different water samples. But arsenic (As) did not show such uniform pattern of accumulation in Bacopa plant tissue. About 50% accumulation was shown by Cd whereas Cr accumulation pattern was not uniform. Accumulation of Cu showed very high rate in almost all samples except water samples collected from paddy field. Mercury also showed variation in the rate of accumulation. Manganese, Ni, Pb and Zn did not show much variation (Table 2).
DISCUSSION
Stained masses deposited in the xylem vessels of plants treated with 0.1µM, 1.0µM and 10µM HgCl2 solutions (Fig. 3, Q P; Fig. 2, L ) respectively. They were absent in plants grown in both the controls ie. Hoagland solution and Distilled water (Fig. 1 A, B&C) and in lower concentrations of 0.05µM and 0.01µM HgCl2 as well (Fig. 3, R S). A comparable result was reported in Phragmites australis in which dark brown deposits (stained with safranin) were observed in stem and root cells as a result of Cd treatment (Ederli et al., 2004). The treatment with 0.1µM of HgCl2 was detected as the threshold level of pollutants in the growth medium. Although the primary site of action of heavy metal is the root system, quick translocation from roots to the shoot via. the apoplastic pathway and shoot as primary target of metal toxicity stress have been reported in plants (Bell et al., 1991;
In addition to the stained deposits in the xylem vessels, the stem tissues of B. monnieri treated with higher concentrations of HgCl2 showed aerenchyma formation whereas control plants exhibited only very limited aerenchyma which is characteristic of aquatic plants (Fahn 1982). The increased aerenchyma development within a short period in the stem of plants treated with HgCl2 may be due to hypoxia stress caused by Hg because hypoxia triggers ethylene production which increases cellulase activity resulting in cell wall disintegration and formation of aerenchyma (Fahn 1982). According to Buchanan et al., (2000) aerenchyma formation is induced by stresses and involves agonistic or antagonistic signal transduction pathways in plants. Nevertheless, heavy metal stress in B . monnieri is expressed not only as aerenchyma formation but as blocks of xylem vessels also. Another important impact of HgCl2 stress on B.
monnieri is increased stomatal index due to the involvement of stomata in the liberation of mercury from the plant body (Hussain 2007).
Drastic anatomical changes have been reported in Triticum aestivum treated with HgCl2, but safranin staining did not show any deposition of stained masses even at a concentration of 0.5 to 2mM HgCl2 (Satia and Bala 1994). Localization of Hg has been reported by staining with safranin in the cross sections of root, stem and leaves of Chromolaena odorata treated with Hg ( NO3)2 ( Velasco-Alinsug et al., 2005 ).In B. monnieri, block of xylem vessels is shown by localizing safranin-stained masses even at very low concentrations such as 0.1, 1.0 and 10 µ M solutions of HgCl2 while, such deposits are not recognized in the treatments of highly reduced quantities of HgCl2 such as 0.05 and 0.01 µ M, revealing high sensitivity of this plant towards HgCl2 as well as any other contaminants present in all water samples which contained varying quantities of elements such as Al, As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn. But plants treated with 1M. Solutions of NH4Cl as well as NH4PO4 do not show the above type xylem deposits, it is possibly due to the lack of contaminants in the medium.
Moreover, according to Hussain-Koorimannil et al. (2010) occurrence or accumulation of heavy metals in general and Hg, Cd, Pb and As in particular in the plant body of B. monnieri grown in different natural habitats may cause health hazards since this genus is an important, widely used medicinal plant (Wealth ofIndia 1948; Singh et al., 1980; Nair 1987), the cultivation of which is usually done in aquatic environment or marshy areas commonly used for anthropogenic and industrial waste water disposal and hence the plants are highly contaminated with heavy metals. According to Moore et. al. (1995) accumulation of mercury varies considerably among plants and maximum amount is translocated and accumulated in plant species growing in very wet conditions.
Список литературы Bacopa monnieri (L.) Pennell -a good biomarker of water pollution/contamination
- Anonymous. (1948) Wealth of India Raw materials Vol. 1 CSIR New Delhi.
- Bell, P.F., Chaney, R.L. and Angle, J.S. (1991) Free metal activity and total metal concentrations as inducers of micro nutrient availability to barley (Hordeum vulgare L.) Klages. Plant Soil., 130, 51-62.
- Bowler, C., Montagu, M.V. and Inze, D. (1992) Superoxide dimutase and stress tolerance. Annu Rev Plant Mol Biol., 43, 83-116.
- Buchanan, B.B., Gruisseum, W. and Jones, R.L. (2000) Biochemistry and Molecular Biology of Plants. American Society of Plants Physiologists.
- Cseh, E. (2002) Metal permeability, transport and efflux in plants. In: M.N.V. Prasad and K. Strazalka, eds, Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants. London. Kluwer Academic Publishers, 1-36.
- Ederli, L., Reale, L., Ferrauti, F. and Pasqualini, S. (2004) Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol Plant., 121, 66-74.
- Fahn, A. (1982.) Plant Anatomy. 3rd ed. Pergamon Press Oxford.
- Hussain, K. (2007) Ecophysiological Aspects of Bacopa monnieri (L.) Pennell Thesis, submitted to the Calicut University.
- Hussain-koorimannil., Abdussalam, A.K., Ratheesh-Chandra, P.and Nabeesa-salim (2010). Bioaccumulation of heavy metals in Bacopa monnieri (L.) Pennell growing under different habitat. Int. J. Ecol. Dev., 15, 67-73.
- Johansan, D.A. (1940) Plant Microtechnique, McGraw Hill, New York.
- Kabata-Pendias, A. and Pendias, H. (1992) Trace Elements in Soils and Plants, 2nd Ed. CRC Press, Florida.
- Moore, T.R., Bubier Heges, J.L.A. and Flett, R.J. (1995) Methyl and total mercury in wet land plants, experimental lakes area, North western Ontario. J. Environ. Annual., 24, 845 -850.
- Mor, I.R., Gokani, S.J., Chanda, S.V. (2002) Effect of mercury toxicity on hypocotyls elongation and cell wall loosening in Phaseolus seedlings. J. Plant Nutr., 25, 843-860.
- Nair, K.K. (1987) Medhya Rasayana Drug Brahmi -its botany, chemistry and uses. J. Econ. Tax. Bot., 11, 359-365.
- Orcutt, D.M., Nilson, E.T. (2000) Physiology of Plants Under Stress -Soil and Biotic Factors, John Wiley & Sons, INC, New York.
- Setia, R.C., Bala, R. (1994) Anatomical changes in root and stem of wheat (Triticum aestivum L.) in response to different heavy metals. Phytomorphology, 44, 95-104.
- Shaw, B.P. (1995) Effect of mercury and cadmium on the activities of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol Plant., 37, 587-596.
- Singh, R.H., Lallan Singh., Zen, S.P. (1980) Studies on anti anxiety effects of the Medhya rasayana drug Brahmi (Bacopa monnieri Linn.) Part II. Experimental Studies. J. Res. Ind. Med. Yoga Homoeopath., 14 (3-4), 1-6.
- Velasco-Alinsug, M.P., Rivero, G.C., Quibuyen, T.A.O. (2005) Isolation of mercury-binding peptides in vegetative parts of Chromolaena odorata. Z Naturforsch, 60c: 252-259.


