Biochemical insights into pigeon pea protease inhibitors for agricultural pest control
Автор: Chandre Mahesh, Borse Tushar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.21, 2025 года.
Бесплатный доступ
Basically, plant uses a variety of bioactive secondary metabolites & defensive proteins to defend themselves against herbivores & fungal diseases. Protease inhibitors / Antiprotease, which can act as insect antifeedant proteins are among these defence proteins. These inhibitors are natural substances present in plants which act by changing the conformation of enzyme proteases present in insect gut thereby effect on insect digestive system; we can call this as a natural defence system of plants against insects. In this research work i.e. on Pigeon pea, around 14% to 100% loss in the farmer’s production has been observed due to insect pest attack. So by epistemology point of view, I thought to extract this natural anti -protease compounds from plants & use it as a natural defendant against insects that attack crop. Thus, total protease inhibitors were extracted using 0.1M Sodium Phosphate buffer (pH 7.5). Protease inhibitor was partially purified to homogeneity by employing conventional protein purification techniques such as Ammonium sulphate precipitation and Dialysis process. Further Partially purified proteins were quantitatively analyzed using Lowry’s Method. Among all the dialyzed fractions (10% to 100%), the fraction saturated with 60% Ammonium sulfate precipitation salt shown highest amount of protein i.e. 4924ug. Pigeon pea seed samples underwent SDS-PAGE (15%) to determine the molecular weights of the proteins. The samples exhibited distinct bands within the range (245-11 kDa), indicating differences in molecular weight due to the extraction process, which resulted in high-molecular-weight (HMW) peptides. The SDS-PAGE analysis further demonstrated the formation of low-molecular-weight (LMW) peptides with masses below 12 kDa. Pigeon pea seeds also have the ability to inhibit trypsin activity. These inhibitors can inhibit up to 59% of trypsin activity. Among all fractions, F6 (60%) showed the highest inhibitory effect on trypsin, whereas fraction F2 (20%) had the lowest inhibition. Similarly total activity and yield percentage in fraction 60% was found to be 4038 umol/min/ml and 83.25 % respectively.
Antifeedant proteins, chemical pesticide, protease inhibitors, secondary metabolites
Короткий адрес: https://sciup.org/143184738
IDR: 143184738
Текст научной статьи Biochemical insights into pigeon pea protease inhibitors for agricultural pest control
With an area of roughly 32.35 lakh hectares, pigeon peas ( Cajanus cajan (L.) Millsp.) are the second most widely grown crop in India (Gupta et al. , 1991). Because of its low input needs, good agronomic adaptation for growth, and excellent source of firewood, it is anticipated to overtake other pulse crops as the most common one in the near future. (Dwivedi, 1986). This pulse's English name comes from Barbados, an island in the Caribbean Sea where pigeons were fed the seeds. (Maesen, 1990). Pigeon pea is also known as red gram or Congo pea, depending on the area. This pulse has a somewhat contradictory origin. Modern genetic data supports the idea that the species originated in East India, despite the widespread belief that it is native to Africa. (Naik, 2019). About 5.5 million tons of pigeon peas are produced worldwide, making up 5.8% of all pulse production. With 90% of the world's pigeon pea production, India is both the biggest producer and consumer (Tapal et al. , 2019). 18 to 28 weight percent (dry basis) of pigeon pea seeds are made up of proteins. The leaves were also found to have a high protein content (19.4 weight percent, dry basis). (Yang et al. , 2020). Researchers are fascinated by plant PIs because to their growing application in the biotechnological and medicinal sectors. Pants have developed adaptation mechanisms over time that give them considerable defence against a variety of adverse situation such as insects and phytopathogens (Krishnan et al. , 2015).
Natural substances called protease inhibitors are found in large quantities in the seeds and tubers of plants of the Solanacea, Leguminosae, and Graminea families (Connors et al., 2002). Protease inhibitors (PIs) are crucial for controlling proteolytic activity and are involved in a number of biological processes that are connected to cell physiology and metabolism. When Mickel and Standish noticed in 1947 that some insect larvae could not develop normally on soybean products, they began to look into the potential use of protease inhibitors (PIs) in plant protection. Later, it was discovered that the trypsin inhibitors included in soybeans were harmful to Tribolium confusum flour beetle larvae. (Lipke et al., 1954). PIs attach to the digestive enzymes in the insect's gut and prevent them from working, which slows down the insect's development and limits the amount of amino acids it can digest proteins. (Azzouz et al., 2005). Because of the large concentration of cysteine residues in disulphide bridges, plant PIs exhibit remarkable resistance to heat treatment and high stability against changes in ionic strength, pH, proteolysis, and denaturing agents. (Cotabarren et al., 2019).
The majority of PPIs bind with the protease's active site to produce a stable inhibitor protease complex that has no enzymatic activity (Norton, 1991). PPIs' defensive properties depend on inhibiting proteases that are either released by bacteria or found in insect stomachs, which lowers the availability of amino acids required for growth and development.(Lawrence and Koundal, 2002). PIs are classified into four mechanistic classes according on their inhibitory efficacy and selectivity. Metallocarboxy PIs, aspartic PIs (pepstatins), serine PIs (serpins), and cysteine PIs (cystatins). The largest of these is the serine PI(SPI) family. (Rakashanda et al. 2013). Through the suppression of protein digestion, these proteins disrupt the metabolism of vital amino acids in pests. (Hilder et al. , 1992). It has been demonstrated that PIs target a specific class of proteinase(s) in the insect stomach that these proteins impede the growth and development of lepidopteran pests. (Boulter, 1993; Pandey et al. , 2014).
Protease inhibitors have been proposed as new substances with antimicrobial potential. Inhibitors of proteolytic enzymes have natural biological activities, e.g., antiviral, antibacterial, antiparasitic, or antitumor effects.(Szałapata et al., 2017). Half of the world’s population still depends completely on plants and their medicinal products. Until now, plants have served as starting raw materials for numerous drugs on the market. From ancient time, plant extracts containing proteolytic enzymes have been used in traditional medicine (De Feo, 1992). Proteolytic enzymes are also valuable targets for the development of novel drugs for infectious diseases because they belong to major virulent factors of infectious agents with important roles in their development, reproduction and interactions with host/invertebrate vector tissues. (Renslo et al., 2006). As therapeutic agents, protease inhibitors have been investigated in the past decade chiefly for the treatment of human immunodeficiency virus (HIV) and hypertension. They are commonly used in combination therapy with reverse transcriptase inhibitors to reduce the viral load in HIV positive individuals; however, they show formidable efficacy even when used in monotherapy (Arribas et al., 2005).
MATERIALS AND METHODS
Extraction of crude protein from Pigeon pea
20Gm of Seeds were crushed in blender and seed powder was defatted by transferring it into a clean conical flask containing Acetone. The suspension was kept in shaking incubator for overnight. Defatted, dried seed powder was mixed with 10 volume of 0.1 M Sodium Phosphate buffer (pH 7.5) containing 2% of PVP and 0.02g PMSF and incubated overnight on rotary shaker. The suspension was filtered and centrifuged at 12,000 rpm for 20 min.
Partial purification of Crude proteins
Then supernatant was used for purification by Ammonium sulphate precipitation method. Saturated ammonium sulfate salt was added to the supernatant (crude extract) to obtain a precipitate formed at 10– 100% saturation with respect to this salt. The pellet was collected from all fractions .The pellet was dissolved in 5 ml of 0.1 M HEPES buffer and The (NH4)2SO4 was removed by the process of dialysis using the extraction buffer stirred gently with magnetic stirrer to improve solute exchange and the dialysis buffer was changed once in 3 hr for 2 times. The dialysed ten fractions were stored at 40 ºC and used for further process.
Protein Concentration
Protein concentrations of all dialyzed fractions were determined by Lowry et al . (1951) method. Bovine Serum Albumin was used as the standard to determine protein concentration.
Detection of PIs by the agar well diffusion method
The agar well diffusion method (Rutwika et al. 2023) was employed to assess PI activity in crude extracts and dialysed fractions (10% to 100%). To prepare the agar plates, 1gm of agar was heated and allowed to cool to approximately 55ºC and 0.1g of casein was added. Once solidified, wells were created using a well borer. Enzyme and PI mixtures were prepared in three different volume ratios 3:1, 1:1, and 1:3 (v/v) with the final reaction volume adjusted using 0.1 M HEPES buffer at pH 7.8. Each well was loaded with 20 μl of these mixtures, while trypsin served as the positive control and buffer as the negative control. The plates were incubated overnight at 37 °C. The next day, 10% TCA was added to the wells and left for 30 minutes. Casein hydrolysis was visually examined based on the formation of clear zones around the wells. A smaller hydrolysis zone compared to the trypsin control indicated inhibition, confirming the presence of PIs.
Dot-blot/spot test
The dot-blot/spot test was performed to evaluate the effectiveness of a protease inhibitor against trypsin using X-ray film as a substrate (Pichare and Kachole, 1994; Padul et al. , 2012). Three different enzymeinhibitor ratios were prepared: 1 (1:3), 2 (1:1), and 3 (3:1) v/v. The reaction mixture was adjusted using 0.1 M Tris-HCl buffer (pH 7.8) for trypsin, and the total volume was brought to 20 μl with the same buffer and loaded on X-ray film. The spotted film was then incubated at 37 °C for 20 minutes, rinsed with tap water, and air-dried. The extent of gelatin hydrolysis varied based on the inhibitor’s potency, producing distinct visual patterns on the film.
Visualization of isoforms of PIs by SDS-PAGE:
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a discontinuous buffer system, as described by Laemmli (1970). The gel matrix consisted of a 5% stacking gel layered over a 10% resolving gel. Bromophenol blue was employed as a tracking dye to monitor protein migration. The samples were prepared under nondenaturing conditions, ensuring that their native structures remained intact. Electrophoresis was conducted under a constant voltage of 150 V until the separation process was complete. Following electrophoresis, the gel was carefully removed and Gel were fixed in Coomassie brilliant blue (CBB) under Room temperature and distained to visualize bands.
Proteinase inhibitory activity
Proteinase inhibitory activity was evaluated following the method described by Paulino da Silva et al. (2001), using N-α-benzoyl-DL-arginine p-nitroanilide (BApNA) as the substrate. Initially, a mixture of 10 µL trypsin and 50 µL inhibitor was preincubated in 50 mM Tris-HCl buffer (pH 8.2) for 10 minutes at room temperature, with a total reaction volume of 990 µL. The enzymatic reaction was initiated by adding 250 µL of 1 mM BApNA, allowing substrate hydrolysis to proceed. To arrest the reaction, 200 µL of acetic acid was introduced. The release of p-nitroaniline, indicative of proteolytic activity, was quantified by measuring absorbance at 410 nm. The inhibitory activity was determined by calculating the difference in enzymatic activity between assays performed in the presence and absence of the inhibitor. One unit of trypsin inhibitory activity (TIA) is defined as the activity that inhibits or reduces one unit of enzyme activity.
RESULTS AND DISCUSSION
Inhibitor Isolation and Partial purification
Crude soluble protein extract obtained from the mature Pigeon pea seeds were initially precipitated at 10% to 100% saturation with ammonium sulphate and protein fractions (F1 to F10) were obtained. The fractions were then subjected to desalting with dialysis.
Total Protein Concentration
The total Protein concentrations of all dialyzed fractions were determined. A standard graph using Bovine serum albumin was drawn. Among all the dialyzed fractions (10% to 100%), the fraction saturated with 60% ammonium sulphate precipitation salt shown highest amount of protein i.e. 4924 µg.
Detection of PIs by the agar well diffusion method
The agar well diffusion detection of protease inhibitors (PIs) was carried out to detect the protease inhibitory activity of crude extract (Rutwika et.al.2023). The trypsin and inhibitor (Crude extract) was loaded on wells in 1:1 ratio. The formation of a clearing zone, resulting from casein hydrolysis by trypsin, was observed. A reduction in the clearing zone was noted when trypsin was incubated with an inhibitor. The results of PI activity detection using the agar well diffusion method are presented in Figure 3.
Dot-blot/spot test
The dot-blot test was conducted to assess the effectiveness of crude protease inhibitors (PIs) against trypsin, utilizing gelatin-coated X-ray film. (Pichare and Kachole 1994; Padul et al., 2012). 10ul concentrations of the enzyme trypsin and 10ul of inhibitor were prepared. The resulting samples were loaded onto X-ray film. After incubating for 20 min at 37 °C, the film was washed with tap water and dried in air. The enzyme and inhibitor produced different patterns of gelatin hydrolysis on the X-ray film depending on the efficacy of inhibitor. The inhibition pattern was observed visually (Figure 4).
Visualization of isoforms of PIs by SDS-PAGE:
In vertical gel electrophoresis, proteins are separated based on their molecular weight. In this study, pigeon pea samples underwent SDS-PAGE (15%) to determine the molecular weights of the proteins. Lane 1, containing the protein marker, displayed a molecular weight range of 245–11 kDa, covering various protein sizes. Lane 2, representing the samples, exhibited distinct bands within the same range (245–11 kDa), indicating differences in molecular weight due to the extraction process, which resulted in high-molecular-weight (HMW) peptides. The SDS-PAGE analysis further demonstrated the formation of low-molecular-weight (LMW) peptides with masses below 12 kDa.
Proteinase inhibitory activity
The inhibition of trypsin activity by protease inhibitors extracted from Pigeon pea was studied. As shown in Table no. 2, Pigeon pea seeds have the ability to inhibit trypsin activity. These inhibitors can inhibit up to 59% of trypsin activity. Among all these (F1 to F10) fractions, F6 (60%) showed the highest inhibitory effect on trypsin, whereas fraction F2 (20%) had the lowest inhibition. Similarly total activity and yield percentage in fraction
60% was found to be 4038 µmol/min/ml and 83.25 % respectively.
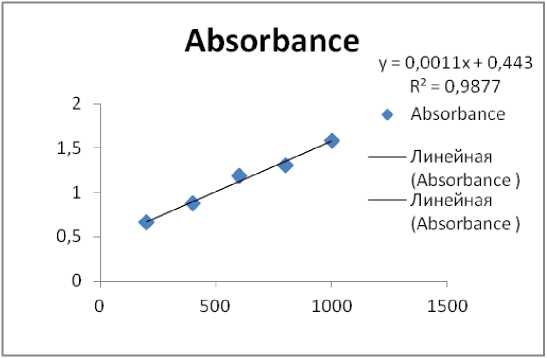
Figure 1. Standard graph for Protein Estimation
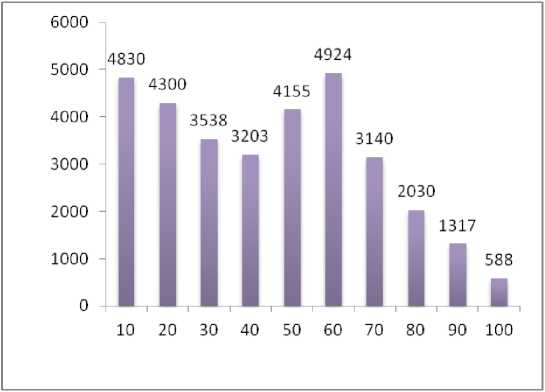
Figure 2. Amount of protein in different fractions (10% to 100%)
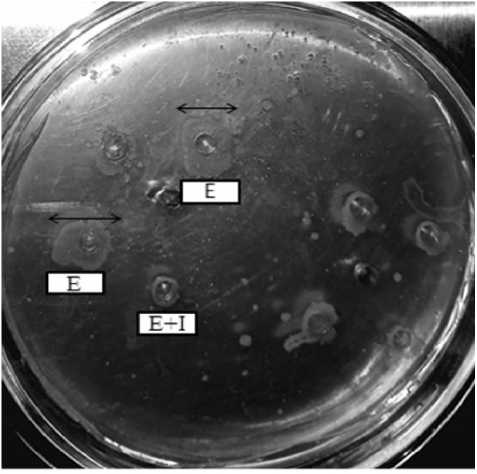
Figure 3. Detection of PI activity by the agar well diffusion method. Different ratios of Enzyme trypsin (E) and Crude extract (I) were prepared and loaded in wells on casein containing agar.
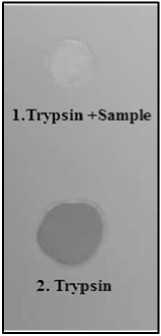
Figure 4. Dot blot assay of Protease inhibitor extracted from pigeon pea seeds. 1. The faint spot (Gelatin) indicates total inhibition of enzyme while dark spot 2. Indicates no inhibition.
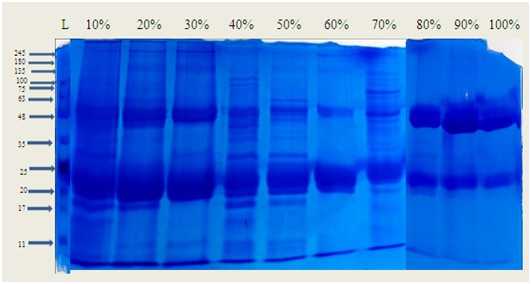
Figure 5. Protein profile of pigeon pea seed extract by Sodium dodecyl sulphate polyacrylamide gel electrophoresis(SDS-PAGE) Lane 1:Standard molecular weight marker. Lane 2 to 10 different fractions of crude seed extracts (10% to 100%)
Table 1. - Molecular weight of different proteins present in Partially purified protease inhibitors
|
Protein Ladder |
F1 (10%) |
F2 (20%) |
F3 (30%) |
F4 (40 %) |
F5 (50%) |
F6 (60%) |
F7 (70%) |
F8 (80%) |
F9 (90%) |
F10 (100%) |
|||||||||||
|
Cm |
KDa |
Cm |
KDa |
Cm |
KDa |
Cm |
KDa |
Cm |
Kda |
Cm |
KDa |
Cm |
KDa |
Cm |
KDa |
Cm |
KDa |
Cm |
KDa |
Cm |
KDa |
|
0.2 |
245 |
0.3 |
180 |
0.3 |
180 |
0.3 |
180 |
0.2 |
245 |
0.2 |
245 |
0.2 |
245 |
0.6 |
87 |
1.4 |
51 |
1.4 |
51 |
1.4 |
51 |
|
0.3 |
180 |
0.5 |
100 |
0.5 |
100 |
0.5 |
100 |
0.3 |
180 |
0.3 |
180 |
0.3 |
180 |
0.7 |
75 |
2.0 |
34.3 |
2.7 |
20 |
2.7 |
20 |
|
0.4 |
135 |
0.8 |
62 |
0.8 |
62 |
1.4 |
51 |
1.4 |
51 |
1.4 |
51 |
1.4 |
51 |
1.3 |
53 |
2.7 |
20 |
||||
|
0.5 |
100 |
1.4 |
51 |
0.9 |
61 |
1.5 |
48 |
1.5 |
48 |
1.5 |
48 |
1.5 |
48 |
1.4 |
51 |
3.6 |
11 |
||||
|
0.7 |
75 |
1.5 |
48 |
1.1 |
60 |
1.6 |
47 |
1.6 |
47 |
1.6 |
47 |
1.6 |
47 |
1.5 |
48 |
||||||
|
1.0 |
63 |
1.6 |
47 |
1.4 |
51 |
2.5 |
22 |
1.8 |
35 |
1.8 |
35 |
1.8 |
35 |
1.9 |
35.2 |
||||||
|
1.4 |
51 |
1.8 |
35 |
1.5 |
48 |
2.8 |
19 |
2.1 |
31.5 |
2.1 |
31.5 |
2.1 |
31.5 |
2.2 |
25 |
||||||
|
1.8 |
35 |
2.0 |
34 |
1.6 |
47 |
3.0 |
17 |
2.3 |
26.7 |
2.3 |
26.7 |
2.3 |
26.7 |
2.8 |
19 |
||||||
|
2.2 |
25 |
2.7 |
20 |
2.5 |
22 |
3.1 |
15 |
2.5 |
22.7 |
2.5 |
22.7 |
2.5 |
22.7 |
3.2 |
14 |
||||||
|
2.7 |
20 |
3.0 |
17 |
2.8 |
19 |
3.8 |
10 |
||||||||||||||
|
3.0 |
17 |
3.3 |
13 |
3.0 |
17 |
||||||||||||||||
|
3.6 |
11 |
3.1 |
15 |
||||||||||||||||||
Table 2. Purification of protease inhibitor from pigeon pea seeds
|
Fraction No. |
Purification step(NH 4 SO 4 ) (%) |
Total Activity (umol/Min/mil) |
% Inhibition |
Total Protein content(ug) |
Specific activity(mg) |
Yield (%) |
Purificatio n fold |
|
F1 |
10 |
800 |
11.05 |
4830 |
0.15 |
16 |
3.75 |
|
F2 |
20 |
422 |
5.8 |
4300 |
0.09 |
8.7 |
2.2 |
|
F3 |
30 |
520 |
7.1 |
3538 |
0.14 |
10.7 |
3.5 |
|
F4 |
40 |
2827 |
39 |
3203 |
0.78 |
58 |
19.5 |
|
F5 |
50 |
3139 |
43 |
4155 |
0.65 |
64.72 |
16.2 |
|
F6 |
60 |
4038 |
55.8 |
4924 |
0.72 |
83.25 |
18 |
|
F7 |
70 |
3041 |
42 |
3140 |
0.93 |
62.70 |
23.2 |
|
F8 |
80 |
2870 |
39.6 |
2030 |
1.29 |
59.1 |
32.2 |
|
F9 |
90 |
2780 |
38.4 |
1317 |
1.94 |
57.3 |
48.5 |
|
F10 |
100 |
3010 |
41.5 |
588 |
3.03 |
62.06 |
75.7 |
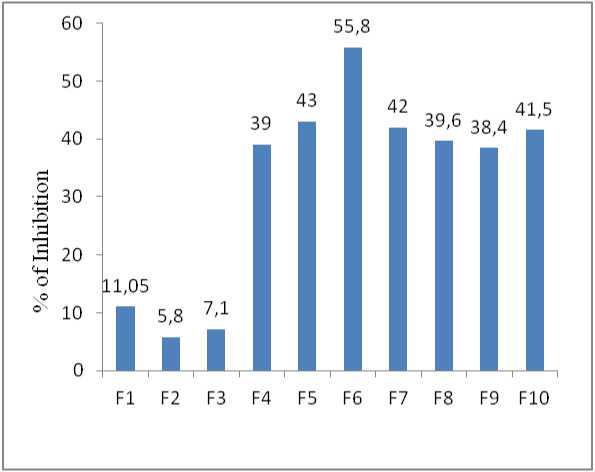
Figure 6. Percent of trypsin inhibition by crude extract fractions (protease inhibitors)
CONCLUSION
This study focuses on the extraction, characterization, and partial purification of protease inhibitors from pigeon pea seeds. The total protein content was quantitatively estimated to be 4924 µg/ml. SDS-PAGE analysis revealed that the separated proteins had molecular weights ranging from 245 to 11
kDa. The protease inhibitors exhibited approximately 60% inhibitory activity against trypsin. Further work will be directed to purification and characterization of Protease inhibitory activity against proteases present in larvae gut.
ACKNOWLEDGEMENT
Seeds of Pigeon pea provided by Mahatma PhuleKrishi Vidyapeeth,Rahuri,Maharashtra is greatfully acknowledged. Authors also thanks Vidya Pratishthan’s Arts, Commerce and Science college, Baramati, Pune, Maharashtra and Savitribai Phule Pune university, Pune for extending the research facilities required to accomplished this work.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.


