Bioethanol to hydrogen membrane surface characteristics change study
Автор: Sidorov Aleksandr, Kosivtsov Yurii, Doluda Valentin
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Химические науки
Статья в выпуске: 12 т.8, 2022 года.
Бесплатный доступ
The problem of the gradual transformation of the modern economy towards greater production and consumption of ‘green’ energy requires a significant revision of existing technologies. One of the possible ways to develop green energy is the use of hydrogen as the most environmentally friendly fuel. Hydrogen can be obtained both by electrolysis, using solar energy, and using biorenewable raw materials, which can be used as ethanol, biogas, peat, agricultural waste. At the same time, for regions with a low level of illumination, the production of hydrogen by electrolysis of water using electricity generated by solar panels is inaccessible, and therefore the processing of biorenewable raw materials can take a leading position. Bioethanol is a large-capacity product with a proven production technology that widely uses waste from agriculture and wood processing. Ethanol can be used as a feedstock for hydrogen generation by means of catalytic pyrolysis or catalytic steam reforming. Membrane-catalytic steam reforming of ethanol with the production of hydrogen makes it possible to obtain hydrogen without the use of an additional purification step, however, the efficiency and stability of the membrane becomes the determining parameter that ensures the efficiency of the entire process. The degradation of inorganic membranes during catalytic steam reforming is closely related to the change in porosity as a result of hydrolysis of the membrane surface. In this connection, the study of the physicochemical properties of membranes during operation can make a significant contribution to the development of stable catalytic membranes for hydrogen production. The article presents the results of studying the physicochemical properties of an inorganic membrane for ethanol steam reforming by the method of low-temperature nitrogen adsorption. The Langmuir, Brunauer-Emmett-Taylor, t-plot and Barrett-Joyner-Halenda models were used to estimate the surface change. An increase in the surface area of mesopores during the operation of the membrane was determined.
Membranes, adsorption, bioenergy
Короткий адрес: https://sciup.org/14126178
IDR: 14126178 | УДК: 544.47 | DOI: 10.33619/2414-2948/85/09
Текст научной статьи Bioethanol to hydrogen membrane surface characteristics change study
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 544.47
Green and sustainable economy determine essential demand for green energy production [1, 2]. One possible way to solve this problem is to develop method for hydrogen production from renewable resources [3, 4]. Green hydrogen can be produced using water electrolysis and bio renewable resources catalytic pyrolysis, gasification and transformation [5]. Bioethanol can be considered as bio renewable resource easily produce from agriculture and wood processing wastes and applicable for hydrogen production [6, 7]. Bioethanol can be converted into hydrogen using ethanol water gas shift reaction. Efficiency of the process is determined catalysts activity and selectivity [8]. Typically, Pt, Pd, Ni, Fe, Cu, Zn, Al containing catalysts are used to provide this process [9]. Catalytic process of bioethanol to hydrogen transformation can be combined with membrane proses of hydrogen separation [10]. The main problem of such systems is catalyst deactivation due to surface carbonization and active metals degradation [11]. Therefor insight in surface change during catalytic membrane operation is essential for understanding ways to decrease membranes deactivation.
Nitrogen physisorption can be considered as effective method for obtaining membranes surface characteristics and determination of surface change during membrane work. Typically, nitrogen physisorption measurements are provided using static volumetric method, were nitrogen adsorption determined by mass balance equations and pressure drop in sample sell. For evaluation of obtained data several models can be used including Langmuir, BET, BJH [12] and t-plot methods can be used. Langmuir model includes adsorption of one molecular layer on solid surface (1).
p _ 1 p
V^+й™
Where p — system pressure, Pa; V a — volume of adsorbed gas; V m — volume of adsorbed gas forms monomolecular layer; b — correlation coefficient.
Most frequently used Brunauer, Emmett, and Teller model includes possibility of gas multilayer condensation (2).
_Л_ = ±+£-1Й(2)
va(po-p) vmc Vmc\pj
Where P 0 — saturation pressure of gas; C — gas adsorption constant;
Barrett-Joyner-Halenda (BJH) method is based on Kalvin equation (3).
^In^U'C
VP
Where ν — molar volume; σ — liquid surface tension; C — is mean curvature defined on formula 4.
C =- +
Г 1
^ 2
Where r — interfacial curvature;
The equation 4 can be converted into equation 5.
p 2yvcos6
ln^=-^^
Where ɣ — interfacial tension, θ — contact angle of condensed liquid with wall.
t-plot model uses equation (6) to evaluate micropores volume, external surface area and micropore surface area.
t=
J
13.99 log 1o (y)+0.034
wheret — film thickness;
Application of discussed models for surface characterization gives possibility for evaluation of membranes surface degradation during its work.
Materials and Methods
Specific surface area and porosity were determined using the following instruments: Becman coulter SA3100 surface area and pore size distribution analyzer (Coulter corporation, Miami, Florida), sample preparation device: Becman coulter SA-prep (Coulter corporation, Miami, Florida). For analysis, the sample is placed in a pre-weighed quartz cuvette, which is installed in the SA-PREP™ sample preparation instrument. Sample preparation parameters: temperature — 120℃; gas — nitrogen; preparation time — 60 min. After completion of sample preparation, the cuvette is cooled and weighed, and then transferred to the analytical port of the BECMAN COULTER SA 3100™ instrument. Gas was sequentially supplied to the analytical cell and the equilibrium pressure in the system was determined.
Results and Discussions
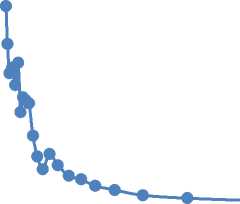
Initial membrane characterized by Langmuir surface area 1.3 m2/g, BET surface area 2.9 m2/g, t-plot surface area 25.3 m2/g. BJH-pore volume distribution (Figure 1) shows that most part of mesopores have diameter less than 6 nm.
0,005
0,0045 •
0,004
0,0035
0,003
0,0025
0,002
0,0015
0,001
0,0005 0
-10 10 30 50 70 90 110 130
Avarage diameter, nm
Figure 1. Pore volume distribution for initial inorganic membrane
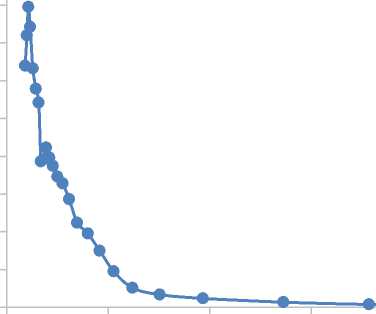
Membrane operation for 50 h on stream results in increase of Langmuir surface area 4.7 m2/g, BET surface area up to 12.3 m2/g, t-plot surface area 78.4 m2/g. BJH-pore volume distribution (Figure 2) shows shift of average pore diameter to 20 nm.
0,0045
0,004
0,0035
0,003
0,0025
0,002
0,0015
0,001
0,0005
0 20 40 60 80 100
Avarage diameter, nm
Figure 2. Pore volume distribution for membrane from ethanol to hydrogen reaction operation for 50 h on stream
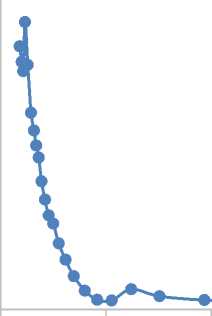
Membrane operation for 100 h on stream results in increase of Langmuir surface area 7.4 m2/g, BET surface area up to 24.9 m2/g, t-plot surface area 53.9 m2/g. BJH-pore volume distribution (Figure 3) shows small shift of average pore diameter to region less than 15 nm.
0,004
0,0035
0,003
0,0025
0,002
0,0015
0,001
0,0005
0 20 40
♦■
Avarage diameter, nm
Figure 3. Pore volume distribution for membrane from ethanol to hydrogen reaction operation for 100 h on stream
Membrane surface degradation during methanol to hydrogen reaction process shows partial increase of membrane mesoporosity and microporosity that can be explained by partial hydrolysis of membrane surface with water steam forming during ethanol to hydrogen transformation process.
Conclusions
Membrane surface degradation is a complex problem for inorganic catalytic membranes. Ethanol to hydrogen catalytic transformation process provided on inorganic membranes strongly suffers from membrane degradation because of high exothermic effect and high amount of water forming during the process. After 50 hours on stream Langmuir surface area increase from 1.3 m2/g to 4.7 m2/g, BET surface area increase form 2.9 m2/g to 12.3 m2/g and t-plot surface area increase from 25.3 m2/g to 78.4m2/g. After 100 hours on stream Langmuir surface area increase to 7.4 m2/g, BET surface area increase to 24.9 m2/g and t-plot surface area decrease to 53.9m2/g.
The reported study was funded by Russian Foundation for Basic Research (RFBR), project number 20-08-00433 А.
Список литературы Bioethanol to hydrogen membrane surface characteristics change study
- Ahmed Z., Ahmad M., Murshed M., Shah M. I., Mahmood H., Abbas S. How do green energy technology investments, technological innovation, and trade globalization enhance green energy supply and stimulate environmental sustainability in the G7 countries? // Gondwana Research. 2022. V. 112. P. 105-115. https://doi.org/10.1016/j.gr.2022.09.014
- Daaboul J., Moriarty P., Palmer G., Honnery D. Making energy green–A method for quantifying the ecosystem maintenance energy and the green energy return on energy invested // Journal of Cleaner Production. 2022. V. 344. P. 131037. https://doi.org/10.1016/j.jclepro.2022.131037
- Eicke L., De Blasio N. Green hydrogen value chains in the industrial sector – Geopolitical and market implications // Energy research & social science. 2022. V. 93. P. 102847. https://doi.org/10.1016/j.erss.2022.102847
- Duan W., Khurshid A., Nazir N., Khan K., Calin A. C. From gray to green: Energy crises and the role of CPEC // Renewable Energy. 2022. V. 190. P. 188-207. https://doi.org/10.1016/j.renene.2022.03.066
- Mazzeo D., Herdem M. S., Matera N., Wen J. Z. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems // Renewable Energy. 2022. V. 200. P. 360-378. https://doi.org/10.1016/j.renene.2022.09.057
- Santoyo-Castelazo E., Santoyo E., Zurita-García L., Luengas D. C., Solano-Olivares K. Life cycle assessment of bioethanol production from sugarcane bagasse using a gasification conversion Process: Bibliometric analysis, systematic literature review and a case study // Applied Thermal Engineering. 2023. V. 219. P. 119414. https://doi.org/10.1016/j.applthermaleng.2022.119414
- Bender L. E., Lopes S. T., Gomes K. S., Devos R. J. B., Colla L. M. Challenges in bioethanol production from food residues // Bioresource Technology Reports. 2022. P. 101171. https://doi.org/10.1016/j.biteb.2022.101171
- Dolgikh L. Y., Zhokh A. A., Trypolskyi A. I., Stolyarchuk I. L., Pyatnitsky Y. I., Strizhak P. E. Conceptual design of an autonomous catalytic generator based on bioethanol steam reforming over the ferrite catalyst // Sustainable Chemistry for Climate Action. 2022. P. 100010. https://doi.org/10.1016/j.scca.2022.100010
- Wang S., He B., Wang Y., Wu X., Duan H., Di J., Xie X. Hydrogen production from the steam reforming of bioethanol over novel supported Ca/Ni-hierarchical Beta zeolite catalysts // International Journal of Hydrogen Energy. 2021. V. 46. №73. P. 36245-36256. https://doi.org/10.1016/j.ijhydene.2021.08.170
- Iulianelli A., Liguori S., Vita A., Italiano C., Fabiano C., Huang Y., Basile A. The oncoming energy vector: Hydrogen produced in Pd-composite membrane reactor via bioethanol reforming over Ni/CeO2 catalyst // Catalysis Today. 2016. V. 259. P. 368-375. https://doi.org/10.1016/j.cattod.2015.04.046
- Wang B., Yu X., Chang J., Huang R., Li Z., Wang H. Techno-economic analysis and optimization of a novel hybrid solar-wind-bioethanol hydrogen production system via membrane reactor // Energy Conversion and Management. 2022. V. 252. P. 115088. https://doi.org/10.1016/j.enconman.2021.115088
- Gibson N., Kuchenbecker P., Rasmussen K., Hodoroaba V. D., Rauscher H. Volumespecific surface area by gas adsorption analysis with the BET method // Characterization of Nanoparticles. Elsevier, 2020. P. 265-294. https://doi.org/10.1016/B978-0-12-814182-3.00017-1


