Биохимические методы синтеза оптически активного пантолактона (обзор)
Автор: Миронова А.В., Гришко В.В., Ившина И.Б.
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Микробиоогия
Статья в выпуске: 6, 2005 года.
Бесплатный доступ
Рассмотрены биохимические методы, обеспечивающие эффективное разделение рацемического пантолактона с участием дегидрогеназ, лактонгидролаз, липаз, карбоксильных эстераз или целых клеток микроорганизмов. Обоснована перспективность использования актинобактерий рода Rhodococcus в качестве новых продуцентов гидролитических ферментов
Короткий адрес: https://sciup.org/147204360
IDR: 147204360 | УДК: 57.088.6
Текст научной статьи Биохимические методы синтеза оптически активного пантолактона (обзор)
Оптически активный пантолактон является ключевым интермедиатом в процессе промышленного получения витаминов группы В и таких бактерицидных агентов, как пантотенол и пантоилтаурин. В тонком органическом синтезе пантолактон широко используется как вспомогательный хиральный агент для получения практически ценных соединений: при дерацемизации непротеиногенных аминокислот (Calmes et al., 1997), энантиоселективном протонировании енолятов (Calmes et al., 2001), производных изокумарина с антиангиогенной и противоопухолевой активностью (Kanoh et al., 2003), в синтезе таких макролидов, как акутифицин, эпотиолоны А и Б (Akbutina et al., 2001); а также в реакции диастерео-селективного гидрирования пиридинов и енаминов (Douja et al., 2003).
Промышленное разделение рацемического пантолактона основано на взаимодействии спирта со стехиометрическим количеством хирального амина. Как правило, с этой целью используются дорогостоящие алкалоиды растительного происхождения. Среди эффективных методов химического синтеза (Я)-пантолактона следует также отметить каталитическое гидрирование кетопантолактона в присутствии платины (Diezi et al., 2003) или ком-, плексов переходных металлов. Описана принципиальная возможность синтеза (Я)-пантолактона на основе ^-замещенных пивальдегидов (Effenberger et al., 1995), эфедрина (Sunil et al., 2003) и 2-метилпропен-2-ола-1 (Upadhya et al., 1999).
В качестве альтернативы многостадийным химическим методам синтеза оптически активного пантолактона разрабатываются более экономичные биохимические подходы, основанные на использовании каталитической активности чистых фермент ных препаратов или целых клеток микроорганизмов. Так, для одностадийного кинетического разделения рацемического пантолактона используются окислительно-восстановительные биотрансформации пантолактона в присутствии специфических дегидрогеназ прокариотных и эукариотных организмов, гидролиз лактонгидролазами внутримолекулярной эфирной связи или карбоксильными эстеразами и липазами межмолекулярной сложноэфирной связи ацильных производных пантолактона.
Окислительно-восстановительные биотрансформации пантолактона
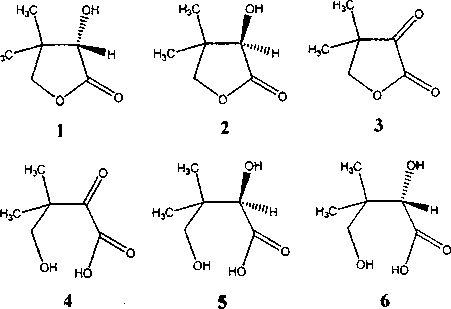
S. Shimizu с соавт. (1987) показано, что стереохимическое превращение (5)-пантолактона 1 в (7?)-пантолактон 2 эффективно протекает под действием дегидрогеназы целых клеток Rhodococcus erythro-polis IFO 12540. Данный процесс включает несколько стадий: 1 - биокаталитическое окисление (S)-пантолактона 1 в кетопантолактон 3; 2 - спонтанный неферментативный гидролиз кетопантолактона 3; 3 - ферментативное восстановление образующейся кетопантоевой кислоты 4 до (Я)-пантоевой ки-
слоты 5; 4 - химическая лактонизация последней в (Я)-пантолактон 2.
С целью увеличения химического и оптического выходов (Я)-пантолактона 2 разработан (Shimizu et al., 1987) альтернативный метод последовательного применения в качестве биокатализаторов бактериальной культуры Nocardia asteroides AKU 2103, обеспечивающей образование кетопантоевой кислоты 4, и дрожжей Candida parapsilosis IFO 0784, ответственных за асимметрическое восстановление кислоты 4 в (Л)-пантоевую кислоту 5. Индуктором дегидрогеназной активности при этом служит 1,2-пропандиол.
Выявлено (Kataoka et al, 1992), что наиболее эффективные дегидрогеназы, катализирующие стереоселективное превращение (5)-пантолактона 1 в кетопантолактон 3, продуцируются представителями родов Nocardia, Rhodococcus и Corynebac-terium. При изучении свойств пантоиллактонде-гидрогеназы из N. asteroids AKU 2103 установлено, что ингибирующее влияние на дегидрогеназ-ную активность фермента оказывают цианид натрия, хлориды некоторых металлов (Мп+2, Со+2), а также типичный ингибитор альдегидредуктаз -барбитал. В качестве конкурирующего субстрата в реакции окисления (£)-пантолактона 1 выступает (7?)-пантолактон 2, в то время как изменение концентрации кетопантоевой 4, (7?)-пантоевой 5 или (5)-пантоевой 6 кислот не оказывает какого-либо влияния на ферментативную активность дегидрогеназы. Максимальная каталитическая активность фермента из N. asteroids AKU 2103, выделенного японскими исследователями (Kataoka et al., 1992), наблюдается при температуре 40°С и pH 9,0-9,5.
Гидролиз внутримолекулярной сложноэфирной связи пантолактона
7 8
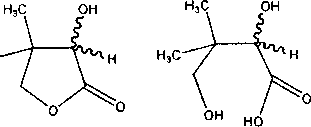
Представители мицелиальных грибов родов Fusarium, Gibberella и Cylindrocarpon продуцируют лактонгидролазы, катализирующие стереоселек-тивный гидролиз внутримолекулярной сложноэфирной связи одного из энантиомеров (Я,5)-пан-толактона 7 (Kataoka et al., 1995; Ogawa et al., 1999). Прямое влияние на лактонгидролазную активность оказывают условия культивирования микроорганизмов. Так, глюкоза, фруктоза, сахароза, мочевина, хлорид и сульфат аммония подавляют рост и каталитическую активность мицелия, в то время как микроорганизмы, выращенные в глицеринсодержащей среде с добавлением полипептона, не накапливают соединений, влияющих на их ферментативную активность (Kataoka et al., 1995).
Скрининг штаммов, обладающих лактонгидролазной активностью, проводят, регистрируя уровень pH реакционной смеси, содержащей в качестве субстрата пантолактон. Индикатором лактонгидролазной активности служит факт закисления среды культивирования в результате образования пантоевой кислоты (Kataoka et al., 1995).
Необходимым требованием для оптимизации процесса ферментативного гидролиза (ЛД-панто-лактона 7 и обеспечения высокой оптической чистоты целевой (5)-пантоевой кислоты 6 является поддержание pH реакционной смеси, поскольку лактонгидролазы сохраняют ферментативную активность в довольно узком (7,0-7,5) интервале pH. Контроль за реакцией среды посредством добавления щелочи (например гидроокиси натрия) недопустим, поскольку приводит к увеличению значений pH реакционной смеси и, как следствие, неферментативному гидролизу пантолактона. Оптическая чистота (S)-пантоевой кислоты 6 в этом случае не превышает 54-68%. В то же время при использовании карбоната натрия или гидроокиси аммония оптическая чистота (5)-пантоевой кислоты 6 достигает 92-94%. Следует отметить, что выделенный из реакционной смеси непрореагировавший (А)-пантолактон 2 является энантиомерно однородным (ее 99%) и может быть использован в практике асимметрического синтеза (Kataoka et al., 1995).
Установлено, что под действием лактонгидролазы из F. oxysporum AKU 3702 на (7?,5)-панто-лактон 7 в соответствующую (Я)-пантоевую кислоту 5 (ее 91-96%) превращается исключительно (/?)-энантиомер 1 (Shimizu, Kataoka, 1996). Данная реакция является обратимой, равновесие устанавливается при pH 6,0 и соотношении молярных концентраций (7?)-пантолактона 2 и (Я)-пантоевой кислоты 5 1:1. Штамм F. oxysporum AKU 3702 демонстрирует относительную субстратную специфичность и катализирует реакции обратимого стереоспецифичного гидролиза широкого круга лактонов (галактоно-, гулоно-, эритроно-, манноно-у-лактоны и т. д.). Интересно отметить, что гидролитическому расщеплению подвергаются лактоны только с (^-конфигурацией гидроксильной группы, в то время как (5)-энантиомеры оказываются нереакционноспособными либо играют роль кон-курентно-ингибирующего субстрата.
В настоящее время для промышленного синтеза (Я)-пантолактона 2 с успехом используют мицелий F. oxysporum AKU 3702, иммобилизованный в альгинат кальция (Shimizu, Kataoka, 1996). Полученный биокатализатор отличается высокой стабильностью и выраженной гидролитической активностью. Практически полная (48%) конверсия (Л)-пантолактона 2 достигается через 21 ч при температуре 30°С и pH 6,8-7,2. При концентрации (/?,^-пантолактона 7 в среде, равной 350 г/л, опта- ческая чистота целевой (А)-пантоевой кислоты 5 составляет 90-97%. В описанных условиях реакции сохраняется более 90% начальной активности катализатора после 180 циклов использования.
Установлено (Kataoka et al., 1996), что лактон-гидролаза из F. oxysporum AKU 3702 содержит 2 моль Са2+ на каждый моль фермента. Добавление солей кальция, в частности СаС12, в среду биотрансформации способствует повышению активности и стабильности катализатора, что приводит к увеличению скорости процесса лактонизации в 3 раза. Выход целевого продукта реакции через 16 ч составляет 30%, в то время как в условиях отсутствия СаС12 - лишь 9%. Интересно отметить, что оптическая чистота (Л)-пантолактона 2 в обоих случаях практически не различается и достигает 90%.
Описан процесс гидролиза (7?)-пантолактона 2 под действием представителей вида F. moniliforme (Tang et al., 2002). При этом в качестве носителя наряду с альгинатом кальция авторами использовались k-каррагенин и желатин. В результате проведенных исследований установлено, что при катализе как иммобилизованными, так и свободными клетками F. moniliforme SW-902 оптимальными условиями для проявления ферментативной активности являются температура 55°С и pH 7,5. При этом иммобилизованный мицелий отличается более высокой стабильностью, сохраняя до 88% начальной лактонгидролазной активности при температуре 60°С и pH 7,5.
\ Обратный процесс - реакция лактонизации рацемической пантоевой кислоты 8 - с образованием энантиомерно обогащенного (ее 90%) (Я)панто-лактона 2 под действием лактонгидролазы из F. oxysporum AKU 3702 наиболее эффективно протекает в условиях двухфазной системы “органический растворитель - вода” (Kataoka et al., 1996). Максимальная ферментативная активность достигается при использовании в качестве органической фазы этилацетата или метилэтилкетона. В случае использования этилацетата в реакцию вступает до 50% (/?)-пантоевой кислоты 5, при этом оптическая чистота образующегося (Я)-пантолактона 2 составляет 85-90%. В случае использования водной среды лактонизации подвергается лишь 25% субстрата. Преимущество двухфазной системы обусловливается сдвигом равновесия в сторону образования (Я)-пантолактона 2 благодаря экстракции последнего в органическую фракцию. Оптимальные условия описанной реакции - температура 30°С и pH 5,0.
Гидролитическое расщепление сложных эфиров пантолактона
Липазы и микробные эстеразы в синтезе оптически активного пантолактона
Эстеразы и липазы - гидролитические ферменты, обеспечивающие кинетическое расщепление межмолекулярной сложноэфирной связи. Известно, что в присутствии ацильных доноров эстеразы и липазы катализируют обратный процесс - реакцию этерификации спиртов. Высокая трансформирующая активность и стабильность позволяют использовать указанные ферменты с применением органических растворителей, что выгодно отличает данные биокатализаторы от дегидрогеназ и лактонгидролаз, обеспечивающих образование энантиомерно однородного пантолактона в условиях водной среды.
В настоящее время разработано несколько экспресс-методов для выявления гидролитически активных культур, в том числе культур, способных к стереоселективному гидролизу сложных эфиров пантолактона. Так, индикаторный экспресс-метод (Коронелли и др., 1986) основан на использовании в качестве субстрата интенсивно окрашенного индофенилацетата, гидролиз которого приводит к обесцвечиванию среды, что свидетельствует об эстеразной активности исследуемых микроорганизмов.
96: R=F Юб: R=F
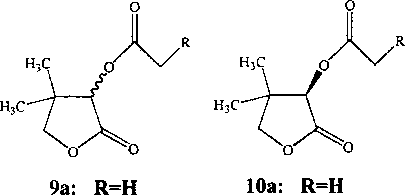
В методе М.Т. Reetz и C.J. Ruggeberg (2000) в качестве субстрата использован фторацетатный эфир пантолактона 96. Образующаяся в процессе гидролиза фторацетата пантолактона 96 токсичная фторуксусная кислота подавляет развитие микроорганизмов, что свидетельствует об эстеразной активности биокатализатора.
Метод отбора ферментов или штаммов-продуцентов эстераз, способных к превращению ацетата пантолактона 9а в пантолактон 1, основан на использовании качественной реакции с индикатором бромтимоловый синий. Изменение окраски реакционной среды из синего цвета в желтый свидетельствует о закислении среды, а следовательно, о расщеплении исходного субстрата до пантолактона и уксусной кислоты. Использование данного метода позволило М. Baumann с соавт. (2000) провести быстрый скрининг эстеразной активности широкого набора ферментных препаратов, а также целых клеток микроорганизмов и выявить наиболее активные (Aspergillus niger, A. oryzae и Bacillus stearothermo-philus) в отношении ацетата пантолактона 9а. В результате проведенных исследований установлено, что отобранные культуры катализируют 47-56% конверсию исходного субстрата с образованием энантиомерно обогащенных ацетата (А)-пантолактона 10а (83-99% ее) и (5)-пантолактона 1 (78-95% ее).
В результате проведенного экспресс-скрининга среди штаммов актинобактерий из Уральской профилированной коллекции алканотрофных микроорганизмов iegmcol) отобраны представители актинобактерий рода Rhodococcus, катализирующие селективный гидролиз ацетата пантолактона 9а (Гришко и др., 2005). На основе наиболее активных культур R. ruber ИЭГМ 381 и Л rhodochrous ИЭГМ 654 получены УФ-моди-фицированные бактериальные клетки, обеспечивающие образование (5)-пантолактона 1 (89,2% ее).
Отобранные в результате скринирования гидролитически активные ферменты и культуры, как правило, используются для стереоселективной этерификации спиртов. В отличие от процесса гидролиза, который предусматривает дополнительную стадию ацилирования рацемического спирта, реакция этерификации является одностадийной. L. Haughton с соавт. (2000) описано выделение из (7?,5)-пантолактона 7 под действием липазы из Candida cylindracea с использованием винилацетата или винилакрилата в качестве ацильного донора. Интересно отметить, что температурный режим реакции не оказывает значительного влияния на скорость реакции и выход целевого продукта -данный фермент остается активным в интервале температур от 25 до 45°С. Изменение концентрации фермента от 5 до 20% приводит к увеличению выхода ацилированного (Я)-пантолактона 2 с 11 до 70%. Вместе с тем при высоких (более 20%) концентрациях фермента снижается оптическая чистота целевого продукта.
Использование эстеразной активности актинобактерий рода Rhodococcus в процессе синтеза практически ценных соединений
В качестве новых источников липаз и эстераз, катализирующих кинетическое разделение рацемического пантолактона 7, в основном рассматриваются эукариотные микроорганизмы, а также представители рода Pseudomonas (Baumann et al., 2000), использование которых наряду с явными преимуществами имеет ряд недостатков. Так, при возможности получения богатой биомассы обнаруживается мицелиальный характер роста грибов или выявляются факторы патогенности среди гра-мотрицательных микроорганизмов. В то же время отдельные работы по исследованию эстеразной активности представителей рода Rhodococcus, отличающихся типично бактериальным характером роста и выраженной ферментативной активностью в отношении труднодоступных органических субстратов, свидетельствуют о перспективности использования родококков в процессах расщепления сложноэфирной связи ацильных производных как алифатических, так и циклических спиртов.
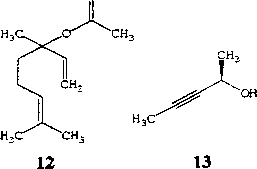
Так, для получения линалоола 11, широко применяемого в качестве ароматизирующего компонента в парфюмерной промышленности, могут быть использованы специфические ферменты -(й)-линалилацетат гидролаза из штамма R. ruber DSM 43338 или (5)-линалилацетат гидролаза из
Rhodococcus sp. NCIMB 11216, катализирующие стереоселективный гидролиз ацетата (R, ^-линалоола 12 (Pogorevc et al., 2000). Необходимо отметить, что целые клетки родококков обладают более низкой стереоспецифичностью (Е=4,2) по сравнению с очищенным ферментом (Е>100).
о
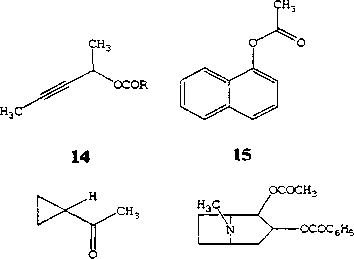
16 17
(7?)-3-пентин-2-ол 13 используется в качестве хирального агента в тонком органическом синтезе и может быть получен в энантиомерно обогащенной форме (ее 97,8%) в результате гидролиза ацетатного (Я^-эфира 14 под действием целых клеток Gordonia (R. rubropertincta) AKU NOC082 (Ogawa, Xie, 1999).
M. Gudelj с соавт. (1998) описано разделение сложных эфиров (ацетата, пропионата, бутирата, хлорпропионата) ароматических спиртов - а- 15 и Р-нафтолов под действием фермента, выделенного из Rhodococcus sp. NCIMB 11216.
Труднодоступность циклопропанола обусловливает ограниченное применение данного соединения в качестве хирального синтона в органическом синтезе. Описано несколько способов получения энантиомерно однородной формы циклопропанола, но ни один из них не может быть использован в качестве эффективного метода. P.L.A. Overbeeke с соавт. (2003) разработан биотехнологический способ ферментативного расщепления циклопропилметилкетона 16 до циклопропанола (химический выход 42 %) под действием целых клеток R. erythropolis DSM 1069. Предполагается, что эта реакция включает окисление по Байер-Виллегеру, сопровождаемое гидролизом промежуточного ацетата циклопропанола. Данная технология может применяться в промышленности и не требует значительных затрат на реагенты, в отличие от описанных ранее химических методов синтеза циклопропанола.
Биологическое действие известного алкалоида 2-карбометокси-З-бензоилокситропана (кокаин) 17 может быть подавлено в результате гидролиза молекулы под действием специфического фермента кокаинэстеразы из Rhodococcus sp. (сосЕ) (Asxenzi et al., 2003).
T. В. Коронелли с соавт. (1986) среди широкого набора углеводородокисляющих бактериальных культур отобраны штаммы, наиболее активно катализирующие процесс расщепления индофенилацетата 18 и принадлежащие к роду Rhodococcus. Выявлено, что ферментные препараты эстераз ро-дококков отличаются повышенной термостабильностью, более того, их каталитическая активность увеличивается на 75% после дополнительной термообработки.
Заключение
Таким образом, в настоящее время разработаны биокатализаторы, обеспечивающие эффективное разделение рацемического пантолактона. Однако катализируемые ими процессы предполагают использование культур мицелиальных грибов, высокочувствительных ферментов, они проходят в несколько стадий. Практически не изучена активность целых клеток микроорганизмов в реакциях этерификации пантолактона с использованием органических растворителей. Преимущество неводных сред проявляется в возможности использования в качестве субстратов трансформации липофильных соединений, осуществления синтеза энантиомерно однородных соединений в одну стадию и смещения равновесия реакции ацилирования в сторону образования целевого продукта.
На наш взгляд, в качестве наиболее перспективных биокатализаторов в реакциях стереоселек-тивной этерификации пантолактона следует рассматривать актинобактерии рода Rhodococcus. Высокая активность и термостабильность эстераз, простота и дешевизна сред для получения клеточной массы позволяют использовать родококки в качестве потенциальных продуцентов гидролитических ферментов.
Список литературы Биохимические методы синтеза оптически активного пантолактона (обзор)
- Гришко В.В., Миронова А.В., Ившина И Б. Биокаталитическое разделение рацемического ацетата пантолактона с использованием целых клеток родококков//Катализ в промышленности. 2005. № 6.
- Коронелли Т.В. и др. Эстеразная активность углеводородокисляющих бактерий//Микробиология. 1986. Т. 55, вып. 5. С. 883-884.
- Akbutina F.A. et al.. Geminal dimethyl-substituted fimctionalized C4-synthons from pantolactone//Russian Journal of organic chemistry. 2001. V. 37, № 5. P. 695-699.
- Asxenzi P., Clementi E., Polticelli F. The Rhodococcus sp. cocaine esterase: a bacterial candidate for novel pharmacokinetic-based therapies for cocaine abuse II lUBMB Life. 2003. V. 55, № 7. P. 397-402.
- Asxenzi P., Clementi E., Polticelli F. The Rhodococcus sp. cocaine esterase: a bacterial candidate for novel pharmacokinetic-based therapies for cocaine abuse IIШВМВ Life. 2003. V. 55, № 7. P. 397-402.
- Baumann M, Hauer B.H., Bornscheuer U.T. Rapid screening of hydrolases for the enantioselective conversion of 'difficult-to-resolve' substrates II Tetrahedron: Asymmetry. 2000. V. 11, № 23. P. 4781-4790.
- Calmes M., Daunis J., Mai N. Asymmetric synthesis of a-amino acids via diastereoselective addition of (R)-pantolactone to their ketens II Tetrahedron: Asymmetry. 1997. V. 8, № 10. P. 1641-1648.
- Calmes M., Glot C, Marlines J. Investigation into the enantioselective protonation of enolate Schiff bases with (R)-panto]actone II Tetrahedron; Asymmetry. 2001. V. 12, № 1. P. 49-52.
- Diezi S. et al. Inversion of enantioselectivity in the hydrogenation of ketopantolactone on platinum modified by ether derivatives of cinchonidine II Tetrahedron: Asymmetry. 2003. V. 14, № n. p. 2573-2577.
- Douja N. et al. Heterogeneous diastereoselective hydrogenation of pyridine and corresponding enamine covalently bound to pantolactone II J. Mol. Catal. A: Chem. 2003. V. 210, № 2. P. 205-209.
- Effenberger F., Eichhorn J., Roos J. Enzyme catalyzed addition of hydrocyanic acid to substituted pivalaldehydes -a novel synthesis of (R)-panto lactone II Tetrahedron: Asymmetry. 1995. V. 6, № 1. P. 271-282.
- Gudelj M. et al. Novel Rhodococcus esterases by genetic engineering II J. Mol. Catal. B: Enzym. 1998. V. 5,Xo 1-4. P. 261-266.
- Kanoh N, Tomatsu A., Nishikawa T. Practical deracemization of NM-3, a synthetic angiogenesis inhibitor II Tetrahedron: Asymmetry. 2003. V. 14, № 10. P. 1251-1253.
- Haiighton L. et al. Enzymatic kinetic resolution of pantolactone: relevance to chiral auxiliary chemistry II Tetrahedron: Asymmetry. 2000. V. 11, № 8. P. 1697-1701.
- Kanoh N. et al. Practical deracemization of NM-3, a synthetic angiogenesis inhibitor II Tetrahedron: Asymmetry. 2003. V. 14, № 10. P. 1251-1253.
- Kataoka M. et al. Optical resolution of racemic pantoic acid through microbial stereoselective lactonization in an organic solvent/water two-phase system II Enzyme Microb. Technol. 1996. V. 19, № 4. P. 307-310.
- Kataoka M. et al. Lactonohydrolase-catalyzed optical resolution of pantoyl lactone: selection of a potent enzyme producer and optimization of culture and reaction conditions for practical resolution//Appl. Microbiol. Biotechnol. 1995. V. 44, № 3-4. P. 333-338.
- Kataoka М., Shimizu S., Yamada Н. Purification and characterization of a novel FMN-dependent enzyme. Membrane-bound L(-i-)-pantoyl lactone dehydrogenase from Nocardia asteroides II Eur. J. Biochem. 1992. V. 204, № 2. P. 799-806.
- Ogawa J., Shimizu S. Microbial enzymes: new industrial appUcations from traditional screening methods II Trends Biotechnol. 1999. V. 17, № 1. P. 13-21.
- Ogawa J., Xie S.-X., Shimizu S. Production of (R)-3-pentyn-2-ol through stereospecific hydrolysis of racemic 3-pentyn-2-ol esters with microbial enzymes//Appl. Microbiol. Biotechnol. 1999. V. 51, № l. p. 53-57.
- Overbeeke P.LA. et al. Biocatalytic synthesis of cyclopropanol from cyclopropyl methyl ketone using whole cells of Rhodococcus erythropolis II J. Mol. Catal. B: Enzym. 2003. V. 21, № 1-2. P. 51-53.
- Pogorevc M. et al. Novel carboxyl esterase preparations for the resolution of linalyl acetate II Monatsh. Chem. 2000. V. 131, № 6. P. 639-644.
- Reets. M. Т., Ruggeberg C. J. A screening system for enantioselective enzymes based on differential cell growth II Tefrahedron: Asymmetry. 2000. V. 11, №23. P. 4781-4790.
- Shimizu S. et al. One-step microbial conversion of a racemic mixture of pantoyl lactone to optically active D-(-)-pantoyl lactone II Appl. Environ. Microbiol. 1987. V. 53. P. 519.
- Shimizu S., Kataoka M. Optical resolution of pantolactone by a novel fimgal enzyme, Lactonohydrolase II Ann. N. Y. Acad. Sci. 1996. V. 799. P. 650-658.
- Shimizu S., Kataoka M. Structure, fimction, and application of microbial lactonases II Chimia. 1996. V. 50, №9. P. 409-410.
- Sunil V.P., Bhattacharyya A. Enantioselective synthesis of pantolactone analogues from an ephedrinederived morpholme-dione II Tetrahedron. 2003. V. 59, №18. P. 3275-3282.
- Upadhya T.T., Gurunath S., Sudalai A. A new and short enantioselective synthesis of (R)-pantolactone II Tetrahedron: Asymmetry. 1999. V. 10, № 15. P. 2899-2904.
- Y.-X. Tang et al. Kinetic resolution of DLpantolactone by immobilized Fusarium moniliforme SW-902 II Process Biochem. 2002. V. 38, № 4. P. 545-549.


