Biomarkers for modeling of cancer-specific tumor-associated macrophages ex vivo
Автор: Sudarskikh T.S., Larionova I.V., Rakina M.A., Kzhyshkowska J.G.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 4 т.23, 2024 года.
Бесплатный доступ
Introduction. Tumor-associated macrophages (TAMs) are essential innate immune cells in the tumor microenvironment. TAMs can stimulate cancer cell proliferation and primary tumor growth, angiogenesis, lymphangiogenesis, cancer cell invasiveness in vessels and metastatic niche formation as well as support chemotherapy resistance. TAMs are phenotypically diverse both in various cancer localizations and in intratumoral heterogeneous compartments. Tumor-specific modeling of TAMs is necessary to understand the fundamental mechanism of pro- and anti-tumor activity, to test their interaction with existing therapies, and to develop TAM- targeted immunotherapy. Aim of study: To investigate cancer-specific transcriptomic features of ex vivo human TAM models. Material and Methods. Here we compared transcriptomic profiles of TAMs for breast, colorectal, ovarian, lung, and prostate cancers ex vivo . Human monocytes were isolated from buffy coats, and then stimulated by the tumor cell conditioned medium ex vivo . Using real-time PCR, we quantified the expression of key TAM biomarkers including inflammatory cytokines, scavenger-receptors, angiogenesis-regulating genes, and matrix remodeling factors.
Tumor-associated macrophages, m1/m2 classification, cytokines, scavenger-receptors
Короткий адрес: https://sciup.org/140307090
IDR: 140307090 | УДК: 616-006:612.017.1:576.38 | DOI: 10.21294/1814-4861-2024-23-4-54-65
Текст научной статьи Biomarkers for modeling of cancer-specific tumor-associated macrophages ex vivo
Macrophages are essential components of the innate immune system playing crucial role in inflammation and cancer [1]. Macrophages are generally divided into two polarization states: classically activated pro-inflammatory macrophages 1 (M1) and alternatively activated anti-inflammatory macrophages 2 (M2) [2, 3]. In tumor microenvironment (TME), Ml macrophages are classified as antitumor and are able to recognize and kill cancer cells, while M2 macrophages promote tumor growth [3]. M1 and M2 polarization is triggered by diverse stimuli from the microenvironment, including growth factors, cytokines, chemokines, components of extracellular matrix (ECM), and other molecules [4, 5]. M1 macrophages express nitric oxide, reactive oxygen species, number of pro-inflammatory factors (IL-ie, IL-6, IL-12, IL-23, CXCL9, CXCL10, CXCL11, and TNF-α), and surface receptors including molecules of the main histocompatibility complex [MHC], CD68, CD80, CD86, and MARCO. Oppositely, M2 macrophages abundantly express anti-inflammatory factors, including IL-10, TGF-β, CCL14, CCL17, CCL18, CCL22, CCL24, arginase-1, CD206, CD204, and CD163 [4, 5].
Tumor-associated macrophages (TAMs) stimulate angiogenesis, metastasis, tumor cell immunity evasion, tumor cell proliferation and tumor growth, which has been shown in numerous in vitro and in vivo studies [1, 3, 6]. The predominance of M2 macrophages in the TME correlates with poor prognosis in lung cancer [7, 8], colorectal cancer [9], breast cancer [6], ovarian cancer [10], and other cancers. In the TME, M2 macrophages contribute to the survival of cancer cells and reduce their apoptosis [11], while M1 macrophages, on the contrary, promote apoptosis of cancer cells [8].
Many studies performed using cancer patient material demonstrate more complex diversity of TAMs inside the tumor that doesn`t conform to generally accepted M1/M2 in vitro classification [2, 12]. Moreover, state-of-the-art technologies of single cell and spatial analysis open the perspectives to distinguish novel subpopulations of TAMs in different cancers [13, 14]. Despite the fact that in vitro studies of TAM activities have been performed for many years, recent advances allowed improvement and modify such models.
In our study, we generated TAMs for breast, colorectal, ovarian, lung, and prostate cancers using CD14+ human monocytes by stimulating them with cancer cell conditioned medium. We analyzed major TAM biomarkers, indicating pro-tumor activity, widely used for the analysis in vitro and in vivo . The advances and limitations of our models were demonstrated and discussed.
Aim of study: To investigate cancer-specific tran-scriptomic features of ex vivo human TAM models.
Material and Methods
Cell cultures
Tumor cell lines were obtained from the bioresource collection of vertebrate cell cultures of the Institute of Sciences of the Russian Academy of Sciences (St. Petersburg, Russia) and from the collection of The Global Bioresource Center, the American Type Culture Collection (ATCC, USA).
The following tumor cell lines were used for the study: MCF7 (breast adenocarcinoma, subtype LumA), BT474 (breast adenocarcinoma, subtype LumB), MDA-MB-231 (breast adenocarcinoma, triple-negative cancer), SKBR3 (breast adenocarcinoma, expressing human epidermal growth factor receptor 2 (HER2) cancer), SW837 (rectal adenocarcinoma), SKOV3 (ovarian serous cystadenocarcinoma), РС3 (prostate adenocarcinoma), and A549 (lung adenocarcinoma) [15]. Each cell line was cultured in recommended growth medium supplemented with 1 % antibiotic penicillin/streptomycin (PanEco, Russia) and 10 % fetal bovine serum (FBS) (Capricorn, Germany). Before use, the FBS was inactivated at 56 °C for 30 min. MCF7, MDA-MB-231 and A549 cell lines were grown in DMEM medium (Gibco, USA); BT474, SKBR3, SKOV3, and PC3 cell lines were grown in RPMI1640 medium (Gibco, USA); SW837 cell line was cultivated in Leibovitz’s medium (Gibco, USA). Conditioned cancer cell supernatants were collected and filtered through sterile syringe filters with low protein binding ability having a 0.22 µm pore size (Millipore, USA). Filtered tumor cell line conditioned media were frozen at –80 °С until the stimulation of freshly isolated monocytes.
Monocyte isolation
Human monocytes were obtained from the Siberian Federal Scientific and Clinical Center of the Federal Medical and Biological Agency (Seversk, Russia). Monocytes were isolated from the buffy coats of ten healthy donors using magnetic sorting (MACS)
with CD14-positive selection. The buffy coats were diluted with PBS free of Ca2+ and Mg2+ (Gibco, ThermoFisher) at a ratio of 1:1. The resulting suspension (30 ml) was layered on 15 ml ficoll density 1.077 (PanEco, Russia). The mononuclear fraction after centrifugation was collected from the ficoll/serum interphase, transferred to fresh tubes, and washed twice with PBS. The washed cells were resuspended in 5 ml PBS and applied to a percoll-based continuous gradient consisting of 13.5 ml percoll (Sigma, USA), 1.5 ml 10x Earl’s minimum basic medium (Sigma, USA) and 15 ml Spinner’s medium (Sigma, USA). After centrifugation, the fraction in the upper part of the gradient enriched in monocytes up to 60–80 % was collected and washed 3 times with PBS. The monocyte-enriched fraction was used for magnetic sorting of cells using human CD14 MicroBeads (Miltenyi Biotech, USA). The purity was 95–98 % that was monitored by analysis of the surface expression of the monocyte marker CD14 by flow cytometry (CytoFLEX, Beckman Coulter, USA).
Modeled system for tumor-associated macrophages
A model system for tumor-associated macrophages (TAMs) was obtained by the stimulation of primary human monocytes with cytokines (M-CSF 10 ng/µl [RP01221, ABclonal, China] and IL-4 10 ng/µl [RP00995, ABclonal, China]), and Dexamethasone 108M (D4902, Sigma, USA) and cancer cell conditioned media as has been described in our recent study [16]. Monocytes were cultivated at a concentration of 1x106 cells/ml. Tumor cell supernatants were added to freshly isolated monocytes in an amount of 20 % of the total volume of the medium. As a control, monocytes were incubated with 20 % of empty cancer cell culture medium (DMEM, Leibovitz’s or RPMI1640). Cells were cultured for 6 days at 37°C. After 6 days of cell incubation, the medium was removed, the plates with cells were placed on ice, and lysis buffer was added. Cell lysates were used for RNA isolation and further cDNA synthesis.
Quantitative real time PCR
RNA isolation from TAM samples was carried out according to the standard method using RNeasy Mini Kit (#74004, Qiagen, USA). cDNA synthesis was performed using RevertAid First Strand cDNA Synthesis Kit (#K1621, Thermo Fisher Scientific, USA) with random hexanucleotide primers in accordance with the instructions for the kit. Gene expression was measured using Taqman technology and normalized to housekeeping gene expression for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). 2-∆∆CT algorithm was used to calculate the expression of genes of interest. First, the difference in threshold cycle between the target and reference genes was calculated:
ΔCT = CT(a target gene)-CT(a reference gene).
Second, the difference between the target and reference samples was calculated:
ΔΔCT = ΔCT(a target sample)-ΔCT(a reference sample).
Finally, to work out the gene expression we did 2 to the power of negative ∆∆Ct:
Fold gene expression = 2^-(∆∆Ct).
Primers were designed using Vector NTI Advance 11.5.4 (Vector NTI, RRID:SCR_014265) and NCBI database. The primers were synthesized by DNA Synthesis (Moscow, Russia). qRT-PCR was performed using AriaMx Real-Time PCR thermocycler (RRID:SCR_019469, Agilent Technologies, USA).
The expression of genes that functionally refer to the pro- and antitumor TAM polarization was analyzed: IL1β, CXCL8, IL6, MRC1, CD163, MARCO, CXCL10, CCL2, MMP9, SPP1, SPARC, S100A4, IL10, Stab1, CHID1, CHI3L2, CHI3L1, CCL18, VEGFA, TGFb, SOCS3, CCLR2, and GATA3 (Table 1).
Statistical analysis
Statistical analysis was performed using Statistica 8.0 for Windows and GraphPad Prism 8.4.2. Shapiro– Wilk test was used for normality. The Mann–Whitney test for independent groups was implemented. Results of real-time PCR were presented using GraphPad Prism 8.4.2 software. Results were considered to be significant with p<0.05.
Results
The major aim of the present study was to find significant differences in the phenotype of TAMs activated by the stimuli from the conditioned medium (CM) of different cancer cell lines: breast cancer (luminal A, luminal B, triple-negative breast cancer
Table 1/Таблица 1
Sequences of primers with original design Последовательности праймеров оригинального дизайна
Gene/ Ген
Sequence/Последовательность
Gene/ Ген
Sequence/Последовательность
F 5'- GCCAGCCGAGCCACATC-3'
GAPDH R 5'- GGCAACAATATCCACTTTACCAGA-3'
FAM-CGCCCAATACGACCAAATCCG-BHQ1
F 5'- CTCGCTCAGCCAGATGC -3'
CCL2 R 5'- ACTTGCTGCTGGTGATTCTTC -3'
FAM-CAATGCCCCAGTCACCTGCTGT-BHQ1
F 5'-CCAAGCCAGGTGTCATCCT-3'
CCL18 R 5'-CTTCAGGTCGCTGATGTATTTCT-3'
FAM-CCACTTCTTATTGGGGTCAGCACAGATC -BHQ1 F 5'- GGCACCAGAGGATGAATATGATG-3'
CCRL2 R 5'- GCGTCATACTTGTCACATTGC-3'
FAM-CATAGAAGGTGAACTGGAGAGCGATGAG-BHQ1
F 5'-TAGTGAGTGTGGGCACAAGG -3'
CD163 R 5'-CCGACTGCAATAAAGGATGA-3'
FAM-CACAACAGGTCGCTCATCCCG-BHQ1
F 5'- CCAGGTGGCAAAGAACCA -3'
CHID1 R 5'- TGGGTGAGCATGTGGATGA -3'
FAM-CGATGGCTTCGTGGTGGAGGT-BHQ1
F 5'- CTCAAGAACAGGAACCCCAA -3'
CHI3L1 R 5'- GGCTATCTTGGAAAATCTTTGAG -3'
FAM-TTGTCTGTCGGAGGATGGAACTT-BHQ1
F 5'- AACAACAAGGTTATCATCAAGGAC -3'
CHI3L2 R 5'- TTTGGGATTCTTGGTTTTGAG -3'
FAM-AGTGAAGTGATGCTCTACCAGACCAT-BHQ1 F 5'- CACTGTGTGTAAACATGACTTCC -3'
CXCL8 R 5'- GGTGGAAAGGTTTGGAGTATGT -3'
FAM-CAGTTTTGCCAAGGAGTGCTAAAGAACT-BHQ1 F 5'- AGTGGCATTCAAGGAGTACCT -3'
CXCL10 R 5'- CTAAAGACCTTGGATTAACAGGTT -3'
FAM-TGATGCAGGTACAGCGTACAGTTCTA-BHQ1 F 5'- CCTCATTAAGCCCAAGCGAA -3'
GATA3 R 5'- AGAGTGTGGTTGTGGTGGT -3'
FAM-CAGCCAGGAGAGCAGGGACG-BHQ1
F 5'-TGGCTTATTACAGTGGCAATG-3'
IL1B R 5'-TGGAAGGAGCACTTCATCTC-3'
FAM-CCATCAGCTTCAAAGAACAAGTCA-BHQ1
F 5'- CTGGATTCAATGAGGAGACTTG -3'
IL6 R 5'- GCTTGTTCCTCACTACTCTCAAA -3'
FAM-TCATCACTGGTCTTTTGGAGTTTGA-BHQ1
F 5'- GCTGGAGGACTTTAAGGGTTA -3'
IL10 R 5'- TGTCTGGGTCTTGGTTCTCA -3'
FAM-AGCCTTGTCTGAGATGATCCAGTTT-BHQ1
F 5'- CAGGGAGTGAAAGGATCTTCT -3'
MARCO R 5'- GGACACTGAGTTTTCACCTCTT -3'
FAM-AGCAAGGAGTAAAGGGAGAAAAAGG-BHQ1 F 5'- CTTCCAGTACCGAGAGAAAGC -3'
MMP9 R 5'- GCAGGATGTCATAGGTCACG-3'
FAM-TTCTGCCAGGACCGCTTCTACTG-BHQ1
F 5'- CTGCGACAGTAAACGAGGCT -3'
MRC1 R 5'- TGTTTGAATCGTTGCTGGAGG -3'
FAM-TGCCAGACACGATCCGACCCTTCC-BHQ1
F 5'- GATTCTACTCTGTGCCTCCTGA-3'
SOCS3 R 5'- GCTGAGTATGTGGCTTTCCTATG-3'
FAM-AGAGATTCGCCTTAAATGCTCCCTGTCC-BHQ1 F 5'- ACCTGACATCCAGTACCCTGA -3'
SPP1 R 5'-ACGGCTGTCCCAATCAGAAG-3'
FAM-TGGAAAGCGAGGAGTTGAATGGTGCATACA-BHQ1
F 5'- CTGTGACCTGGACAATGACAA -3'
SPARC R 5'- ATCCTTGTCGATATCTCTGCTTG -3'
FAM-TACATCGCCCTGGATGAGTGGG-BHQ1
F 5'- TCCGCCTCCTGGAATATAAG -3'
STAB1 R 5'- CAGCTCATCCTGCGACAG -3'
FAM-TTCACCATCTTCGTGCCGCA-BHQ1
F 5'- TCTTGGTTTGATCCTGACTGC -3'
S100A4 R 5'- CACCCTCTTTGCCCGAGTA -3'
FAM-CATGGCGTGCCCTCTGGAGAA-BHQ1
F 5'- CCTGGACACCAACTATTGCTT -3'
TGFb R 5'- TCCTTGCGGAAGTCAATGTAC -3'
FAM-TCCACGGAGAAGAACTGCTGCG-BHQ1
F 5’- GAATCATCACGAAGTGGTGAA -3’
VEGFA R 5’- TGGCTTGAAGATGTACTCGAT -3’
FAM-CGAGACCCTGGTGGACATCTT-BHQ1
Note: created by the authors.
Примечание: таблица составлена авторами.
Table 2/Òàблицà 2
Differential expression of macrophage-related genes in models of human ex vivo TAMs
Äиффåðåнциàльнàÿ эêñпðåññиÿ гåнîв в мîäåльныõ ÎÀΜ чåлîвåêà ex vivo
|
Genes |
MCF-7 |
BT-474 |
MDA-MB-231 |
SKBR-3 |
SW-837 |
SKOV-3 |
PC-3 |
A-549 |
|
Inflammatory cytokines/Воспалительные цитокины |
||||||||
|
Il1b |
0.0708 |
0.6829 p=0.0185 |
1.1886 p=0.0264 |
0.7584 p=0.0185 |
0.0301 |
0.0556 |
0.304 p=0.0433 |
0.7093 |
|
IL6 |
0.642 |
4.018 |
15.284 p=0.0653 |
15.354 |
-5.558 |
1.058 |
2.651 |
11.284 p=0.0947 |
|
CXCL8 |
0.0891 |
0.184 |
0.5903 |
0.9206 p=0.0052 |
0.5997 p=0.0753 |
0.2799 p=0.0524 |
0.5866 |
0.7385 |
|
IL10 |
0.2717 |
0.0953 |
0.868 p=0.035 |
0.2802 |
-0.3514 |
-0.228 |
0.2129 |
-0.0962 |
|
Macrophage recruitment and polarization/Рекрутирование и поляризация макрофагов |
||||||||
|
CCL2 |
3.561 |
2.52 |
6.063 p=0.0435 |
8.249 p=0.0355 |
1.008 |
1.369 |
10.849 p=0.0115 |
5.981 p=0.0101 |
|
CCRL2 |
0.0668 |
-0.0964 |
-0.1146 |
0.4802 |
-0.046 |
0.2832 p=0.0892 |
0.1275 |
-0.0833 |
|
GATA3 |
0.3042 |
-0.0948 |
2.0198 p=0.0814 |
0.5335 |
0.1705 |
-0.1682 |
0.2665 |
0.6828 |
|
SOCS3 |
0.05546 |
0.19895 |
0.24187 |
0.21025 |
-0.03503 |
0.00763 |
0.02615 |
0.01563 |
|
CXCL10 |
-2.3641 |
0.5281 |
-2.1778 |
9.3161 p=0.043 |
-0.1853 |
0.9031 |
3.6161 p=0.0748 |
-2.674 |
|
CHID1 |
0.1138 |
-0.0456 |
-0.10158 |
-0.017 |
-0.0513 |
-0.0697 |
0.0218 |
-0.13091 |
|
Scavenger receptors/Скавенджер-рецепторы |
||||||||
|
STAB1 |
-0.0851 |
0.1487 |
-0.0062 |
0.0135 |
-0.2077 |
-0.3165 |
0.7053 |
-0.1652 |
|
CD163 |
-0.0022 |
0.469 |
0.4436 |
-0.1097 |
-0.1522 |
-0.1923 |
0.347 |
0.1672 |
|
MRC1 |
-0.718 |
0.053 |
0.709 |
-0.122 |
-0.753 |
-0.393 |
1.029 p=0.0787 |
-0.517 |
|
MARCO |
0.09016 |
0.2859 |
0.46816 |
0.0458 |
-0.1616 |
-0.002 |
0.244 |
0.00527 |
|
Angiogenesis-regulating proteins/Белки-регуляторы ангиогенеза |
||||||||
|
SPP1 |
-0.1136 |
-0.2487 |
-0.2316 |
-0.6193 |
1.8967 |
2.269 |
0.025 |
-0.2781 |
|
SPARC |
-0.1954 |
-0.1845 |
-0.381 |
-0.3367 |
0.5231 |
0.0771 |
-0.3005 |
-0.3865 |
|
p=0.0856 |
p=0.001 |
p=0.0719 |
p=0.0753 |
p=0.022 |
p=0.0056 |
|||
|
S100A4 |
-0.6318 |
-0.3758 |
-0.6588 |
-0.282 |
-0.1381 |
-0.2738 |
-0.3761 |
-0.627 |
|
p=0.0435 |
p=0.0606 |
p=0.0041 |
p=0.0504 |
p=0.0416 |
p=0.0122 |
p=0.0764 |
||
|
VEGFA |
-0.1784 |
0.1331 |
-0.0798 |
0.4883 p=0.0892 |
0.3102 p=0.0753 |
-0.018 |
0.0415 |
0.2266 |
|
CHI3L1 |
0.0719 |
-0.2876 |
-0.0873 |
-0.2283 |
0.0471 |
-0.2348 |
-0.38 |
-0.2332 |
|
CHI3L2 |
-0.1279 |
0.2445 |
0.3945 |
0.2328 |
-0.1195 |
0.08 |
0.0608 |
0.0507 |
|
Matrix remodeling and invasion/Ремоделирование матрикса |
и инвазия |
|||||||
|
MMP9 |
-0.0091 |
0.2479 |
0.3284 |
0.5292 p=0.0892 |
0.0717 |
0.2582 |
0.2861 |
-0.0266 |
|
TGFb |
-0.0806 |
0.117 |
-0.1939 |
0.0345 |
-0.1822 |
-0.0232 |
-0.0405 |
-0.2692 |
|
CCL18 |
1.251 |
4.623 |
4.593 |
-2.241 |
-0.182 |
-2.633 |
-2.269 |
4.122 |
Notes: fold changes are indicated for all stimulations in comparison with control; statistically significant changes are marked in bold; values with minus indicate decreased expression compared to control, values without minus – increased expression; created by the authors.
Примечания: кратность изменений для всех стимуляций указана по сравнению с контролем; значимые различия выделены жирным шрифтом; значения с минусом указывают на снижение экспрессии по сравнению с контролем, без минуса – на повышение; таблица составлена авторами.
[TNBC], and HER2-positive subtypes), colorectal cancer, prostate cancer, lung cancer, and ovarian cancer. The comparative analysis was performed in groups of CM-stimulated TAMs versus TAMs stimulated by empty media.
The most activated state was observed in TAMs modeled for breast cancer. Statistically significant up-regulation of' pro-inflammatory cytokines IL1b and
CXCL8 as well as anti-inflammatory cytokine IL10 was found in breast cancer-modeled TAMs compared to control (Table 2, Fig. 1). The mRNA expression of pro-migratory factors CCL2 and CXCL10 were elevated in modeled TAMs (Table 2, Fig. 2). In contrast, downregulation of angiogenesis-regulating genes SPARC and S100A4 was detected in breast cancer TAMs compared to control media (Table 2, Fig. 1 supplementary).
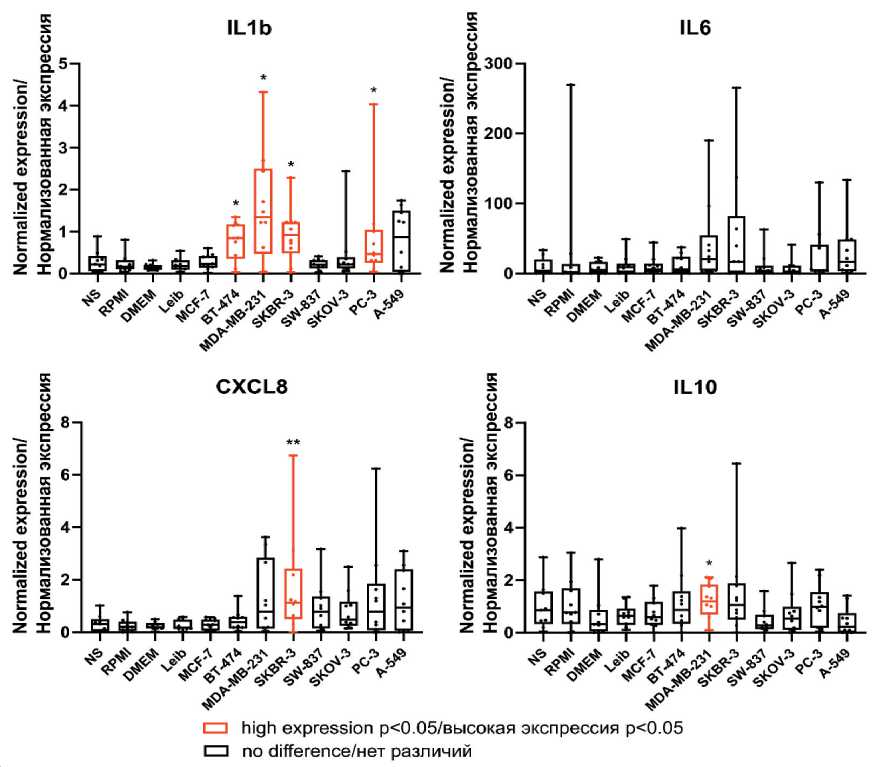
Fig. 1. The mRNA expression of inflammation-related genes in human modeled TAMs.
Notes: * – p<0.05, ** – p<0.01; created by the authors
Рис. 1. Экспрессия мРНК факторов, связанных с воспалением, в модельных ОАМ человека. Примечания: * – р<0,05, ** – р<0,01; диаграммы выполнены авторами
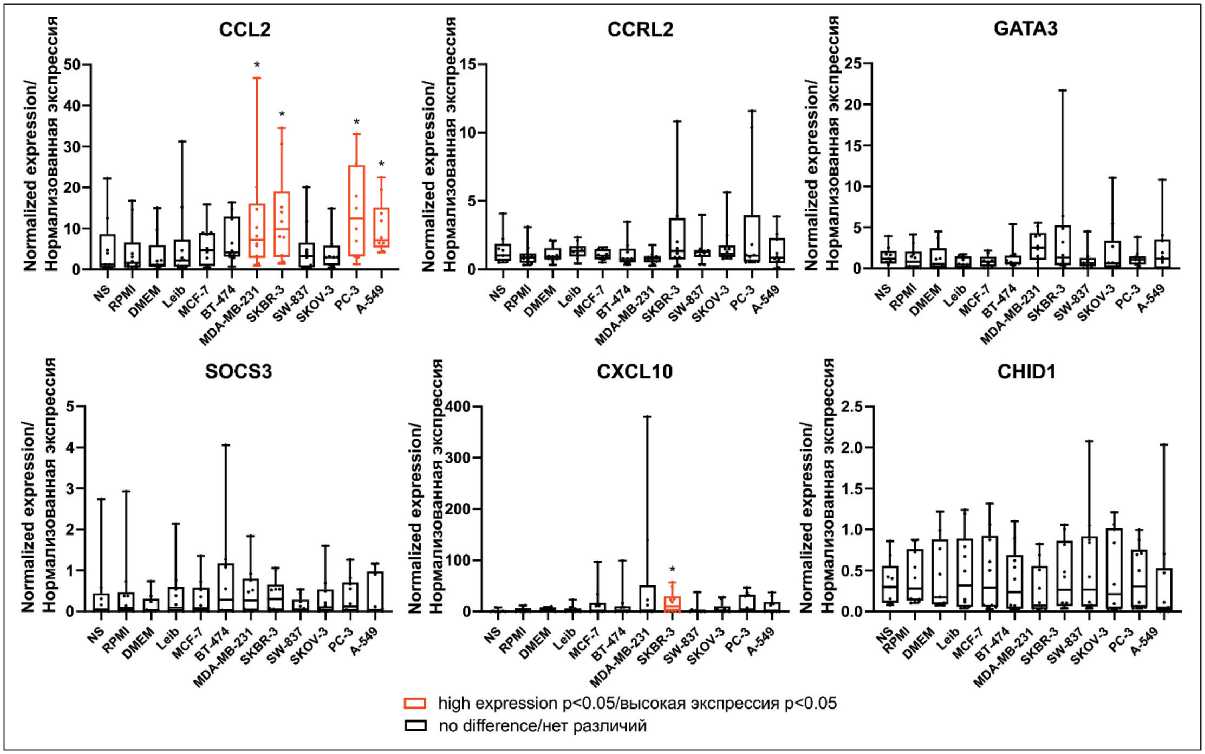
Fig. 2. The mRNA expression of pro-migratory factors in human modeled TAMs. Notes: * – p<0.05; created by the authors
Рис. 2. Экспрессия мРНК про-миграционных факторов в модельных ОАМ человека. Примечания: * – р<0,05; диаграммы выполнены авторами
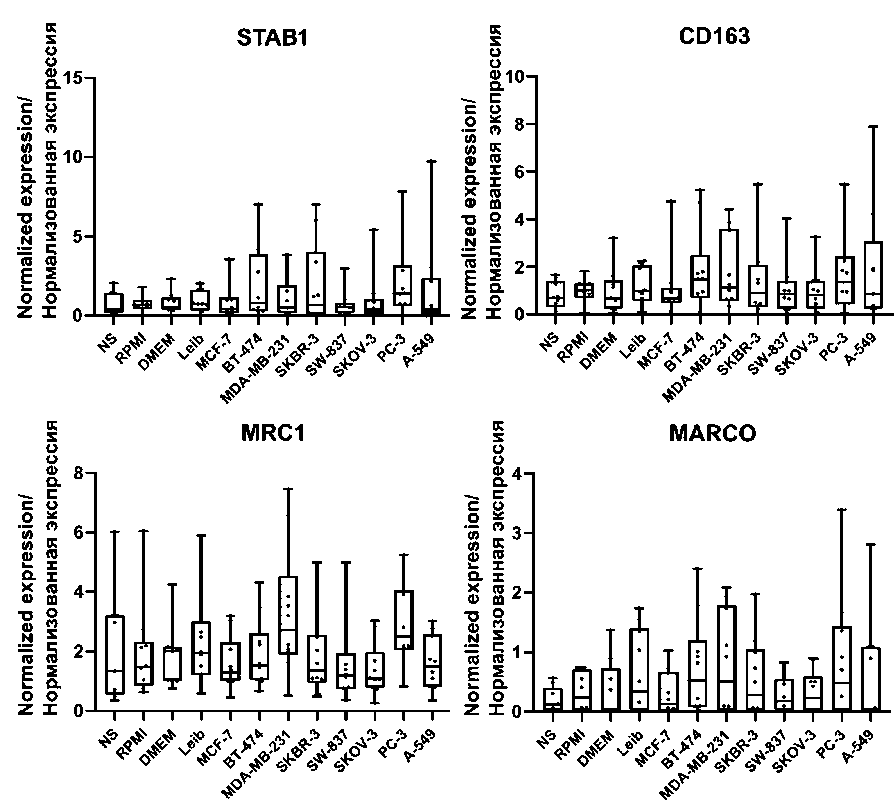
Fig. 3. The mRNA expression of scavenger receptors in human modeled TAMs.
Note: created by the authors
Рис. 3. Экспрессия мРНК скавенджер-рецепторов в модельных ОАМ человека. Примечание: диаграммы выполнены авторами
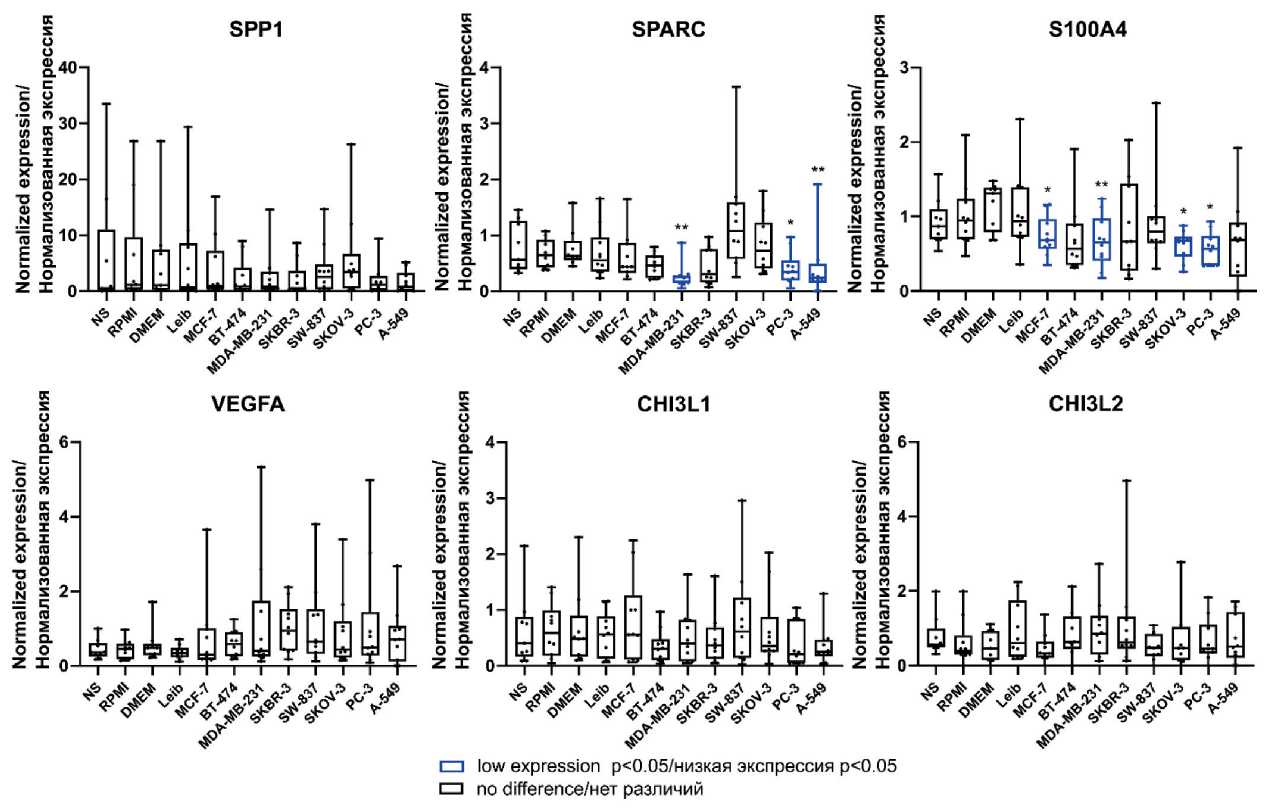
Fig. 1 supplementary. The mRNA expression of angiogenesis-regulating genes in human modeled TAMs.
Notes: * – p<0.05, ** – p<0.01; created by the authors
Рис. 1, дополнительный. Экспрессия мРНК факторов, регулирующих ангиогенез, в модельных ОАМ человека. Примечания: * – р<0,05, ** – р<0,01; диаграммы выполнены авторами
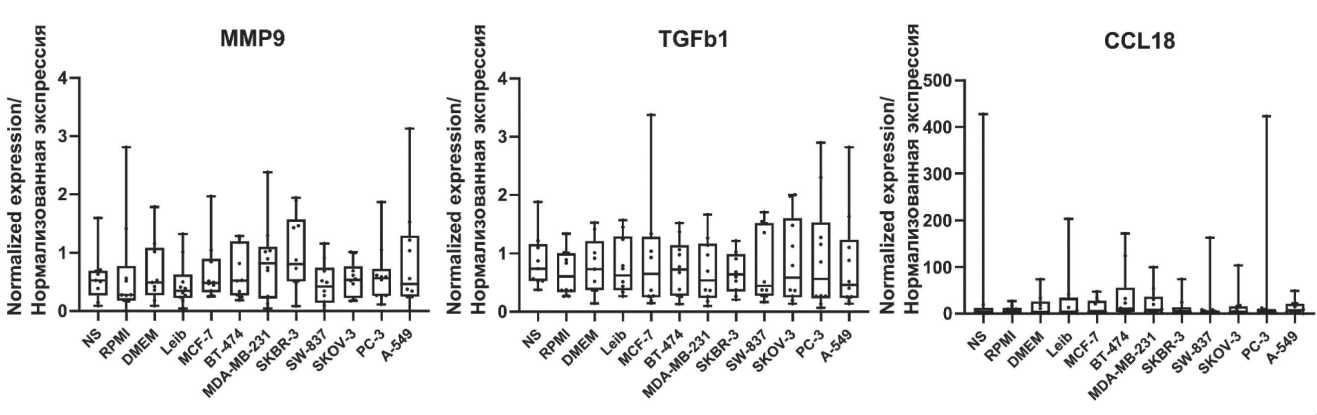
Fig. 2 supplementary. The mRNA expression of matrix remodeling and invasion factors in human modeled TAMs.
Note: created by the authors
Рис. 2, дополнительный. Экспрессия мРНК факторов ремоделирования матрикса и инвазии в модельных ОАМ человека. Примечание: диаграммы выполнены авторами
The elevated expression of IL1b and CCL2 was observed in TAMs stimulated by CM of HER2-positive (SKBR-3) (0.920 [0.480; 1.226] vs 0.162 [0.102; 0.318], p=0.018 for IL1b and 9.870 [3.012; 19.04] vs 1.621 [0.526; 6.573], p=0.035 for CCL2 ) as well as triple-negative (MDA-MB-231) (1.342 [0.472; 2.501] vs 0.1534 [0.083; 0.208], p=0.026 for IL1b and 7.208 [2.811; 16.07] vs 1.145 [0.638; 5.957], p=0.043 for CCL2 ) cell lines, correspondingly, but not of hormonedependent luminal subtypes (Table 2, Fig. 1, Fig. 2). Accumulating evidence demonstrated the differences in triple-negative breast cancer (TNBC), or basal-like subtype, compared to luminal subtypes [17, 18]. In our model, CCL2 expression was also elevated in TAMs of non-luminal subtypes. Up-regulation of pro-inflammatory cytokines CXCL8 (1.122 [0.497; 2.421] vs 0.201 [0.051; 0.404], p=0.005) and pro-migratory factor CXCL10 (9.913 [0.826; 30.06] vs 0.596 [0; 6.626], p=0.043) was detected in TAMs stimulated by CM of HER2-positive (SKBR-3) cell lines, but up-regulation of anti-inflammatory cytokine IL-10 (1.190 [0.694; 1.846] vs 0.322 [0.057; 0.877], p=0.035) was observed only in TAMs stimulated by CM of triple-negative (MDA-MB-231) cell lines (Table 2, Fig. 1, Fig. 2). In addition, in our model, angiogenesis-regulating genes ( SPARC, S100A4 ) [19] were downregulated in TAMs stimulated by CM of luminal A (MCF-7) (0.683 [0.569; 0.967] vs 1.315 [0.794; 1.385], p=0.043 for S100A4 ) and triplenegative (MDA-MB-231) cell lines (0.251 [0.146; 0.285] vs 0.632 [0.556; 0.902], p=0.001 for SPARC and 0.656 [0.406; 0.979] vs 1.315 [0.794; 1.385], p=0.004 for S100A4 ) (Table 2, Fig. 1 supplementary).
Similar to breast cancer-stimulated macrophages, the expression of IL1b (0.466 [0.258; 1.044] vs 0.162 [0.102; 0.318], p=0.043), CCL2 (12.47 [3.164; 25.44] vs 1.621 [0.526; 6.573], p=0.011), SPARC (0.345 [0.190; 0.555] vs 0.645 [0.427; 0.921], p=0.022), S100A4 (0.570 [0.343; 0.741] vs 0.9463 [0.695; 1.241], p=0.012) was statistically significant differed in TAMs of prostate cancer, compared to the control macrophages (Table 2, Fig. 1, Fig. 2, Fig. 1 supplementary).
In modeled lung cancer-associated TAMs, CCL2 was also upregulated compared (7.126 [5.382; 15.14] vs 1.145 [0.638; 5.957], p=0.010) to control media-stimulated TAMs (Table 2, Fig. 2). CCL2-CCR2 axis, which is a prominent activator of monocyte/macrophage recruitment, facilitate lung cancer progression [20, 21]. In addition, S100A4 was downregulated (0.688 [0.194; 0.924] vs 1.315 [0.794; 1.385], p=0.005) in TAMs stimulated by CM of lung cancer cell line (A-549) (Table 2, Fig. 1 supplementary).
However, tumor-induced macrophages in our model didn`t show any changes in the expression of general markers of TAMs, that belong to scavengerreceptors ( CD163, MRC1, MARCO, Stab1 ) [22] (Table 2, Fig. 3) and markers associated with matrix remodeling (Table 2, Fig. 2 supplementary).
Discussion
It is well known that the TME can determine the polarization state of TAMs [4, 23]. M1/M2 paradigm has existed for more than 30 years and it reflects two extreme states of macrophage polarization – pro-inflammatory M1 and anti-inflammatory M2 [24]. In the context of tumor microenvironment, these states were defined as anti-tumor and pro-tumor, consequently [2, 24]. Recently, with the introduction of many advanced technological methods such as singlecell and spatial analysis we can look deeper into tumor composition to dissect multiple cell phenotypes with a distinct functional activity [25, 26]. Following that, a large diversity of dynamic cell subpopulations within a tumor has been revealed. Most recently collected single-cell data suggest that up to 10 subsets of TAMs may be distinguished, depending on the cancer type and localization [27, 28]. These studies allowed identifying new macrophage markers. Interestingly, generally accepted markers of scavenger-receptor macrophages [2] are not always in the top genes of TAM subsets, or aren’t even present in the gene signature [29]. Nevertheless, researchers continue to use them in many in vitro and in vivo studies as differential markers of M1 and M2 macrophages.
Well-known existing in vitro models for human macrophage analysis include either stable macrophage cell lines (e.g. THP-1 cells) or monocyte-derived macrophages [30]. However, most of the methods are based on the use of limited differentiation and polarization factors which determine the monocyte to macrophage transition [31]. It becomes clear that macrophages obtained in vitro do not reflect that intricate phenotypic diversity which develops under the influence of tumor environment [2, 12]. This can explain the limitations of existing macrophage targeting and inability of multiple approved clinical trials to overcome therapeutic failure [32, 33]. Thus, translatable and reproducible in vitro models for human macrophages are under intensive investigation [31].
In our study, we used an experimental ex vivo model of TAMs, which, in our opinion, can most accurately reflect the influence of tumor factors on macrophages. For this purpose, we treated human monocyte-derived macrophages with M2-polarizing cytokines and conditioned tumor supernatants. Generation of monocyte-derived TAMs subjected to the tumor-conditioned media presented more features that are similar to in vivo TAMs, compared to macrophages generated by cytokine stimulation alone [34]. The main question we raised was whether this model can adequately reflect the diversity and real functional activity of TAMs in the TME. The most pronounced up-regulation of macrophage-associated markers was detected in breast cancer TAMs. Accumulating evidence demonstrated the differences in triple-negative breast cancer (TNBC), or basal-like subtype, compared to luminal subtypes [17, 18]. Basal-like breast cancer cells determine more prominent differentiation of THP-1 cells compared to luminal cancer cells [35]. In vitro stimulation of macrophages with basal-like cells induced mixed M1/M2 phenotype [35] that is similar to observations made by us. However, gene expression analysis did not show differences in the expression of the main markers including scavengerreceptors ( CD163, MRC1, MARCO, Stab1 ) or markers associated with matrix remodeling, although, elevated expression of these markers was observed in human tumor tissue TAMs and was significantly correlated with outcome of cancer patients [2, 19, 36].
Different studies show the variety among the induced phenotypes of TAMs. This depends on both the type of the model system and the differentiating and polarizing factors that are used to skewer macrophages into a pro-tumor phenotype. For instance, authors revealed that IL-34 and CXCL4 have similar functions to M-CSF and activate the same pathway for differentiation of macrophages in vitro [31]. Monocyte-derived macrophages stimulated by IL-34 acquire macrophage-like morphology and express CD45 and CD68. But, IL-34-treated macrophages show increased phagocytic capacity and higher IL-10
and CCL-17 production after stimulation, and CXCL4-activated macrophages have more pro-inflammatory features compared to M-CSF-stimulated ones [31]. Authors of another study determined that CD40 and CD64 are the most distinctive makers for human M1 macrophage activation and CD163 and MR for M2 macrophage activation ex vivo [37]. They also revealed that adherent and floating macrophages could have different activation state. Thus, the expression of MR and CD40 was indicative for adherent cells compared to the floating cells. Distinct activation states of human monocyte-derived macrophages were found when they matured with M-CSF or GM-CSF [37]. Monocyte-derived macrophages (MDMs) differentiated under serum-free conditions displayed greater phagocytic properties and tumor promoting activity for M2 macrophages in contrast to MDMs differentiated in the presence of serum [38]. Monocyte isolation method can also affect the phenotype of resulting macrophages ex vivo [39]. Expression of CD163 and CD14 were significantly lower on monocytes isolated by CD14positive selection and by simple plastic adhesion, compared to untouched peripheral blood mononuclear cells. MDMs from plastic adhesion were M1-skewed (CD80high HLA-DRhigh CD163low), whereas MDMs obtained by negative selection were M2-skewed (CD80low HLA-DRlow CD163high) [39].
Moreover, in the majority of studies insufficient attention is given to the components of culture media used for cancer cell passaging that can contain diverse growth factors affecting macrophage phenotype. In our ex vivo models, we provide the necessary control and clearly differentiate between the effects of empty cancer cell culture medium and conditioned cancer cell culture medium.
It becomes clear that traditional in vitro models cannot reproduce the genetic, phenotypical and functional features of macrophage environment in vivo . In recent review, authors provided the idea of new model systems where macrophages are first generated according to their ontogenetic identity and then induced to obtain their tissue-specific entity. One of such methods is generating macrophages from induced pluripotent stem cells (iPSCs) [30]. The use of 3D organotypic tumor models combining components of tumor microenvironment including macrophages has also become more popular [40, 41]. Authors of recent study developed 3D multicellular tumor spheroid (MCTS) model comprising of cancer cells, fibroblasts, and human monocyte-derived macrophages [42]. They demonstrated the importance of TAM-containing models in drug screening of anti-cancer immunotherapies targeting macrophages [42].
Conclusion
In summary, monocyte-derived macrophages stimulated with cancer cell-conditioned medium can, to a certain extent, allow modeling of cancer-specific programming of TAMs. This model enables analysis of the differential expression of genes that are involved in the major regulatory activities of TAMs that support primary tumor growth and its metastatic potential. These gene groups cover inflammatory cytokines, regulators of macrophage recruitment and polarization, scavenger receptors, regulators of angiogenesis and extracellular matrix remodeling, as well as cancer cell invasion. Our model system is valuable to examine agents reprogramming key TAM pro-tumoral activities, and for the reproducible analysis of mechanistic events that program tolerogenic status of TAMs towards
Список литературы Biomarkers for modeling of cancer-specific tumor-associated macrophages ex vivo
- Malekghasemi S., Majidi J., Baghbanzadeh A., Abdolalizadeh J., Baradaran B., Aghebati-Maleki L. Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment. Adv Pharm Bull. 2020; 10(4): 556-65. https://doi.org/10.34172/apb.2020.066.
- Larionova I., Tuguzbaeva G., Ponomaryova A., Stakheyeva M., Cherdyntseva N., Pavlov V., Choinzonov E., Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front Oncol. 2020; 10. https://doi.org/10.3389/fonc.2020.566511.
- Munir M.T., Kay M.K., Kang M.H., Rahman M.M., Al-Harrasi A., Choudhury M., Moustaid-Moussa N., Hussain F., Rahman S.M. TumorAssociated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int J Mol Sci. 2021; 22(12): 6526. https://doi.org/10.3390/ijms22126526.
- Boutilier A.J., Elsawa S.F. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021; 22(13): 6995. https://doi.org/10.3390/ijms22136995.
- Wu K., Lin K., Li X., Yuan X., Xu P., Ni P., Xu D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol. 2020; 11. https://doi.org/10.3389/fimmu.2020.01731.
- Monteiro L.N., Rodrigues M.A., Gomes D.A., Salgado B.S., Cassali G.D. Tumour-associated macrophages: Relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Vet J. 2018; 234: 119-25. https://doi.org/10.1016/j.tvjl.2018.02.016.
- Hwang I., Kim J.W., Ylaya K., Chung E.J., Kitano H., Perry C., Hanaoka J., Fukuoka J., Chung J.Y., Hewitt S.M. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020; 18(1): 443. https://doi.org/10.1186/s12967-020-02618-z.
- Zheng X., Weigert A., Reu S., Guenther S., Mansouri S., Bassaly B., Gattenlöhner S., Grimminger F., Pullamsetti S., Seeger W., Winter H., Savai R. Spatial Density and Distribution of Tumor-Associated Macrophages Predict Survival in Non-Small Cell Lung Carcinoma. Cancer Res. 2020; 80(20): 4414-25. https://doi.org/10.1158/0008-5472.CAN-20-0069.
- Wei C., Yang C., Wang S., Shi D., Zhang C., Lin X., Liu Q., Dou R., Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019; 18(1): 64. https://doi.org/10.1186/s12943-019-0976-4.
- Tan Q., Liu H., Xu J., Mo Y., Dai F. Integrated analysis of tumorassociated macrophage infiltration and prognosis in ovarian cancer. Aging (Albany NY). 2021; 13(19): 23210-32. https://doi.org/10.18632/aging.203613.
- Genin M., Clement F., Fattaccioli A., Raes M., Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015; 15(1): 577. https://doi.org/10.1186/s12885-015-1546-9.
- Cassetta L., Pollard J.W. Tumor-associated macrophages. Curr Biol. 2020; 30(6): 246-8. https://doi.org/10.1016/j.cub.2020.01.031.
- Mulder K., Patel A.A., Kong W.T., Piot C., Halitzki E., Dunsmore G., Khalilnezhad S., Irac S.E., Dubuisson A., Chevrier M., Zhang X.M., Tam J.K.C., Lim T.K.H., Wong R.M.M., Pai R., Khalil A.I.S., Chow P.K.H., Wu S.Z., Al-Eryani G., Roden D., Swarbrick A., Chan J.K.Y., Albani S., Derosa L., Zitvogel L., Sharma A., Chen J., Silvin A., Bertoletti A., Blériot C., Dutertre C.A., Ginhoux F. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity. 2021; 54(8): 1883-900. https://doi.org/10.1016/j.immuni.2021.07.007.
- Ma R.Y., Black A., Qian B.Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022; 43(7): 546-63. https://doi.org/10.1016/j.it.2022.04.008.
- Dai X., Cheng H., Bai Z., Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017; 8(16): 3131-41. https://doi.org/10.7150/jca.18457.
- Larionova I., Kiselev A., Kazakova E., Liu T., Patysheva M., Iamshchikov P., Liu Q, Mossel D.M., Riabov V., Rakina M., Sergushichev A., cancer cells. However, in order to achieve the full specificity of TAM phenotypes 3D modelling is needed to create the most physiologically relevant context for macrophage interactions with the extracellular matrix, cancer cells and other cells of tumor microenvironment. In this regard, the rapidly developing field of organoids is highly promising direction which will allow to recreate three-dimensional multicellular composition of tumor tissue, and also to model not only cancerspecific but also patient-specific TAM phenotypes and study their functions. Bezgodova N., Vtorushin S., Litviakov N., Denisov E., Koshkin P., Pyankov D., Tsyganov M., Ibragimova M., Cherdyntseva N., Kzhyshkowska J. Tumor-associated macrophages respond to chemotherapy by detrimental transcriptional reprogramming and suppressing stabilin-1 mediated clearance of EGF. Front Immunol. 2023; 14. https://doi.org/10.3389/fimmu.2023.1000497.
- Sun N., Gao P., Li Y., Yan Z., Peng Z., Zhang Y., Han F., Qi X. Screening and Identification of Key Common and Specific Genes and Their Prognostic Roles in Different Molecular Subtypes of Breast Cancer. Front Mol Biosci. 2021; 8. https://doi.org/10.3389/fmolb.2021.619110.
- Hollmén M., Roudnicky F., Karaman S., Detmar M. Characterization of macrophage - cancer cell crosstalk in estrogen receptor positive and triplenegative breast cancer. Sci Rep. 2015; 5(1): 9188. https://doi.org/10.1038/srep09188.
- Kazakova E., Rakina M., Sudarskikh T., Iamshchikov P., Tarasova A., Tashireva L., Afanasiev S., Dobrodeev A., Zhuikova L., Cherdyntseva N., Kzhyshkowska J., Larionova I. Angiogenesis regulators S100A4, SPARC and SPP1 correlate with macrophage infiltration and are prognostic biomarkers in colon and rectal cancers. Front Oncol. 2023; 13. https://doi.org/10.3389/fonc.2023.1058337.
- Roblek M., Protsyuk D., Becker P.F., Stefanescu C., Gorzelanny C., Glaus Garzon J.F., Knopfova L., Heikenwalder M., Luckow B., Schneider S.W., Borsig L. CCL2 Is a Vascular Permeability Factor Inducing CCR2- Dependent Endothelial Retraction during Lung Metastasis. Mol Cancer Res. 2019; 17(3): 783-93. https://doi.org/10.1158/1541-7786.MCR-18-0530.
- Schmall A., Al-Tamari H.M., Herold S., Kampschulte M., Weigert A., Wietelmann A., Vipotnik N., Grimminger F., Seeger W., Pullamsetti S.S., Savai R. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med. 2015; 191(4): 437-47. https://doi.org/10.1164/rccm.201406-1137OC.
- Kazakova E., Iamshchikov P., Larionova I., Kzhyshkowska J. Macrophage scavenger receptors: Tumor support and tumor inhibition. Front Oncol. 2023; 12. https://doi.org/10.3389/fonc.2022.1096897.
- Larionova I., Kazakova E., Patysheva M., Kzhyshkowska J. Transcriptional, Epigenetic and Metabolic Programming of Tumor-Associated Macrophages. Cancers (Basel). 2020; 12(6): 1411. https://doi.org/10.3390/cancers12061411.
- Hourani T., Holden J.A., Li W., Lenzo J.C., Hadjigol S., O’BrienSimpson N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front Oncol. 2021; 11. https://doi.org/10.3389/fonc.2021.788365.
- Li X., Wang C.Y. From bulk, single-cell to spatial RNA sequencing. Int J Oral Sci. 2021; 13(1): 36. https://doi.org/10.1038/s41368-021-00146-0.
- Longo S.K., Guo M.G., Ji A.L., Khavari P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat Rev Genet. 2021; 22(10): 627-44. https://doi.org/10.1038/s41576-021-00370-8.
- Liu Z., Gao Z., Li B., Li J., Ou Y., Yu X., Zhang Z., Liu S., Fu X., Jin H., Wu J., Sun S., Sun S., Wu Q. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology. 2022; 11(1). https://doi.org/10.1080/2162402X.2022.2085432.
- Lin C., Yang H., Zhao W., Wang W. CTSB+ macrophage repress memory immune hub in the liver metastasis site of colorectal cancer patient revealed by multi-omics analysis. Biochem Biophys Res Commun. 2022; 626: 8-14. https://doi.org/10.1016/j.bbrc.2022.06.037.
- Wu S.Z., Al-Eryani G., Roden D.L., Junankar S., Harvey K., Andersson A., Thennavan A., Wang C., Torpy J.R., Bartonicek N., Wang T., Larsson L., Kaczorowski D., Weisenfeld N.I., Uytingco C.R., Chew J.G., Bent Z.W., Chan C.L., Gnanasambandapillai V., Dutertre C.A., Gluch L., Hui M.N., Beith J., Parker A., Robbins E., Segara D., Cooper C., Mak C., Chan B., Warrier S., Ginhoux F., Millar E., Powell J.E., Williams S.R., Liu X.S., O’Toole S., Lim E., Lundeberg J., Perou C.M., Swarbrick A. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021; 53(9): 1334-47. https://doi.org/10.1038/s41588-021-00911-1.
- Lee C.Z.W., Kozaki T., Ginhoux F. Studying tissue macrophages in vitro: are iPSC-derived cells the answer? Nat Rev Immunol. 2018; 18(11): 716-25. https://doi.org/10.1038/s41577-018-0054-y. Erratum in: Nat Rev Immunol. 2018; 18(11): 726. https://doi.org/10.1038/s41577-018-0060-0.
- Luque-Martin R., Mander P.K., Leenen P.J.M., Winther M.P.J. Classic and new mediators for in vitro modelling of human macrophages. J Leukoc Biol. 2021; 109(3): 549-60. https://doi.org/10.1002/JLB.1RU0620-018R.
- Lopez-Yrigoyen M., Cassetta L., Pollard J.W. Macrophage targeting in cancer. Ann N Y Acad Sci. 2021; 1499(1): 18-41. https://doi.org/10.1111/nyas.14377.
- Wang S., Yang Y., Ma P., Huang H., Tang Q., Miao H., Fang Y., Jiang N., Li Y., Zhu Q., Tao W., Zha Y., Li N. Landscape and perspectives of macrophage-targeted cancer therapy in clinical trials. Mol Ther Oncolytics. 2022; 24: 799-813. https://doi.org/10.1016/j.omto.2022.02.019.
- Benner B., Scarberry L., Suarez-Kelly L.P., Duggan M.C., Campbell A.R., Smith E., Lapurga G., Jiang K., Butchar J.P., Tridandapani S., Howard J.H., Baiocchi R.A., Mace T.A., Carson W.E. 3rd. Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J Immunother Cancer. 2019; 7(1): 140. https://doi.org/10.1186/s40425-019-0622-0.
- Stewart D.A., Yang Y., Makowski L., Troester M.A. Basal-like breast cancer cells induce phenotypic and genomic changes in macrophages. Mol Cancer Res. 2012; 10(6): 727-38. https://doi.org/10.1158/1541-7786.MCR11-0604.
- Larionova I., Kazakova E., Gerashchenko T., Kzhyshkowska J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers (Basel). 2021; 13(13): 3253. https://doi.org/10.3390/cancers13133253.
- Vogel D.Y., Glim J.E., Stavenuiter A.W., Breur M., Heijnen P., Amor S., Dijkstra C.D., Beelen R.H. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014; 219(9): 695-703. https://doi.org/10.1016/j.imbio.2014.05.002.
- Rey-Giraud F., Hafner M., Ries C.H. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS One. 2012; 7(8). https://doi.org/10.1371/journal.pone.0042656.
- Nielsen M.C., Andersen M.N., Møller H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology. 2020; 159(1): 63-74. https://doi.org/10.1111/imm.13125.
- Golabek A., Kaczmarek M., Dondajewska E., Sakrajda K., Mackiewicz A., Dams-Kozlowska H. Application of a three-dimensional (3D) breast cancer model to study macrophage polarization. Exp Ther Med. 2021; 21(5): 482. https://doi.org/10.3892/etm.2021.9913.
- Rebelo S.P., Pinto C., Martins T.R., Harrer N., Estrada M.F., Loza-Alvarez P., Cabeçadas J., Alves P.M., Gualda E.J., Sommergruber W., Brito C. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials. 2018; 163: 185-97. https://doi.org/10.1016/j.biomaterials.2018.02.030.
- Helleberg Madsen N., Schnack Nielsen B., Larsen J., Gad M. In vitro 2D and 3D cancer models to evaluate compounds that modulate macrophage polarization. Cell Immunol. 2022; 378. https://doi.org/10.1016/j.cellimm.2022.104574.


