Ca2+ induced lipid peroxidation accentuates aging in rat brain mitochondria
Автор: Mohan Kumar B. S., Mahaboob Basha P.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.21, 2025 года.
Бесплатный доступ
Aging in rats is multifactorial and include decline in biological, physiological, psychological and social changes. The antioxidant imbalance is one of the commonest factors during aging and age-related diseases. Several studies have identified the participation of various antioxidant’s differential activity levels in the brain. However, Ca2+ is known to alter the homeostatic balance of these antioxidants. Lipid peroxidation is the chief marker of membrane architecture disruption in the aging and neurodegenerative diseases. In the current study, we have focused on the contribution of Ca2+ participation, its concentration and effect on the lipid peroxidation at various stages of rat’s life. We have observed that LPO induction is increased in mitochondria isolated from aged rats in comparison to young adult and neonatal rats. Treatment of 100 to 300uM Ca2+ has shown a steady increase in lipid peroxidation of up to 20% in mitochondria isolated from aged rats while neonates and young rats have displayed differential levels of peroxidation from 17% to 15%. Thus, alleviating role of Ca2+ in lipid peroxidation in aging and neurodegeneration.
Lipid peroxidation, calcium, aging, rat brain, mitochondria
Короткий адрес: https://sciup.org/143183773
IDR: 143183773
Текст научной статьи Ca2+ induced lipid peroxidation accentuates aging in rat brain mitochondria
Aging has been ascribed to changes at molecular intersections and impairments due to free radical generation (Harman, 1956), alterations in immunity (Hong et al. , 2019; Martínez de Toda et al. , 2019), telomere shortening (López-Otín et al. , 2013) and the presence of genes that play a vital role in senescence (Payne & Chinnery, 2015). More recently, however, a single amalgamated theory that emphasizes gene repair and maintenance systems, and their surrounding is becoming more and more accepted (Rattan, 2006), stressing upon the need for a holistic, cumulative and logical analysis of senescence. However, massive research integration on aging and aging-related processes should be able to address it systematically and simplify theories on aging. More so, as commonly found by many researchers around the globe, one mechanism that is thought to contribute to this age-associated degeneration is the production of free radicals (Cui et al. , 2012; Sanz, 2016; Stepien et al. , 2017). Reactive oxygen species (ROS) are generated at multiple compartments within the cell and as accidental byproducts of multiple enzymes. Significant contributions include proteins that are localized on the plasma membrane, such as a family of oxidases, as well as the activity of various cytosolic enzymes such as cyclo-oxygenase. Peroxisomal lipid metabolism results in ROS release too. Although all these sources add up to the overall oxidative load, mitochondria still occupy the central position producing approximately 90% of total cellular ROS.
An increase in free radical generation usually worsens the mitochondrial physiology, partly via lipid peroxidation, respiratory chain protein oxidation, and mitochondrial DNA damage. Additionally, damaged mitochondrial membranes would shift the cellular metabolism towards glycolysis signifying the tight interplay between bioenergetics, metabolism and aging (Silva et al. , 2013).
MATERIALS AND METHODS
MATERIALS
Ethylene glycol-bis-(-aminoethyl ether) N, N, N1, N1 tetraacetic acid (EGTA), calcium chloride, sodium succinate, N-2-hydroxy ethyl piperazine-n-2-ethane sulfonic acid (HEPES), and dimethyl sulfoxide and epinephrine were obtained from Sigma Chemicals. Other chemicals of analytical grade were obtained from BDH Industries Ltd. and Glaxo Laboratories, Mumbai, India.
Sprague Dawley albino rats were procured from Sri Raghavendra Enterprises, Bangalore, and acclimatized to laboratory conditions (12 hrs dark/light, 28±2oC). Animals were let free to feed on standard food (Amruth Feeds, India) and potable water ad libitum. Animals were maintained following the stipulated guidelines of ICMR - National Institute of Nutrition, Hyderabad, with the approval taken from Animal Ethical Committee, Bangalore University, Bangalore. Rats of three different age groups: 2-3 weeks, 2-3 months and 2-3 years, were chosen to correlate neonatal, young adult and senescent/old stage of the animal. They were acclimatized a week before the commencement of the study.
METHODS
Isolation of Mitochondria:
1,680g for 10 minutes. Mitochondria collected are stored at -700C till further use. All the isolation procedures were carried out at 0 - 40C.
The protein concentration was determined by the Folin-Lowry method. The total protein concentration made up to 1mg/ ml in storage buffer.
Mitochondrial marker assay
Lipid Peroxidation assay
Briefly, 100ug of mitochondria is treated with varying concentration of Ca2+ (0 to 300uM) along with 2ml of TBA: TCA: HCL reagent in 1:1:1 ratio of 0.37% Thiobarbituric acid, 15% Trichloro-acetic acid and 0.25N Hydrochloric acid; placed on a water bath for 10 minutes, later cooled down and spun at 1000 rpm for another 10 minutes. An aliquot of the solution measured at 535 nm with an extinction coefficient of MDA being 1.56x105 m-1/cm-1, was used for calculating LPO activity. LPO activity is measured with and without Ca2+ to delineate the role of Ca2+ in the ageing brain. The values were expressed in nmoles of MDA/ g wet weight of tissue.
RESULTS
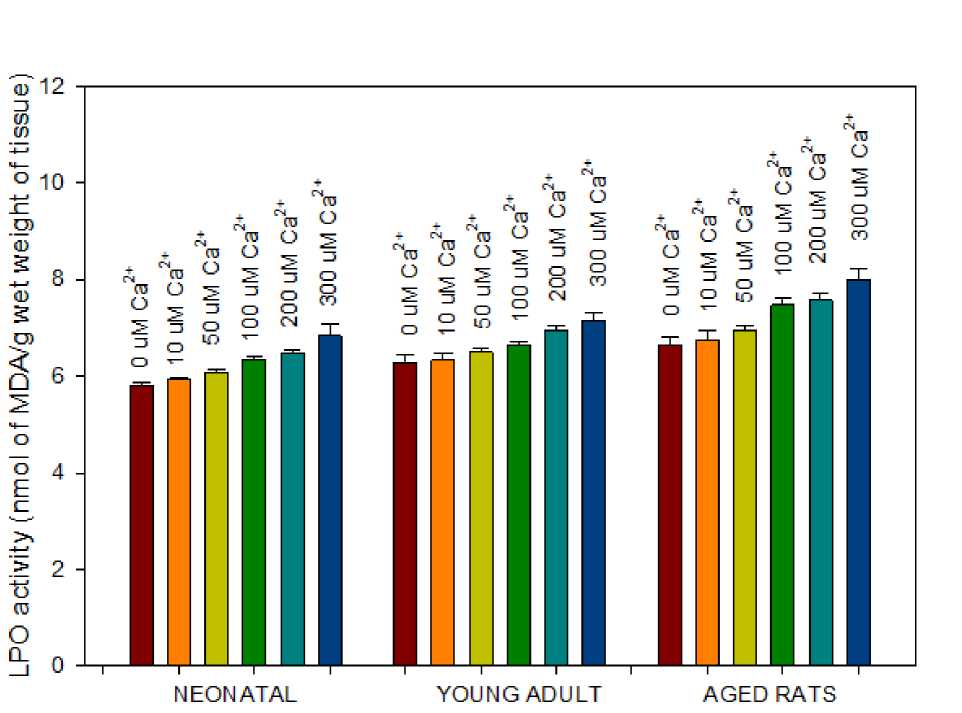
Figure 1 . Effect of Ca2+ on LPO activity of mitochondria isolated from brain tissue of three different age groups viz., neonatal, young adult and aged rats. Mitochondria (100 µg/ml protein) isolated from neonatal rats showed low basal rate of LPO activity in control studies and gradient increase in activity with each treatment of rise in Ca2+ concentration. Data points represent the Mean ± SD of 5 experiments (p<0.001).
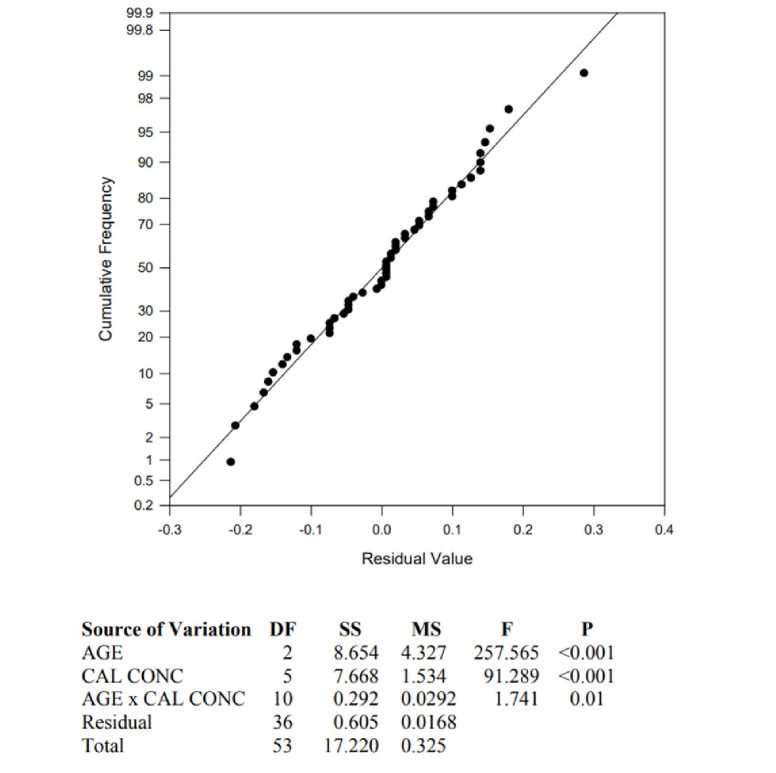
Figure 2. Normal probability plot, illustrating the linear and normal frequency distribution of LPO products formed values to Ca2+ treatment in all three age groups. The table represents the results of Two-Way Analysis of Variance with a significant p=0.01.
DISCUSSION
In our extended study, including the role of Ca2+ points out at the gentle rise in rate of LPO indicating the increased ROS production. The LPO rate, though dismal, is quantitative enough to measure the deleterious effects of ROS, suggesting a direct relationship between ROS and LPO. Lipid peroxidation products are highly reactive and include hydroxyl acids or reactive aldehydes (Gaschler & Stockwell, 2017). Assaying the LPO reaction results in intermediate products like PUFA-derived stable aldehydes such as 4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA) and acrolein (Ayala et al , 2014). Our experiment measuring malondialdehyde indicate the formation of covalent adducts with lipids (Lykkesfeldt, 2007), DNA (Kadiiska et al , 2005), matrix proteins and inner membrane bound proteins as well (Pizzimenti et al , 2013). Hence, LPO involved in loss of membrane bound proteins resulting in loss of structural integrity of the membrane (Ademowo et al , 2017;
CONCLUSION
We studied the effect of Ca2+ on LPO induction in mitochondria isolated from brain tissue of all three age groups viz., neonatal, young adult and aged rats. Aged rats exhibiting an average of ~12% rise in lipid peroxidation to 100 µM Ca2+ is indicative of increased sensitivity to Ca2+ interplay in LPO reaction. Our results are in agreement with Ca2+ actively potentiating LPO (Jain & Shohet, 1981). An application of 300 µM Ca2+ further doubled the rate of lipid peroxidation in aged rats to 20%, which should be significantly altering the phospholipid content in the mitochondrial inner membrane, compromising the membrane stability and architecture. In summary, age related free radical production and accumulation has been suggested to be harmful to major components of the cell and would promulgate aging and age-related diseases like Alzheimer’s disease (Simoncini et al. , 2015). The decrease in LPO activity as seen in young adult rats may be due to stable and healthy adult life, as seen in all animals.
ACKNOWLEDGMENT
Список литературы Ca2+ induced lipid peroxidation accentuates aging in rat brain mitochondria
- Ademowo, O. S., Dias, H. K. I., Burton, D. G. A., & Griffiths, H. R. (2017). Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontology, 18(6), 859-879.
- Ayala, A., Muñoz, M. F., & Argüelles, S. (2014). Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity, 2014, 360438.
- Birch-Machin, M. a, & Turnbull, D. M. (2001). Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods in Cell Biology, 65, 97-117.
- Cui, H., Kong, Y., & Zhang, H. (2012). Oxidative Stress, mitochondrial dysfunction, and aging. Journal of Signal Transduction, 1-13.
- Das, A. M., & Harris, D. A. (1990). Intracellular calcium as a regulator of the mitochondrial ATP synthase in cultured cardiomyocytes. Biochemical Society Transactions, 18(4), 554-555.
- Fernández-Moriano, C., González-Burgos, E., & Gómez-Serranillos, M. P. (2017). Lipid Peroxidation and Mitochondrial Dysfunction in Alzheimer's and Parkinson's Diseases: Role of Natural Products as Cytoprotective Agents. In Neuroprotective Natural Products: Clinical Aspects and Mode of Action (pp. 107-151).
- Flaquer, A., Rospleszcz, S., Reischl, E., Zeilinger, S., Prokisch, H., Meitinger, T. Strauch, K. (2015). Mitochondrial GWA Analysis of Lipid Profile Identifies Genetic Variants to Be Associated with HDL Cholesterol and Triglyceride Levels. PloS One, 10(5), e0126294-e0126294.
- Gaschler, M. M., & Stockwell, B. R. (2017). Lipid peroxidation in cell death. Biochemical and Biophysical Research Communications, 482(3), 419-425.
- Glancy, B., Willis, W. T., Chess, D. J., & Balaban, R. S. (2013). Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry, 52(16), 2793-2809.
- Griffiths, E. J., & Rutter, G. A. (2009). Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochimica et Biophysica Acta - Bioenergetics, 1787(11), 13241333.
- Gutiérrez, A. M., Reboredo, G. R., Mosca, S. M., & Catalá, A. (2006). A low degree of fatty acid unsaturation leads to high resistance to lipid peroxidation in mitochondria and microsomes of different organs of quail (Coturnix coturnix japonica). Molecular and Cellular Biochemistry, 282(1), 109-115.
- Harman, D. (1956). Aging: a theory on free radical radiation chemistry. Journal of Gerontology, 11, 298-300.
- Hong, H., Wang, Q., Li, J., Liu, H., Meng, X., & Zhang, H. (2019). Aging, Cancer and Immunity. Journal of Cancer, 10(13), 3021-3027.
- Jain, S. K., & Shohet, S. B. (1981). Calcium potentiates the peroxidation of erythrocyte membrane lipids. Biochimica et Biophysica Acta (BBA) -Biomembranes, 642(1), 46-54.
- Kadiiska, M. B., Gladen, B. C., Baird, D. D., Germolec, D., Graham, L. B., Parker, C. E..... Barrett, J. C. (2005). Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radical Biology and Medicine, 38(6), 698-710.
- Ku, H. H., Brunk, U. T., & Sohal, R. S. (1993). Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radical Biology and Medicine, 15(6), 621-627.
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6).
- Lykkesfeldt, J. (2007). Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clinica Chimica Acta, 380(1-2), 50-58.
- Martínez de Toda, I., Vida, C., Sanz San Miguel, L., & De la Fuente, M. (2019). Function, Oxidative, and
- Inflammatory Stress Parameters in Immune Cells as Predictive Markers of Lifespan throughout Aging. Oxidative Medicine and Cellular Longevity, 2019, 1-11.
- Niehaus, W. G., & Samuelsson, B. (1968). Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry, 6(1), 126-130.
- Payne, B. A. I., & Chinnery, P. F. (2015). Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochimica et Biophysica Acta - Bioenergetics, 1847(11), 1347-1353.
- Pizzimenti, S., Ciamporcero, E., Daga, M., Pettazzoni, P., Arcaro, A., Cetrangolo, G..... Barrera, G. (2013). Interaction of aldehydes derived from lipid peroxidation and membrane proteins . Frontiers in Physiology , Vol. 4, p. 242.
- Pratic, D. (2002). Lipid peroxidation and the aging process. Science of Aging Knowledge Environment, 2002(50), 5re - 5.
- Rattan, S. I. S. (2006). Theories of biological aging: Genes, proteins, and free radicals. Free Radical Research, 40(12), 1230-1238.
- Rikans, L. E., & Hornbrook, K. R. (1997). Lipid peroxidation, antioxidant protection and aging. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1362(2-3), 116-127.
- Rosenthal, R. E., Hamud, F., Fiskum, G., Varghese, P. J., & Sharpe, S. (1987). Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. Journal of Cerebral Blood Flow and Metabolism, 7(6), 752-758.
- Sanz, A. (2016). Mitochondrial reactive oxygen species: Do they extend or shorten animal lifespan? Biochimica et Biophysica Acta - Bioenergetics, 1857(8), 1116-1126.
- Savitha, S., & Panneerselvam, C. (2006). Mitochondrial membrane damage during aging process in rat heart: Potential efficacy of l-carnitine and dl a lipoic acid. Mechanisms of Ageing and Development, 127(4), 349-355.
- Schlame, M., & Greenberg, M. L. (2017). Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochimica et Biophysica Acta (BBA) -Molecular and Cell Biology of Lipids, 1862(1), 3-7.
- Silva, D. F., Selfridge, J. E., Lu, J., E, L., Roy, N., Hutfles, L..... Swerdlow, R. H. (2013). Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Human Molecular Genetics, 22(19), 3931-3946.
- Simoncini, C., Orsucci, D., Caldarazzo Ienco, E., Siciliano, G., Bonuccelli, U., & Mancuso, M. (2015). Alzheimer's Pathogenesis and Its Link to the Mitochondrion. Oxidative Medicine and Cellular Longevity, 2015, 803942.
- Stepien, K. M., Heaton, R., Rankin, S., Murphy, A., Bentley, J., Sexton, D., & Hargreaves, I. P. (2017). Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. Journal of Clinical Medicine, 6(7), 71.
- Turrens, J. F. (1997). Superoxide production by the mitochondrial respiratory chain. Bioscience Reports, 17(1), 3-8.


