Calculation of the formation process of clinker inside the rotary cement kiln
Автор: Mohamed Yasir A., Kasif A. Elhameed M.O., Alla Elrafie A.A., Elmahadi Muaz Musa
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Химическая технология
Статья в выпуске: 1 (75), 2018 года.
Бесплатный доступ
This study examined the effect of the liquid phase on the heat required for clinker formation and the Coating index, and the relation of the Burning zone temp with the clinker and the heat required for clinker formation. The selection of the liquid phase at 1450 temperatures is beingmaterials difficulty in the Burning, and 1338 is being materials easy in the Burning. All tests and tests were conducted at the Nile Cement Company. The results proved that the more difficult the materials are, the more the Heat required for clinker formation and increase the cost of cement production, and the Coating thickness is weak but strong. The materials easy Burning being little the Heat required for clinker formation and Decreases the cost of cement production, and the Excessive but unstable coating with tendency to form thick ring formation. The found average difference in Heat required for clinker formation between temperature 1338 °C and 1450 °C is 82.26 kJ/kg-clinker representing 2.23% of Total heat input are 3686 kJ/kg-clinker.
Clinker, formation, cement, kiln, rotary
Короткий адрес: https://sciup.org/140229965
IDR: 140229965 | DOI: 10.20914/2310-1202-2018-1-233-239
Текст научной статьи Calculation of the formation process of clinker inside the rotary cement kiln
DOI:
The cement is made of clinker and grinded gypsum and produced from a burned mixture of limestone and clay in certain percentages. A cement kiln is the most vital part of a cement factory whose outcome is cement clinker. Cement is the essential material for civil engineering construction works. Output from the cement industry is directly related to the state the construction business in general and therefore closely tracks the overall economic situation in a region or a country. Cement kilns are used for the gyro-processing stage of manufacturing of Portland and other types of hydraulic cements, in which calcium carbonate reacts with silica-bearing minerals to form a mixture of calcium silicates. Over a billion tons of cement is made per year and cement kilns are the heart of this production process. Their capacities usually define the capacity of the cement plant. As the main energy-consuming and greenhouse-gas-emitting stage of cement manufacture, improvement of their efficiency has been the central concern of cement manufacturing technology. In the recent years, and sustainable use of fossil fuels and renewable enhance attracted much attention worldwide. It is mainly due to high energy costs dictated by oil prices and the strong environmental concerns associated with carbon dioxide (СО 2 ) emissions. The use of process systems engineering tools, enable the alternative generation of more efficient and sustainable processes, Software tools have been widely used for process simulation, integration and optimization, which help process industry companies to achieve their operational excellence goals, such as. Aspen Hysys. A kiln is
basically an industrial oven, and although the term is generic, several quite distinctive designs have been used over the years. The most common one associated with pottery making, both ‘Bottle’ and their very close relatives ‘Beehive’ kilns, were also the central feature of any cement works. Early designs tend to be updraft kilns, which were often built as a straight-sided cone into which the flame was introduced at, or below, floor level. At 70 ft, the dome or bottle shape of the kiln, known as the ‘hovel’, would be quite a prominent landmark. As well as protecting the inner kiln or ‘crown’, the opening at the top of the hovel also used, to remove the smoke and exhaust gases that were produced during the production process. There was a 3–4 ft gap between the outer wall of the hovel and inner shell of the crown. Due to the fact that the 1 ft thick crown wall would expand and contract during firing, it was reinforced by a number of iron bands, known as ‘bunts’. Apart and ran right around the circular oven. The development of downdraft kilns in the early 20th Century proved to be much more fuel efficient and were designed to force more heated air to circulate around the kiln. The design incorporated a gentle curve at the ‘shoulders’ of the kiln, which served to reflect the rising heat from the fire at the bottom of the kiln, back down again over the material. The smoke and exhaust was then sucked out through holes at the bottom of the kiln via a flue, which was connected to a nearby chimney. The chimney would also serve a number of neighboring kilns as well. The kiln would be fired for several days to achieve the required temperature to produce cement clinker. Although the above
methods were successful, the problem with batch kiln was that it was intermittent and once the product had been produced, the fire was allowed to extinguish and the contents allowed to cool. This not only wasted a lot of heat, but also added to the expense of the finished product. Clinker as the main constituent of cement, is composed of various crystal phases, the following of which are the most important. Alite, belite, aluminates and ferrite Alite and belite are calcium silicate phases. Consisting only of СаО and SiО 2 , alite is a tri calcium silicate phase (Са 3 SiО 5 ) and belite a dicalcium silicate phase (Са 2 SiО 4 ). The aluminates phase, formed by pure СаО and pure Аl 2 О 3 , is a tri calcium aluminates phase (Са 3 Аl 2 О 6 ) and the ferrite phase, formed by pure СаО, Аl 2 О 3 and Fе 2 О 3 , is a tetra calcium alumina ferrite phase (Са 4 Аl 2 Fе 2 О 10 ). Table 1 Shows It is common to abbreviate the chemical formulae.
Chemical formulae for clinker
Table 1.
|
Chemical compound Abbreviation |
Clinker phase Abbreviation |
|
СаО C SiО 2 S Аl 2 О 3 A Fе2О 3 F |
Са 3 SiО 5 С 3 S Са 2 SiО 4 С 2 S Са 3 А l2 О 6 С 3 A Са 4 А l2 Fе 2 О 10 С 4 AF Са 12 А l7 О 33 С 12 А 7 |
Pure oxides are only available for laboratory investigations of clinker formation. In the industrial process the raw material contains various impurities. which form clinker phases incorporating impurities or forming solid solutions with minor compounds. Therefore, it is more appropriate to use the phase names “A lite”, “B lite” – and so on, since these express the clinker phases including impurities. Chemical processes of clinkerisation are assumed to comply with the following reaction scheme.
CaCO ^ CaO + CO2
Charge Materials
The main constituents of the raw materials required for cement production are calcium oxide (СаО), silicon dioxide (SiО 2 ), aluminum oxide (Аl 2 О 3 ) and iron oxide (Fе 2 О 3 ). Typical sources of these oxides are limestone, chalk, marl, clays (kaolinite, illite, feldspar) or shale, tuff, oil shale, bauxite and iron ore. These materials often contain alkalis, earth alkalis, heavy metals, sulfate, and supplied, phosphate, fluoride and chloride compositions in lower concentrations. Besides natural materials, waste products from other industrial processes, such as lime sludge or fly ash from coal combustion, can be added. The addition of relatively pure limestone, sand or iron ore might be necessary to adjust for absent chemical compounds and achieve the standards required for cement. A typical chemical composition of four-component raw meal is listed in Table 2. The chemical composition of the raw materials is constantly controlled in plant laboratories.
Physical and Chemical Process of Portland cement Clinker Formation
A lite (С3S)
A lite is the most important clinker phase in cement, since it controls mainly the initial and ultimate strength of cement. Portland cement clinker consists of ca. 50–70 wt. % of a lite .Which contains 71–75 wt. % СаО, 24–28 wt. % SiО 2 and 3– 4 wt. % substituted ions. Typically incorporated ions within the A lite crystal lattice are Мg2+, Аl3+ and Fе3+. Theimpurities in A lite stabilize high temperature polymorphs at low temperatures (below 15 the related decomposition temperature).
Tri calcium Aluminates (С 3 A)
С 3 A is the most reactive component of Portland cement clinker, which contains 5–10 wt. % of the phase. Pure С 3 A consists of 62 wt. % СаО and 38 wt. % Аl 2 О 3 and does not exhibit temperature dependent polymorphs. Table 2. Typical chemical composition of a four component raw meal.
Chemical composition of a four component raw meal
Table 2.
|
Compound |
Limestone (Wt. %) |
Shale (Wt. %) |
Sand (Wt. %) |
Iron Oxide (Wt. %) |
Kiln feed** composition (Wt. %) |
|
Dry material used |
73 |
22,5 |
|||
|
SiО 2 |
1,4 |
37,9 |
4,2 |
0,3 |
|
|
Аl 2 О 3 |
0,5 |
16,5 |
95,0 |
2,7 |
-20,1 |
|
Fе 2 О 3 |
0,2 |
5,1 |
1,4 |
6,6 |
6,3 |
|
СаО |
53,7 |
15,4 |
1,3 |
84,0 |
2,4 |
|
СаСО 3 |
95,9 |
27,5 |
1,0 |
2,7 |
64,4 |
|
Minor* compounds |
2,0 |
13 |
-2,3 |
-6,7 |
-6,8 |
However, ion substitution of Са2+ in the structure of the pure С 3 A causes changes in crystal structure. Typically Са2+ is substituted by Мg2+, 2К+ and 2Na+, Аl3+ by Fе3+ and Si4+, but only the alkali metals affect the structural changes .From a cubic crystal structure (pure С3А) to orthorhombic and monoclinic structures via intermediate structures of lower symmetry. In industrial clinker products, orthorhombic and cubic structures are commonly present polymorphs. The orthorhombic form features dark, prismatic crystals, whereas the cubic polymorph forms fine grains with dendritic ferrite crystals.
Calcium Aluminoferrite (С 4 AF)
Calcium Aluminoferrite constitutes 5–15 wt. % of Portland cement clinker. The pure phase contains 46 wt. % СаО, 21 wt. % Аl 2 О 3 , 33 wt. % Fе 2 О 3 , but in industrial clinker up to 10 wt. % of incorporated oxides appear (mostly МgО). In systems consisting of only СаО, SiО 2 , Аl 2 О 3 and Fе 2 О 3 , with typical Portland cement compositions, melting С 3 A and С 4 AF crystal phases commences at the eutectic at 1338 °C. This is only valid in an absolutely homogeneous mixture. In homogeneities in the raw meal mixture cause a shift of the eutectic toward lower temperatures (and different compositions). As an example, local composition in an Portland cement raw meal mix of 62 wt. % СаО, 15 wt. % Аl 2 О 3 and 23 wt.% SiО 2 melts at a temperature of circa 1170 °C.
In addition, all natural raw materials contain minor compounds, which decrease the melting point of a certain composition. Therefore it is common, that a molten phase occurs in industrial raw meal mixes at temperatures lower than 1338 °C actually, observed the melting of С 3 A and С 4 AF at 1280 °C through in situ studies of clinker formation. To simplify, the following discussion of melt formation will be described for a homogeneous system of pure oxides at a temperature of 1338 °C. The reader should keep in mind that temperatures might shift by 50–100 °C in more typical Portland cement raw meals. The composition of the melt phase at the eutectic point is 54.8 wt. % СаО, 16.5 wt. % Аl 2 О 3 , 6.wt. % Fе 2 О 3 and 22.7 wt. % SiО 2 . Preformed crystalline С 3 A and С 4 AF melt to provide Аl 2 О 3 and Fе 2 О 3 , as well as СаО for the melt phase. SiО 2 is obtained from free SiО 2 particles or, if all has been consumed for belite formation, partially molten crystalline belite.
The extent of С3 A and С4 AF melting at 1338 °C depends on the total clinker raw meal composition, i.e. on the ratio of Аl2О3/Fе2О3 in the total composition., which shows the part of interest for Portland cement clinker compositions in the phase diagram for the four oxides. Since only С3 A and С4 AF melt, the amount of all the other phases, i.e. mainly СаО and С2 S is fixed. It should be emphasized here, that for all three of the following discussed cases, the Аl2О3/Fе2О3 ratio of the molten phase is always 1.38, as it is for the eutectic composition. In the first case, a total clinker raw meal composition with an Аl2О3/Fе2О3 ratio of 1.38 is considered. The Аl2 О3/Fе2 О3 ratio is constant over the whole surface of the plane. Since it is the same ratio as in the molten phase at the eutectic, all crystalline С3 A and С4 AF melts. The liquid phase fulfills two important tasks in the clinker burning process: 1) Acceleration of the clinker phase formation; 2) Prevention of clinker dust formation.
Materials and methodsRaw Materials
The composition of Portland cement varies from plant to plant due both to cement specifications and to the mineralogy of available materials. In general, however, an eutectic mix is sought which minimizes the heat input required for clinkering. The total cost of raw materials, while producing a cement of acceptable performance.
Note that, with a substantial proportion of the raw mix being СаСО 3 , heating either in a kiln or in a laboratory furnace evolves some 35% by weight as СО 2 ; this results in a requirement of approximately 1.5tоn of raw materials to produce 1tоn of clinker, and also requires that analytical data be clearly distinguished between "raw" and "ignited" basis. T raditional kiln fuels are gas, oil or coal. The choice is normally based on price and availability. It must be noted, however, that fuels are usually priced in terms of gross heat (heat available assuming water in combustion product is condensed to recover latent heat of vaporization). In practice, only the net heat is employed (assumes that water in combustion gas is released as vapour).
Methods
Thermo chemical calculations tools aims at benefiting from the specific strengths. Detailed calculation of mass and energy transport conditions inside the kiln including combustion. However, performing chemical calculations within a kiln environment requires a mass balance equation for each phase to be solved, which results in very high computational costs for complex chemical conditions.
An alternative approach to modeling these conditions can be applied, if thermo chemical equilibrium can be assumed to establish in certain zones within the kiln. A numerical equilibrium calculation is then assigned to each of these zones. Mass and energy transport conditions are modeled by means of streams interconnecting the equilibrium zones and thus forming a flow sheet model of the kiln. As the flow sheet model needs to be derived from mass and energy transport calculation results.
Which in turn are strongly influenced by chemical processes, the entire model is solved iteratively. In the following they actually used software tools and equations realization of the modeling.
Equations Clinker Phase
Based on phase relations in the four component system, several equations have been derived to describe the quality and quantity of Portland cement clinker of a known raw material composition. In all equations the chemical compositions are expressed in wt. %. The quality of clinker is often referred to as the amount of free (non-reacted) СаО in the sample, which reduces the strength of concrete.
Up to now many theoretically and empirical derived equations have been developed to calculate the so called “Lime Saturation Factor (LSF)” (Eq. 1). It is used to quantify the amount of СаО in the raw material that can be combined with SiО 2 , Аl 2 О 3 and Fе 2 О 3 to form the main clinker phases С 3 S, С 2 S, С 3 А and С 4 AF. For satisfactory clinker quality LSF should be in the range of 92–98%.
A common equation is given in Eq. 1. Other parameters are the “Silica Ratio (SR of Ms)” (Eq. 2) and the “Alumina Ratio (AR or Ma)” (Eq. 3). The SR, usually in the range of 2 – 3, describes the proportion of the silica phases to aluminates and ferrite phases, and reflects the ratio of solid phases (the silica phases) to the liquid phase, formed by aluminates and ferrite, in clinker. AR expresses the ratio between the aluminates phase and ferrite phase and indicates which of these two phases is forming the Melt phase (for further details see subsection).
LSF =
CaO
2.8 SiG 2 + 1.18 AlG? + 0.65 Fe2G3
SR =
SiО
AlO3 + Fe2O3
AR =
Аl О
FеО
Calculation equations for clinker compounds
C3S = 4.071 x (total CaG -free lime - 0.7 SG3 ) - ^ - 7.6 x SiG3 - 6.718 Al2G 3 -1.43 Fe2G3
C2S = 2.867SiG2 - 0.744C3S(5)
C3A = 2.65Al2G3 -1.69Fe2G3
CAF = 3.04 FeG(7)
Equations associated with the process of clinker production
-
• Burning zone temp:
1300С + 4.51С3S + 3.74С2S – 12.64 С4АF (8)
-
• Coating index, AW AR > 0.64,
AW = C3 A + C4 AF + 0.2 QS + 2 F (9)
(AW normally = 27–32)
When AW < 20, No coating or very less coating When AW > 30, Excessive but unstable coating with tendency to form thick ring formation.
Liquid percentage (by Weight) If AR > 1.38,
% L . P = 6.1 F + M + K + SG3 (10)
If AR < 1.38,
% L.P = 8.2 A - 5.22 F + M + K + N + SO3 (11)
at 1338 deg C If AR < or = 0.64,
L . P = 3.0 A + 2.25 F + M + K + N + SG 3 (12)
At 1450 °C Аl 2 О 3 , К 2 O and Nа 2 O increase the viscosity and Fе 2 О 3 and SО 3 decreases the viscosity.
Heat required for clinker formation by (kJ/kg-clinker)
Q = 17.196( Al 2 G 3 ) + 27.112( MgG ) +
+ 32 ( CaG ) -21.405 ( SiG3 ) -2.468( Fe2G 3) 1
Chemical Analyses of materials feed kiln and Clinker Phase
The chemical Analyses for composition the materials feeding the kiln and the clinker using the traditional method of testing in the chemical analysis laboratory at Rabek cement factory. Taking the samples from the daily report of the chemical tests of the materials feeding of the kiln and clinker in the every hour, 30 samples were selected based on the formation of the liquid phase in the clinker. Taken 15 samples at Burning zone temperature a temperature about to 1338 °C and 15 samples at Burning zone temperature 1450 °C by determine the Burning zone temperature. We collected 15 samples the liquid phase formation at temperature 1450 °C. To calculation liquid phase percentage and the clinker compounds in the samples and the percentage of Coating index in the kiln and the Heat required for clinker formation by (kJ/kg-clinker). By equations and the percentage of the oxides materials feed inside to kiln and the clinker oxides. The oxides were taken from the chemical analysis laboratory. The calculations were carried out by means of the Mat lab program. And we collected 15 samples another the liquid phase formation at temperature 1338 °C, and also The calculations were carried out by means of the Mat lab program and obtain on results, to Comparison between the composition of the liquid phase in temperature (1450–1338) °C.
Results and discussion
Figure 1 and 2 show the calculations of composition results of 15 samples of the percentage clinker compounds and coating index, liquid phase and heat required at liquid phase formation in tеmреrаturе 1450 °C and 1338 °C.
С 3 S Increases by increasing total СаО by 4.07 per 1% of total СаО, And С 3 S Decreases by increasing SiО 2 by 7.6 per of 1% of SiО 2 . Also Decreases by increasing Аl 2 О 3 by 6.9 per 1% of Аl 2 О 3 , decreases with Fе 2 О 3 increase by 1.43 per of 1% of Fе 2 О 3 . Increases in С 3 S leads the Burning zone temp and increase in the Heat required for clinker formation and difficult in the fire and formation of the liquid phase shall be within (23.02–27.05) at 1450 °C and the formation of a medium, strong and constant coating within the limits of (23–28)
The ratio of С 3 S in the difficult liquid phase is within (56.49–68%). A cut in С 3 S leads to an increase in С 2 S, a decrease in the Burning zone temp, a decrease in the heat of the clinker formation, and a slight ease in the fire. And the composition of the liquid phase is within the limits of (16–21) at 1338 °C .the ratio of С 3 S in the easily liquid phase is within (44.77–57%). С 3 S is responsible for the strength of the initial cement in 28 days if decrease proportion reduces the initial strength and affects the quality of cement. The С 3 S increases strength initial but increases Heat required for clinker formation .
С2S Increases by increasing SiО2 by 2.867 per 1% of SiО2. And С2 S decreases by increasing С3S by 0.744 per 1% of С3S, С2S at 1338 °C to the limits value (14–29). At a temperature of 1450 °C this limit reaches (5.5–17). It is the second major cement compound and has a significant impact on the strength after 28 days. This compound has a direct impact on the production of concrete. С3A Increases by increasing Fе2О3 by 2.867 per 1% of Аl2О3. And С3A decreases by increasing Fе2О3 by 1.69 per of 1% of Fе2О3. С4AF Increases by increasing Fе2 О3 by 3.04 per 1% of Fе2 О3 .Increase С4 AF leads to an increase in the liquid phase and reduce the Burning zone temp, the heat of clinker formation and the Increase in Coating index. Coating index Increases by increasing С3A, С4AF, С2S and F. At a temperature of 1450 °C gives a Coating index, within the limits of (23–27) be coating normally .At a temperature of 1338 °C gives a Coating index, within the limits of (29–33), if Coating index > 30, Excessive but unstable coating with tendency to form thick ring formation .Liquid Phase formation at a temperature of 1338 °C Increases by increasing A, SО3 and decrease F, being limits of (16–21) .Liquid Phase formation at a temperature of 1450 °C Increases by increasing A, SО3 and F, being limits of (23–27) leads difficult in the fire .Burning zone temp Increases by increasing С2S by 3.74 per 1% of С2 S and Increases by increasing С3S by 4.51 per 1% of С3S, Decreases by increasing С4AF by 12.64 per of 1% of С4AF. Burning zone temp determines the Transformation of the solid phase for liquid phase at 1338–1450 °C.
Liquid Phase at 1338
- AW
-
- Q
- C3S
- C2S
- C3A
- C4AF
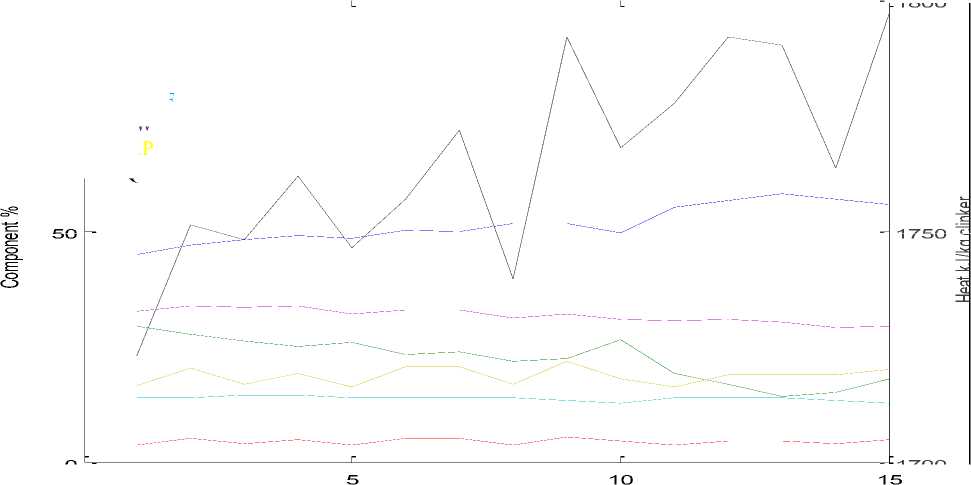
Sample No.
Figure 1. Relationship between clinker compounds and Coating index, liquid phase and Heat required at liquid phase formation in tеmреrаturе1338 °C
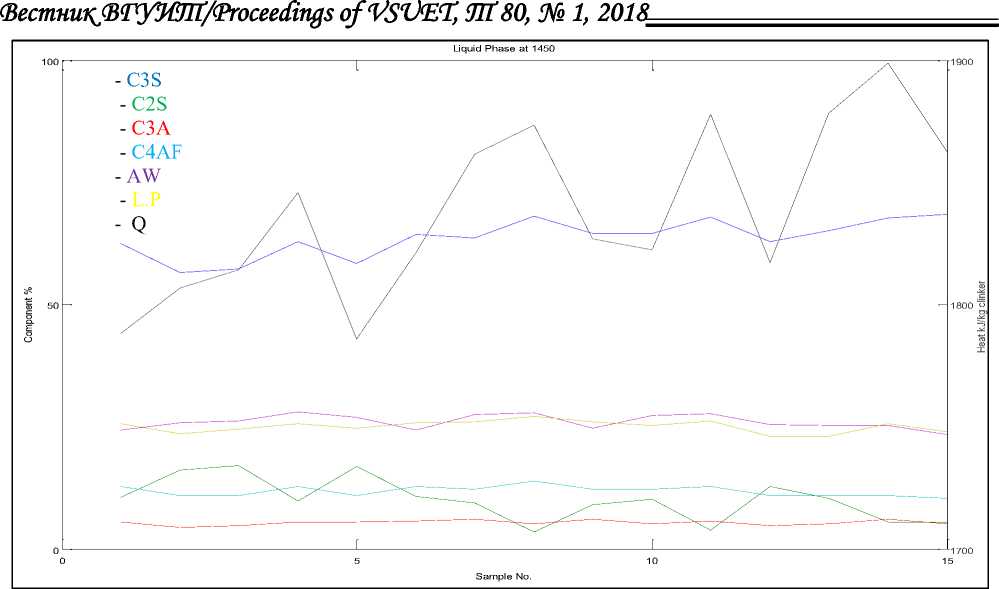
Figure 2. Relationship between clinker compounds and Coating index, liquid phase and Heat required at liquid phase formation in tеmреrаturе1450 °C
Conclusion
Heat required for clinker formation Increases by increasing Аl2О3 by 17.196 per 1% of Аl2О3, increases by increasing МgО by 27.112 per 1% of МgО and increases by increasing СаО by 27.112 per 1% of СаО. And heat required for clinker formation decreases by increasing SiО2 by 21.405 per of 1% of SiО2 and decreases by increasing Fе2О3 by 2.468 per of 1% of Fе2О3. The average difference in heat required for clinker formation between temperature 1338 °C and 1450 °C is 82.26kJ/kg-clinker representing 23.2% of total heat input are 3686 kJ/kg-clinker. The heat required for clinker formation increases with the increase of the liquid phase and the burning zone temp and С3S, decreases by increasing С4AF.
Список литературы Calculation of the formation process of clinker inside the rotary cement kiln
- Abdulkadhum J. K. Al-Yasiri, Estimating the thickness of coating in the burning zone of Cement Kilns includ-ing the aging factor. The Iraqi Journal For Mechanical And Material Engineering. 2012. vol. 12. no.3.
- David Barthelmy. Available at: http://webmineral.com/data/Brownmillerite.html.
- Jolyon Ralph and Ida Chau. Available at: http://www.mindat.org/min 790.html.
- Goswami G., Padhy B.P., Panda J.D. Thermal analysis of spurrite from a rotary cement kiln, Chemistry and Materials Science. Journal of Thermal Analysis and Calorimetry. 2012.
- Goswami G., Padhy B.P., Panda J.D. Thermal analysis of spurrite from a rotary cement kiln. Journal of Ther-mal Analysis and Calorimetry. 2011. vol. 35. no. 4. рр1129-1136.
- Kerdarpour, F., Sufi, T., Goshayeshi, H.R., Mir Sanjari, A. Investigation of Energy Waste in the Cement Indus-try and Solutions to Optimize Energy Consumption. International Journal of Petroleum and Energy. 2013. no.86. pp.52.
- Boateng A.A., Rotary K., Shakeri, M.R., Oghabi M. et al. Volume 1. Tehran, Sharif University, 2011.
- Noshirvani, G.R., Shirvani, M., Nourzadeh, H.R., Saddiqi, S. Calculate and Estimate the Thickness of the Coating of Cement Rotary Kiln. Fourth Conference Condition Monitoring and Diagnostics of Machines. 2010. pp. 1388.
- Cement kiln. Available at: http://en.wikipedia.org/wiki/Talk:Cement kiln.
- Goshayeshi H.Z., Poor F.K. Modeling of Rotary Kiln in Cement Industry. Department of Mechanical Engi-neering, Mashhad Branch. Islamic Azad University. Mashhad. Iran. Energy and Power Engineering. 2016. no. 8. pp. 23-33. Available at: http://www.scirp.org/journal/epe.
- Muhammed M. A. Estimating the Thickness of Kiln Shell Coating at the Burning Zone of Cement Kilns In-cluding the Aging Factor. Journal of Thermal Analysis and Calorimetry. 2016. vol. 35.


