Can the toxicity of asbestos be reduced?
Автор: Habashi Fathi
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 7 т.6, 2013 года.
Бесплатный доступ
Asbestos, an extremely useful natural resource, has been studied extensively mineralogically and for industrial application. Its extraction from ores has been greatly improved with respect to safety in the work place. In spite of that, there is a decisive effort in many countries to ban its use on the grounds that it is a toxic substance. This action resulted in drastic decrease in production and the possibility of destroying the industry. The chelation of asbestos with organic dyes seems to be a promising way to abate its toxicity. Dyeing can be done when the fi bers are slurred in water at ambient conditions in the same way as textile fi bers.
Asbestos, toxicity
Короткий адрес: https://sciup.org/146114791
IDR: 146114791 | УДК: 622.341
Текст научной статьи Can the toxicity of asbestos be reduced?
Dyestuffs have been used for many decades to stain cells and microorganisms for identification purposes [1]. They have also been used to identify clay minerals through a color reaction, e.g., montomorillonite gives a blue color with benzidine but illite does not [2]. Reactions like these were exploited by engineers to separate minerals by flotation [3]. For example, Alizarin Red S is more adsorbed on apatite than calcite, hence separation by flotation can be made possible [4]. Fly ash, which is mainly a silicate, is suggested as a cheap material for removal of certain dyestuffs from textile waste water [5]. Activated charcoal is also used for the same purpose. Dyestuffs are also adsorbed on metallic surfaces to inhibit their corrosion [6] and used to color certain metals on which an anodic oxide film has formed [7]. This shows the diversified reactions of dyestuffs with materials other than textile fibers – their most important application. In the present communication the reaction of dyestuffs with chrysotile asbestos is reviewed with respect to the toxicity issue [8-11].
Structure of absestos
Asbestos is an industrial mineral of great economic importance. It occurs in nature in a variety of forms but the most important is chrysotile which represents more than 90 % of the asbestos used; it is a hydrated magnesium silicate, 3MgO.2SiO2.2H2O. The given formula, however, represents only the ratio of the components and a more exact formula would be Mg 3 (Si 2 O 5 )(OH) 4 [12].
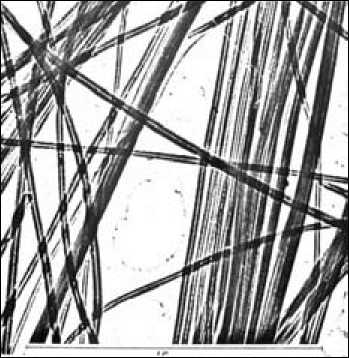
Fig. 1. Asbestos fibers under the microscope. The fibers that are visible to the naked eye are composed of hundreds of thousands mono-fibers called fibrils in close packed parallel arrangement
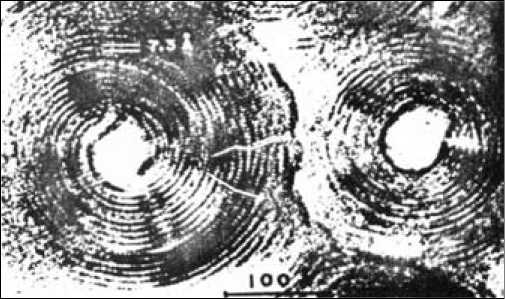
Fig. 2. Cross section of fibrils at high resolution electron microscope showing the scroll-like structure. A typical fiber consists of 12 to 20 layers rolled up like a scroll
The asbestos fibers that are visible to the naked eye are composed of hundreds of thousands monofibers called fibrils in close packed parallel arrangement (Fig. 1). The mono-fibers have a tubular shape formed by the rolling up of a sheet composed of hydrated magnesium silicate. This sheet is composed of silica groups in tetrahedral pyramid form linked to each other to form a first layer and conjugated to octahedral groups of hydrated magnesia. The latter groups are bulkier than their conjugate silica groups and the sheet that they form has to curve to accommodate the excess encumberment of the magnesia. Lateral crystal growth of the sheet eventually leads to the formation of a tube. A typical fiber consists of 12 to 20 layers rolled up like a scroll (Fig. 2). The inner and outer diameters are respectively in the order of 50 to 250 Å. The hydration represents about 14 % of the mass and is in the form of hydroxyl groups linked to the magnesium and covering the external surface (Fig. 3).
A sliver of chrysotile asbestos with cross section 0.1 mm square contains about 20 x 106 fibrils all in parallel orientation. It is possible therefore to strip from asbestos fiber bundle very fine threads, – 780 –
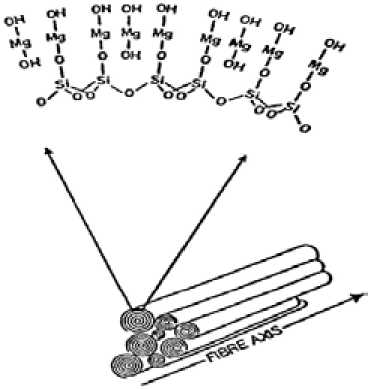
Fig. 3. Schematic illustration of the hydrated magnesium silicate sheets rolled in form of scrolls to form an asbestos fiber. Hydroxyl groups linked to the magnesium and covering the external surface each of which still contains many thousand fibrils. Aqueous suspensions of chrysotile asbestos show a marked alkaline, e.g., 10 g suspended in 1 L of distilled water shows a pH of 9.5 at room temperature. This is due to the reaction:
Mg 3 Si 2 O 5 (OH) 4 + 5 H 2 O → 3 Mg2+ (aq) + 6 OH- (aq) + 2 H 4 SiO 4(aq)
The toxicity issue
Asbestos is claimed by some as a workplace problem that can be adequately addressed with current dust control technology and appropriate practices and not an environmental issue, and by others as a toxic and carcinogenic material that must be banned immediately. It was demonstrated, for example, that heavy smokers in particular are more susceptible to have cancer of the lung; it seems that any fiber lodging in the lung because of its large surface area is capable of adsorbing carcinogenic substances from tobacco smoke. The accumulation of siliceous dust in the lungs may result in causing a disease called silicosis, and this may be cured by surgery. In case of lung cancer due to asbestos, however, this is not possible because the disease spreads rapidly from the lungs to other organs.
As a result of this controversy, the consumption of asbestos in many countries has been decreasing gradually over the past few years, which forced production decline, and in some cases plant shut downs. The industry is looking for substitutes for asbestos, but the long term environmental effects of other minerals or synthetic substitutes that have been proposed are unknown. There are two widely accepted screening toxicity tests for asbestos: hemolytic and cyto-toxicity.
-
□ Hemolytic : In these tests red blood cells are left in contact with certain amount of fiber and after various periods of time, the cells are centrifuged; and the hemoglobin content of the supernatant solution is measured using spectrophotometric methods. Usually, 72 % of the cells undergo hemolysis when contacted with dry fibers, and 65 % when contacted with wet fibers.
-
□ Cytotoxicity : In these tests P 388D 1 cells are incubated with a certain amount of fibers for various periods of time. The number of healthy and dead cells is then measured using Trypan Blue dye. Usually, 81 % of the cells die when contacted with dry fibers and 50 % when contacted with wet fibers.
While hemolysis is a short-term test in which the membrane stability is involved, cytotoxicity as outlined above gives a global picture of the toxicity of the fibers. Hence, the two tests do not necessarily correlate together since the factors involved are not the same.
The chelation of asbestos
Chrysotile asbestos has the advantage of a large surface area and, unlike activated charcoal, is white in color so that the action of dyes can be followed easily. When a sample of asbestos fibers is agitated at room temperature with an aqueous solution of an organic dye, the fibers become immediately colored. When the colored fibers are separated by filtration, dried and examined by the scanning electron microscope they are homogeneous and contain no precipitates. When boiled with distilled water under reflux condenser, they do not lose their color thus indicating that the dye is chemically bound to the fiber. Table 1 gives the color of the dye solution and the color of the asbestos treated by these dyes.
The appearance of new peaks in X-ray diffraction patterns and in infrared spectra together with the absence of precipitates supports the view that chelates are formed with asbestos, apparently with its Mg(OH) 2 component (Fig. 4). This resembles the process of mordant dyeing of textile fibers where Mg(OH)2, Al(OH)3, or Cr(OH)3 are first precipitated on textile fibers then known as “lakes” [13]. Some organic compounds, e.g., bromocresol purple and phenolphthalein, although they are not dyestuffs, they color asbestos. They have nearly similar structure and behave nearly the same way towards asbestos. The toxicity of the colored fibers was measured by the tests mentioned earlier and compared with the uncolored sample. It was found that some dyes, e.g., Thiazol Yellow G and Trypan Blue decreased the toxicity of the fibers (Table 2).
Conclusions
Chrysotile asbestos can be colored by a variety of dyes at ambient conditions like textile fibers forming stable magnesium chelates. Certain dyes render it less toxic as measured by the homolytic
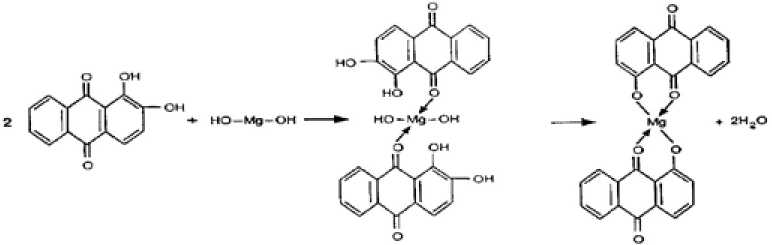
Fig. 4. Formation of a chelate between Mg(OH) 2 and alizarin
Table 1. Color of dye solutions and color of the asbestos treated by these dyes [Habashi, 1992, Awadalla and Habashi, 1990]
|
pH of application |
Color of dye solution |
Color of asbestos |
|
|
Alizarin |
9.5-10 |
Violet |
Blue-violet |
|
5-6 |
Brown |
Brown |
|
|
2-3 |
Yellow |
Yellow |
|
|
Basic Fuchsin |
9.5-10 |
Purple |
Colorless |
|
5-6 |
Red rose |
Pale red |
|
|
2-3 |
Pale red |
Pale red |
|
|
Brilliant Blue R |
9.5-10 |
Colorless |
Blue |
|
6-7 |
Blue |
Blue |
|
|
2-3 |
Blue |
Blue |
|
|
Brilliant Yellow |
9.5-10 |
Red |
Red |
|
6-7 |
Orange |
Orange |
|
|
3-5 1-1.5 |
Yellow Green Precipitate |
Yellow |
|
|
Bromocresol Purple* |
9.5-10 |
Violet |
Blue |
|
6-7 |
Yellow |
Yellow |
|
|
2-3 |
Yellow |
Yellow |
|
|
Crystal Violet |
9.5-10 |
Violet |
Violet |
|
5-6 |
Violet |
Violet |
|
|
2-3 |
Greenish yellow |
Greenish yellow |
|
|
Hydroxyquinoline |
1-2 |
Yellow |
Gray |
|
>2 |
Insoluble |
-- |
|
|
Malachite Green |
9.5-10 |
Greenish blue |
Pale blue |
|
6-7 |
Greenish blue |
Pale blue |
|
|
2-3 |
Green |
Greenish blue |
|
|
Methyl Blue |
9.5-10 |
Colorless |
Blue |
|
6-7 |
Blue |
Blue |
|
|
3-5 |
Blue |
Blue |
|
|
Methylene Blue |
2-10 |
Pale blue |
Pale blue |
|
Methyl Violet |
9.5-10 |
Violet |
Violet |
|
6-7 |
Violet |
Violet |
|
|
1-3 |
Greenish yellow |
Greenish yellow |
|
|
Phenolphthalein* |
9.5-10 |
Pink |
Pink |
|
6-7 |
Colorless |
Pale pink |
|
|
2-3 |
Colorless |
Colorless |
|
|
Thiazol Yellow G |
9.5-10 |
Red |
Yellow |
|
6-7 |
Yellow |
Yellow |
|
|
2-3 |
Orange |
Orange |
|
|
Trypan Blue |
2-9.5 |
Blue |
Blue |
* Not a dye
Table 2. Toxicity of chelated asbestos [Habashi et al., 1988, 1991]
|
Chelating |
Concentration of chelating Toxicity agent on asbestos grade 7 RF66, % Hemolysis Cytotoxicity |
|
None (dry asbestos) |
0 72 81 |
|
None (asbestos suspended in water) |
0 65 50 |
|
Thiazol Yellow G |
0.2 54 59 2.0 1 13 2.1 6 0 2.5* 6 12 |
|
Trypan Blue |
4.0 1 4 |
|
Brilliant Yellow |
0.2 57 85 0.9 40 74 1.0 73 14 |
|
Methyl Blue |
0.5 69 61 2.0 23 51 2.4 12 46 |
|
Alizarin |
0.4 54 40 2.7 46 57 3.9* 90 13 5.3 64 24 |
* Chelating agent contacted with asbestos at the boiling point.
and cyto toxicity tests. This is a promising area of research that may lead to the preparation of colored asbestos that is nontoxic. Since the major application of asbestos, about 70 %, is in the fabrication of asbestos cement whereby the fibers are slurried in water with Portland cement, the addition of a dye in this step is a simple matter. Colored asbestos is stable with respect to boiling water that suggests a chemical reaction with magnesium hydroxide in the silicate structure.


