Capsaicin-induced Thermal Enhancement on Target Tissues in Hyperthermia
Автор: Peng Zeng, Zhong-Shan Deng, Jing Liu
Журнал: International Journal of Image, Graphics and Signal Processing(IJIGSP) @ijigsp
Статья в выпуске: 3 vol.3, 2011 года.
Бесплатный доступ
Local thermal enhancement in target tissue is of great interest in tumor hyperthermia. In this study, we proposed a brand-new thermal enhancement protocol for tumor hyperthermia using heat generated from thermoge-nesis evoked by capsaicin, which can safely deliver a totally localized heating to target tissue. A healthy male volunteer was recruited, whose partial areas of the dorsum of hand and posterior aspect of forearm were smeared with 1% (w/w) capsaicin solution, to determine the increase of ther-mogenesis in human body. In addition, animal experiments on healthy Kunming (KM) mice (20-22g) were performed to test the feasibility and efficacy of capsaicin-induced thermal enhancement. These KM mice were first locally smeared with, subcutaneous or intraperitoneal injected of the same capsaicin solution, and then heated by near infrared laser. Preliminary experiments on the volunteer showed an effec-tive temperature increase in the skin area. Animal experi-ments indicated that distinct thermal enhancement in heat-ing effect, and that the thermal enhancement induced by intraperitoneal injection of capsaicin is more obvious than the other two ways. Thus capsaicin can be used as a poten-tial therapeutic adjuvant to locally enhance heating effects in target tissue during tumor hyperthermia.
Thermal enhancement, thermogenesis, capsai-cin, tumor hyperthermia, TRPV1, thermoregulation
Короткий адрес: https://sciup.org/15012141
IDR: 15012141
Текст научной статьи Capsaicin-induced Thermal Enhancement on Target Tissues in Hyperthermia
Published Online April 2011 in MECS
Regional hyperthermia has long been an important modality of cancer based on the localized heating of tumor tissue to above 43 °C. Different from conventional therapies, namely surgery, radiotherapy and chemotherapy, this therapy takes advantage of the high sensitivity of tumor cell to heat, and induces minor side effect which wins its fame as a “Green Therapy”. Up to now, a wide variety of hyperthermia modalities have been established such as heating by microwave, ultrasound, radiofrequency, electromagnetic source, laser, and hot medium. However, a common characteristic of these methods is that they will inevitably cause thermal damage to the healthy tissues along the path heat energy is transmitted to the tumor and that surrounding the tumor. Therefore, the ul- timate aim of hyperthermia is to optimize the generated temperature distribution within the target tissue such that the tumor is essentially heated, but preferably not any healthy tissue surrounding the tumor. For this reason, thermal enhancement on target tissue involved in hyperthermia is necessary. Along this way, nanoparticles have been introduced to regional hyperthermia [1, 2]. However, this technique is limited by the use of alternating strong electromagnetic field and biological toxic nanoparticles in clinics [3].
In this study, we instead proposed a new physiological approach to locally enhance heating effect on target tissue in hyperthermia by adjuvant use of capsaicin. Previous experiments in rats have showed that capsaicin could be decomposed by metabolic breakdown in liver [4]. Thus, capsaicin-induced thermal enhancement may avoid ill-defined damages aroused by additional physical field or toxic nanoparticles.
Capsaicin, the main pungent ingredient of chili peppers, elicits a heat/burning sensation when it comes in contact with skin, eyes or oral mucosa. It (relative amount: 69%) and dihydrocapsaicin (relative amount: 11%) are the two major capsaicinoids in peppers. Capsaicin has been used as a biochemical pesticide [5], an adjunct in weight management [6] and an ingredient in cosmetics [7]. Medical research also demonstrated its use in eliminating pain [8] and treating some cancers [9]. Previous investigations have observed thermoregulation characterized by rectum temperature when capsaicin was injected intraperitoneally to desensitized rats [10]. A study on the perineal cutaneous area suggests that the heat/burning sensation occurred may be due to the local application of capsaicin [11].
The mechanism of capsaicin-induced pungency is that capsaicin binds its receptor in primary afferent sensory neuron which then triggers the sensory neuron to signal the brain. Its receptor is named Vanilloid Receptor (VR) [12, 13]. Being structurally related to members of Transient Receptor Protein (TRP) family of ion channels, VR is also called Transient Receptor Protein Vanilloid 1 (TRPV 1) or simply named capsaicin receptor [12, 13]. Julius and his colleague first cloned Vanilloid receptor in 1997 and demonstrated its function as a non-selective cation ion channel [14]. A study on VR 1 gene knockout mice proved that the capsaicin receptor is essential for capsaicin-induced pain sensation and for injury-induced thermal hyperalgesia [15]. A recent investigation documented that capsaicin is the first naturally occurring substance found to enhance uncoupled the sarcoplasmic reticulum Ca2+-ATPase (SERCA) without consuming energy for pumping Ca2+, which could finally result in thermogenesis [16]. It indicates that capsaicin might be potentially adjunctive to promote thermal enhancement in hyperthermia. Thereby, we first performed preliminary experimental investigations on the dorsum of hand and the forearm of human by local application of capsaicin solution to test the effect of thermogenesis for thermal enhancement in human body. Then further experiments on animals by local application, intraperitoneal and subcutaneous injection of capsaicin solution were performed to test the feasibility and efficacy of the capsaicin in hyperthermia.
-
II. Materials
-
A. Chemicals and Reagents
-
1. Capsaicin, 8-methy- N -vanillyl-6-nonenamide, crystalline, purity: 80%, purchased from the Aladdin Reagent Inc., Shanghai, China.
-
2. Tween 80 (polysorbate 80), C 12 H 10 CINO 3 , also purchased from the Aladdin Reagent Inc., Shanghai, China
-
3. Urethane (Ethyl carbamate), C 3 H 7 NO 2 , crystalline, purity: equal or greater than 98%, Chemically Pure (CP), purchased from the Sinopharm Chemical Reagent Co., Ltd, China.
-
B. Instruments, Equipents and Software
-
1. Thermal infrared camera, FLIR SC 620, purchased form FILR systems Inc. Characteristics of this thermal infrared camera are listed in Table I.
-
2. Near infrared laser, wavelength: 808nm, peak power output: 10W, purchased from BWT, Beijing, China.
-
3. Commercial post-processing software for thermal infrared images: FLIR research IR.
TABLE I. T hermal I nfrared C amera (FLIR SC620)
|
Parameters |
Range |
|
Field of view (FOV) |
24°× 18° |
|
Minimum focus distance |
0.3m |
|
Focal length |
38mm |
|
Spatial resolution |
0.65mrad |
|
Thermal sensitivity |
65mk@30 C |
|
Accuracy |
±2 °C or 2% of reading |
|
Object temperature range |
-40 ~ 120 C or 0 ~ 400 C |
-
C. Experimental Subjects
-
1. A healthy volunteer, male, 24 years old.
-
2. Laboratory animal, Kunming (KM) mice, purchased from Department of Laboratory Animal Science, Health Science Center, Peking University, Beijing, China. License number is SCXK ( Jing ) 2006-0008. Its characteristics are listed in Table II.
-
TABLE II. L aboratory A nimal (KM M ice )
|
Parameters |
Range (or Definition) |
|
Quality |
SPF/VAF |
|
Type |
Outbreeding |
|
Weight |
20-22g |
|
Week |
6 ~ 8 |
III. Methods
-
A. Reagent Preparation
Crystalline capsaicin is hydrophobic but miscible with organic solvent. Tween 80 is miscible in water, and miscible with ethanol, cottonseed oil, corn oil, ethyl acetate, methanol, and toluene. Tween 80 and ethanol are therefore used to increase dissolve quality of capsaicin. So the 1% (w/w) capsaicin solution were prepared as: 0.1 g crystalline capsaicin was first dissolved by 2 to 3 drops of ethanol as well as 15 drops of Tween 80, and then diluted to 10 ml with physiological saline solution, which was described by Jancsó [17]; the control solution was correspondingly made merely in absence of crystalline capsaicin.
Crystalline urethane was dissolved with physiological saline solution to finally make 5% (w/w) urethane solution as anesthetic. Studies in rats showed that urethane hardly affects respiratory system, blood pressure regulation and heart rate, so it is suitable for studying neural function in both the central and peripheral nervous systems [18].
-
B. Thermometry
Fig. 1 depicts the schematic diagram of experimental facility. All thermal images were taken with the best quality in terms of location and focus during experiments. The surrounding temperature was about 22 + 2°C, humility was 55 ± 5% during the whole experiment, and the surface emissivity was set to 0.98 as the recommendation for biological tissues [19]. Such values are indispensable for post processing of accurate temperature by commercial software (FLIR research IR).
-
C. Human Test
We recruited a healthy male volunteer to check the direct thermal effect by capsaicin on human body. Capsaicin solution were daubed on the local area of the dorsum of left hand (Position 1, as shown in Fig. 3) and on two local areas of the posterior aspect of the left forearm (Position 2 and 3, as shown in Fig. 3) by absorbent cotton; the dorsum of right hand and the posterior aspect of the right forearm was locally smeared with the control solution at the corresponding areas, respectively. The volunteer was asked to keep still during the whole experiment to avoid unnecessary heat caused by movement.

Figure 1. Schematic diagram of experimental facility. (1) a: 808nm near infrared laser; (2) b: thermal infrared camera (FLIR SC620); (3) c: personal computer (HP Pavilion dv2000); (4) d: experimental object; (5) the black line: wire; (6) the orange dualline: wire for data exchanges between personal computer and thermal infrared camera.
-
D. Animal Experiments
The KM mice, an outbred stock derived from Swiss albino mice, are widely employed in Chinese laboratories [20]. Thus they were used in the current work for study of thermal enhancement. All KM mice were separated by sex and randomly housed every two in specified plastic cages using a layer of wood shavings as bedding. They were available to commercial diets and water ad libitum.
A single intraperitoneal injection of anesthetic was taken to every mouse at the dose of 2g/kg. Then each one was fixed on a board and shaved on abdomen and thorax for ruling out disorders by hairs in thermal infrared images. The capsaicin solution was locally applied on the abdomen of mice. Then such KM mice were heated by the 808nm near infrared laser 5cm away from the heating region (abdomen skin) at a power output of 2W. For comparison, other animals were injected subcutaneously or intraperitoneally at a dose of 0.1 ml, and then heated by the same approach, respectively.
IV. Results and Discussion
-
A. Human Test
Fig. 2 presents cutaneous nerves and superficial veins of the dorsum of hand and the forearm. As it shows, basilic vein is distributed under the skin of the posterior aspect of the forearm and dorsal venous rete of hand is broadly distributed under the skin of the dorsum of hand.
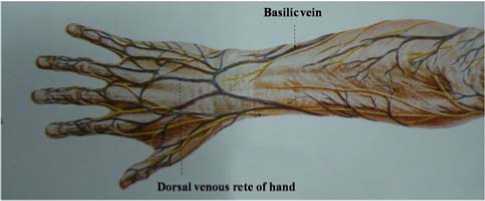
Figure 2. Superficial veins of the posterior aspect of the forearm and the dorsum of hand [21].
Fig. 3 depicts thermal images and temperature response of the dorsum of hand and the forearm after local application of capsaicin solution. As shown in Fig. 3 (a), initially, basilica vein and dorsal venous rete of hand are clearly showed in the thermal infrared image. As time goes on, temperature of areas smeared with capsaicin solution (Position 1-3) increase and these superficial veins trend to be blurry in thermal infrared images. Finally the overall areas of the dorsum of hand and the posterior aspect of forearm augment in temperature; meanwhile, superficial venous are absolutely invisible.
Fig. 3 (b) describes the average temperature responses of such areas (Position 1-3). At the beginning, the temperature of Position 1 (33.5 C ) is lightly higher than those of Position 2 (33.3 C ) and 3 (33.4 C ). Afterward, temperature of Position 2 and 3 ascend more markedly than that of Position1. Ultimately, all three positions level off at respective temperatures. The dynamically stable temperature of Position 3 wins the highest place (35.5 C ); Position 2 takes second place (35.4 C ); Position 1 is the lowest in dynamically stable temperature (35.1 °C).
About twenty minutes after application, heat and burning sensation occurred in these smeared areas (Position 13). Thereafter, this sensation diffused to the areas around. Such irritated sensation becomes even stronger when hot water is applied.
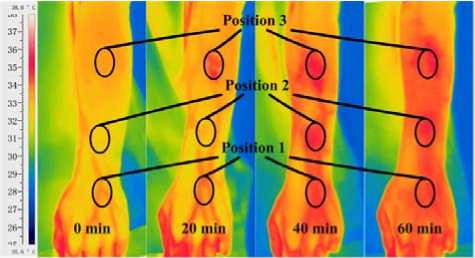
(a) Thermal Infrared images.
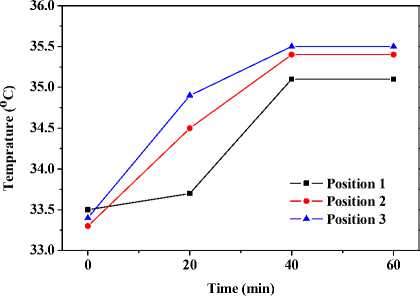
(b) Temperature responses.
Figure 3. Thermal images and temperature responses of the dorsum of hand and the posterior aspect of the forearm locally pre-smeared with capsaicin solution. (1) Position 1: interested skin area of the dorsum of hand; (2) Position 2: interested skin area (near the wrist joint) of the posterior aspect of the forearm; (3) Position 3: interested skin area (near the elbow joint) of the posterior aspect of the forearm.
Vanilloid Receptor has been found to be expressed in many neuronal and non-neuronal locations [12]. But its expression in small-diameter peripheral sensory neurons is at least 30-fold higher than anywhere else [22]. It contains six transmembrane domains, with cytosolic amino and carboxyl termini, and a pore loop between transmembrane domain 5 and 6 as a cation channel, [12] as schematically showed in Fig. 4. When vanilloid receptors in peripheral terminals of primary sensory neurons are activated by capsaicin, pore loop is open, and then cations (calcium ions, sodium ions and others) translocate inwards through the pore loop, which facilitates transmission of information about stimuli to the central nervous system (brain) [23]. This physiological process can be triggered not only by capsaicin but also by increases in temperature in the noxious range. In human test, the heat and burning sensation may be the results of the activation of capsaicin receptor; furthermore, hot water, as a kind of noxious heat stimuli, aggravates the heat/burning sensation.
Generally, contraction of muscle cell triggers the release of calcium ions from sarcoplasmic reticulum lumen to cytoplasm; then, SERCA pumps the calcium ions in the opposite direction by the energy from ATP hydrolysis to relax muscle cell. Mahmmoud suggested that capsaicin could augment the uncoupled SERCA without consuming released energy from ATP hydrolysis and that capsaicin may, therefore, directly evoke thermogenesis [16]. The thermogenesis may account for the temperature increases in capsaicin-applied regions.
In human test, capsaicin applied on Position 1 can permeate into dorsal venous rete of hand, and then be transmitted by blood flow to surrounding skin tissues. Therefore, the residual capsaicin is low in amount. Correspondingly, basilica vein can help transmission of capsaicin applied on Position 2. However the vein distribution density in Position 2 is lower than that of Position 1, thus the effects of transmission is relatively lower. Little capsaicin can be absorbed by skin [8], thereby capsaicin smeared on Position 3 is the slowest in diffusion, and residual capsaicin is the highest in amount. Due to capsaicin-induced thermogenesis, the average temperature of Position 3 is the maximum, whereas the average temperature of position 1 the minimum. And the diffused capsaicin evoked thermogenesis in the vicinity of these smeared areas (Position 1-3).
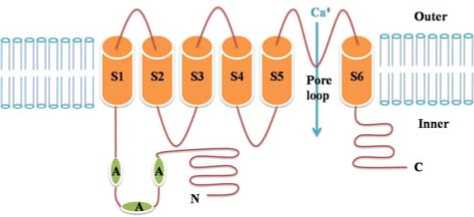
Figure 4. Schematic diagram of the Vanilloid Receptor 1 (modified from [14]). Outer: the outer side of membrane; Inner: the inner side of membrane; A: ankyrin repeats; N: amino terminal; C: carboxyl terminal.
-
B. Animal experiments
Fig. 5 illustrates the contrast of optical image and thermal infrared image of the same KM mouse. The thermal infrared image can show the rough outline of a mouse. In mammals, body core temperature is constant and generally higher than the environmental temperature, so the temperatures on abdomen and thorax temperatures of the mouse are higher than those of limbs and tail.
Fig. 6 presents the average temperature response of abdomen of another mouse after intraperitoneal injec-tionn of anesthetic. The average temperature first decreases and then levels off at around 34.9 C . This may be owing to the reduction of metabolism rate by anesthesia, and therefore a drop in body temperature.
1) Daub with Capsaicin
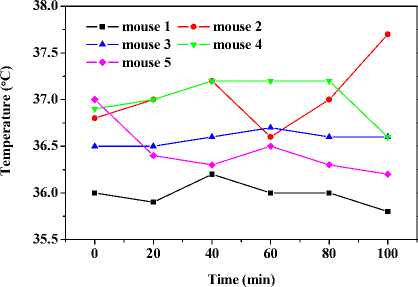
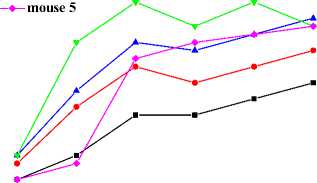
Fig. 7 depicts the maximal temperature responses of abdomens and right feet of mice after local daub of capsaicin solution on abdomen. As showed in Fig. 7 (a), the abdominal temperatures are almost invariable, respectively, in the first 80 minutes. Thereafter, the abdominal temperature of mouse 2 goes up, mouse 3 levels off, mouse 1 and 5 goes down slightly, and mouse 4 declines observably.
Temperatures of limbs and tail of mice rise after local application of capsaicin on abdomen. Fig. 7 (b) describes the average temperature responses of right feet after daub with capsaicin. In the first 40 minutes, average feet temperatures of all five mice ascend. Thenceforth, only mouse 4 fluctuates and then began to decrease in feet temperature, others continue to go up. The maximum temperature increase of the five mice is less than 2 °C during the overall process.
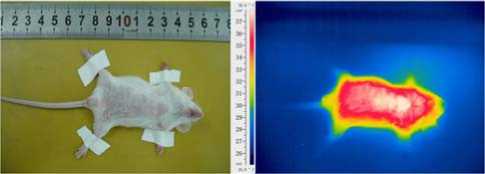
(a) Optical image (b) Thermal infrared image
Figure 5. Contrast of optical image and thermal infrared image of the same KM mouse.
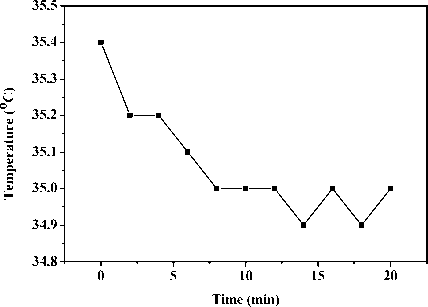
Figure 6. Average local abdominal temperature response of an anesthetic KM mouse.
(a) Temperature responses of abdomen.
32.0
31.5
31.0
30.5
30.0
29.5
29.0
mouse 1 mouse 2
mouse 3 mouse 4
0 20406080100
Time (min)
(b) Temperature responses of right feet shell.
Figure 7. Capsaicin-induced shell temperature responses of abdomen and right feet by local application on abdominal skin of KM mice.
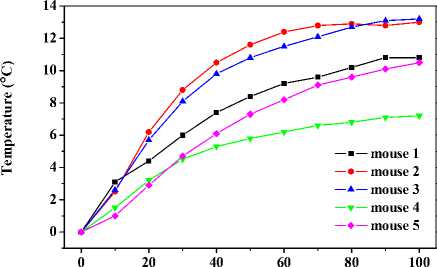
Fig. 8 gives the maximal temperature responses of above five mice heated by 808nm near infrared radiation, respectively. Five response curves can be clearly divided into three groups. Group 1 includes curves from mouse 2 and 3. The initial temperature of the two mice greatly increase, and the final temperature increase is 13 C and 13.2 °C , respectively. Group 2 contains curves from mouse 1 and 5. During the initial period of heating, their temperature increase moderately, and the overall temperature increase is respectively 10.8 C and 10.5 C . The curve of the left mouse, namely mouse 4, belongs to group 3. Its initial temperature goes up slightly, and the final temperature increase is 7.2 C , which is in accord with the heating results from mice smeared with control solution.
It can be concluded from Fig. 7 (a) and (b) that the capsaicin-induced thermogenesis on abdomen by the daub approach is not obvious, and that capsaicin-induced thermogenesis in cells of limbs and tail with the help of blood circulation is notable. In addition, we can infer that all mice were affected by capsaicin at the beginning (in the first 40 minutes) owing to the temperature increase in limbs and tail. 80 minutes later, capsaicin-induced thermogenesis in mouse 4 began to fall remarkably, mouse 1 and 5 went down slightly, mouse 3 maintained, and mouse 2 continued to heighten. Results form Fig. 8 sup- ports such conclusions. Temperature increase of mice with thermogenesis still existing turned out to be higher involving heating by 808nm near infrared radiation. These results preliminary confirm the obvious thermal enhancement by capsaicin in hyperthermia.
Time (s)
Figure 8. Effects of thermal enhancement by locally applied capsaicin solution on local region of abdominal skin when the same abdominal region was being heated by 808nm near infrared laser.
2) Injection of Capsaicin
Fig. 9 shows thermal infrared images of a mouse after intraperitoneal injection of capsaicin solution at a dose of 0.1ml. The first thermal infrared image can only give a rough outline of the mouse, which agrees with Fig. 4. Temperatures of limbs and tail lift from the beginning, and the outline of the mouse gradually become clear in latter thermal infrared images. At the third fourth image (around the third minutes), temperatures of limbs and tail ascend to the maximum. Thereafter, temperatures of body core, limbs and tail start to lower.
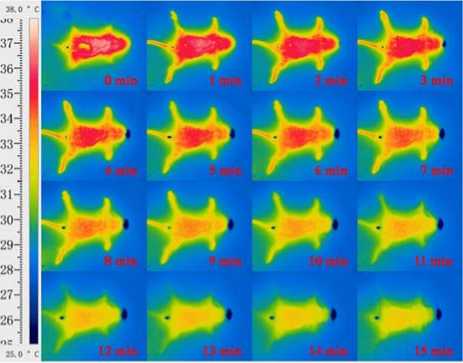
Figure 9. Thermal infrared images of an anesthetic mouse after an intraperitoneal injection of capsaicin solution at the dose of 0.1ml.
In the current work, capsaicin solution was injected in-treperitoneally or subcutaneously to anesthetized mice at a dose of 0.1ml. Fig. 10 shows the temperature responses of feet and abdominal temperature by such two ways of injection to two mice respectively. Both mice reduced in abdomen from the very beginning; and there was a period of temperature increase followed by a period of maintain in temperature and a subsequent temperature decrease in both mice. However, the feet temperature of one mouse, intraperitoneally injected of capsaicin solution, increased earlier than that of the other one.
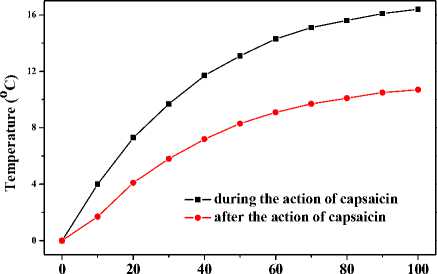
Another two mice were intraperitoneally injected of capsaicin at the same dose. Then, one mouse was heated by 808nm near infrared laser when feet temperature is in the maintained period; the other was also heated in the same way in the declined period. Fig. 11 depicts the abdominal temperature responses of the two mice. It shows that the initial temperature increase of former mouse is larger than that of latter one. The final temperature increase of the former mouse is turned out to be 16.4°C, which is prominently higher than all above effects of thermal enhancement. The final temperature increase of latter mouse is 10.7 C , which is close to those of group 2 in experiments where mice were daubed with capsaicin.
The abdominal temperatures drop when capsaicin is injected intreperitoneally or subcutaneously, which is in line with previous study [10]. Similar to results of applied method, there are temperature increases in both mice, which backing the thermogenesis in feet cell. Because capsaicin diffuses more rapidly through abdominal cavity than subcutaneous tissue, the start of thermogenesis in mice injected of capsaicin intraperitoneally occurs earlier. So the final temperature increases by injected method are higher than those of applied method.
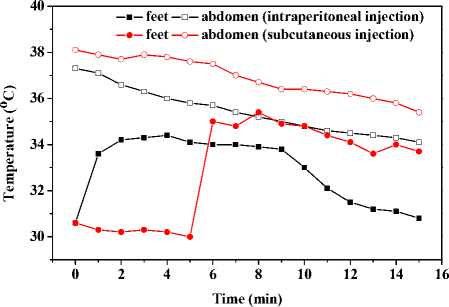
Figure 10. Capsaicin-induced temperature response of abdomens and feet by intraperitoneal or subcutaneous injection at the same dose.
Time (s)
Figure 11. Effects of thermal enhancement by intraperitoneal injected capsaicin solution during different period of action of capsaicin when local region of abdominal skin was being heated by 808nm near infrared laser.
-
V. Conclusions
An effective approach to thermal enhancement in tumor hyperthermia by adjuvant use of capsaicin was proposed in this study. Through experimental investigations on temperature responses of local skin areas of human body smeared with capsaicin solution in low concentration, as well as on abdominal skin of KM mice locally applied with, subcutaneously or intraperitoneally injected of the same capsaicin solution, with or without being subjected to near infrared radiation, the feasibility and efficacy of the capsaicin-induced thermal enhancement method was demonstrated. The experimental results indicate that this novel application of capsaicin can increase temperature caused by capsaicin-evoked thermogenesis, and that the adjuvant use of capsaicin to heating raised the initial and final temperature increase, which can serve as an applicable way to significantly enhance the heating effects of biological tissues during hyperthermia. This current work might be the basis to find a new effective modality for thermal enhancement in hyperthermia.
Acknowledgment
This work was financially supported by the National High Technology Research and Development Program of China (2009AA02Z414) and the National Natural Science Foundation of China (30770578).
Список литературы Capsaicin-induced Thermal Enhancement on Target Tissues in Hyperthermia
- Z. S. Deng, J. Liu, and J. R. Zhang, “Conformal RF abla-tion to reduce ‘dead region’ with adjuvant injection of magnetic micro/nano particles: feasibility study,” Interna-tional Conference on Integration and Commercialization of Micro and Nano-systems, 2007.
- Y. G. Lv, and J. Liu, “Research advancement on magnetic micro/nano particles aimed for tumor target hyperthermia treatment,” Nanomater. Struct., vol. 41, pp. 24-28, 2004. (in Chinese)
- J. Liu, and Z. S. Deng, Physics of tumor hyperthermia, Beijing: Science, 2008. (in Chinese)
- A. Akagi, N. Sano, H. Uehara, T. Minami, H. Otsuka, and K. Izumi, “Non-carcinogenicity of capsaicinoids inB6C3F(1) mice,” Food Chem. Toxicol., vol. 36, pp. 1065-1071, 1998.
- L. G. Copping, The BioPesticide Manual, 2nd ed., British Crop Protection Council, 2001.
- R. J. Bloomer, R. E. Canale, and K. H. Fisher-Wellman, “The potential role of capsaicinoids in weight manage-ment,” Agro Food Ind. Hi-Tech, vol. 20, pp. 33–35, 2009.
- W. Johnson, “Final report on the safety assessment of cap-sicum annuum extract, capsicum annuum fruit extract, cap-sicum annuum resin, capsicum annuum fruit powder, cap-sicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin,” Int. J. Toxicol, col.26, pp. 3–106, 2007.
- H. Knotkova, M. Pappagallo, and A. Szallasi, “Capsaicin (TRPV1 agonist) therapy for pain relief – Farewell or revi-al?” Clin. J. Pain, vol. 24, pp 142–154, 2008.
- A. Mori, S. Lehmann, J. O’kelly, et al., “Capsaicin, a com-ponent of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells,” Cancer Res., vol. 66, pp. 3222–3229, 2006.
- A. Jancsoga, J. Szolcsan, and N. Jancsó, “Irreversible im-pairment of thermoregulation induced by capsaicin and similar pugent substances in rats and guinea-pigs,” J. Phy-siol., vol. 206, pp 495–507, 1970.
- D. Turini, P. Beneforti, M. Spinelli, S. Malagutti, and M. Lazzeri, “Heat/burning sensation induced by topical appli-cation of capsaicin on perineal cutanesous area: New ap-proach in diagnosis and treatment of chronic prostati-tis/chronic pelvic pain syndrome?” Urology, vol. 67, pp. 910–913, 2006.
- M. J. Caterina, “Transient receptor potential ion channels as participants in thermosensation and thermoregulation,” Am. J. Physiol.-Regul. Integr. Comp. Physiol., vol. 292, pp. R64-R76, 2007.
- A. A. Romannosky, M. C. Almeida, A Garami, A. A. Steiner, M. H. Norman, S. F. Morrison, K. Nakamura, J. J. Burmeister, and T. B. Nucci, “The transient receptor poten-tial vanilloid-1 channel in thermoregulation: a thermosen-sor it is not,” Pharmacol. Rev., vol. 61, pp. 228-261, 2009.
- M. J. Caterina, M. A. Schumacher, M. Tominaga, T. A. Rosen, J. D. Levine, and D. Julius, “The capsaicin receptor: a heat-activated ion channel in the pain pathway,” Nature, vol. 398, pp. 816-824, 1997.
- M. J. Caterina, A. Leffler, A. B. Malmberg, W. J. Martin, J. Trafton, K. R. Petersen-Zeitz, M. Koltzenburg, A. I. Bas-baum, and D. Julius, “Impaired nociception and pain sen-sation in mice lacking the capsaicin receptor,” Science, vol. 288, pp. 306-313, 2000.
- Y. A. Mahmmoud, “Capsaicin stimulates uncoupled ATP hydrolysis by the sarcoplasmic reticulum calcium pump,” J. Biol. Chem., vol. 283, pp. 21418–21426, 2008.
- N. Jancsó, A. Jancsoga., and J. Szolcasn, “Direct evidence for neurogenic inlammation and its prevention by denerva-tion and by pretreatment with capsaicin,” Brit. J. Pharma-col. Chemother., vol. 31, pp. 138–151, 1967.
- C. A. Maggi, and A. Meli. “Suitability of urethane anesthe-sia for physiopharmacological investigations in various systems. 1. General-considerations,” Experientia, vol. 42, pp. 109-115, 1986.
- J. Speakman, and S. Ward, “Infrared thermograph: prin-ciples and applications,” 5th International Congress of Ver-tebrate Morphology – Noninvasive Imaging, Bristol Eng-land Gustav Fischer Verlag, pp. 224–232, 1998.
- G. H. Chen, W. J. Wang, L. Q. Zhang, and J. N. Zhou, “Age- and Sex-related disturbance in a battery of sensori-motor and cognitive tasks in Kunming mice,” Physiol. Be-hav., vol. 83,pp. 531-541, 2004.
- G.W. Guo, and X. Wang, Colour Atlas of Human anatomy, Beijing: Renmin Health, 2008. (in Chinese)
- J. F. Sanchez, J E. Krause, and D. N. Cotright, “The distri-bution and regulation of vanilloid receptor VR1 and VR1 5’ splice variant RNA expression in rat,” Neuroscience, vol. 107, pp. 373-381, 2001.
- S. Bevan, and J. Szolcsanyi, “Sensory neuron-specific actions of capsaicin: mechanisms and applications,” Trends in Pharmacol. Sci., vol. 11, pp. 330-333, 1990.


