Changeable watering conditions nonspecifically affect quantitative traits in Fusarium head blight–wheat/barley pathosystem
Автор: Sakr Nachaat, Kurdali Fawaz, Attar Jalal
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.21, 2025 года.
Бесплатный доступ
From an economic and toxicological point of view, Fusarium head blight (FHB), a biotic stress, is one of the most dangerous diseases of wheat and barley worldwide. It is recently known that humidity, an abiotic stress, enhances susceptibility of cereal heads to FHB infection and aggressiveness of Fusarium pathogens; however, whether its effect on pathogenicity in fungi and resistance in hosts is specifically or not is unknown. To end this, pot bio-experiments under natural climatic conditions during the growing season 2022/2023 were conducted to determine the nature of post-anthesis moisture influence on quantitative traits in FHB-wheat/barley interaction. Five durations of spray-irrigation of 0, 7, 14, 21, and 28 days, eight bread wheat, durum wheat and barley cultivars with different levels of FHB and 16 Fusarium isolates of various aggressiveness belonging to four FHB pathogens were involved under split-split-plot design. Evaluation of FHB quantitative traits to the variable watered conditions was through the quantification of Fusarium incidence, DI, Fusarium severity, DS, 21 days after inoculation as well as Fusarium -damaged kernel (FDK) percentages on harvested grain. Changeable watered conditions applied on plant materials of different resistance levels nonspecifically affected aggressiveness, DI, DS and FDK, of the tested Fusarium isolates whatever their pathogenic backgrounds (low, medium and high aggressiveness) and genetic origin (i.e., F. culmorum , F. solani , F. verticillioides , and F. equiseti ). The four susceptible responses, i.e., susceptible, susceptible to moderately susceptible, moderately susceptible and moderately resistant, did not vary also in their relative rankings in the eight tested cereal plants. From a grower’s perspective, prolonged rainy periods following wheat and barley flowering should be a signal to scout wheat and barely crops for FHB symptoms and mycotoxin accumulation, even if pre-flowering conditions were not conducive to FHB. This indicates for the first time that post-anthesis moisture nonspecifically affect development of Fusarium isolates in tissues of wheat and barley heads. Our results have obvious implications for disease epidemiology in complex relationship moisture, resistances of cultivars, and aggressiveness of Fusarium pathogens.
A pot bio-experiment, fusarium species, hordeum vulgare, post-anthesis moisture, resistance, triticum spp
Короткий адрес: https://sciup.org/143184744
IDR: 143184744
Текст научной статьи Changeable watering conditions nonspecifically affect quantitative traits in Fusarium head blight–wheat/barley pathosystem
Globally, bread wheat ( Triticum aestivum ) is a worldwide crop of main nutritional importance and economic value, as it provides 20% of the protein and calories consumed by the world’s population. Annually, T. aestivum it is sown over an area of 219 million ha and yielding over 760 million tons (FAOSTAT, 2023). Durum wheat ( Triticum durum ) is one of the most essential cereal species and is planted in different parts of the world over almost 17 million ha (5% of the total wheat grown globally), with a worldwide production of 38.1 million tonnes in 2019. The largest producer is the European Union, followed by Canada, Turkey, United States, Algeria, Mexico, Kazakhstan, Syria, and India (FAOSTAT, 2023). T. durum production and cultivation locations are concentrated in the Mediterranean basin. This type of wheat is preferred for preparing high quality pasta, semolina, and flour products because of the high gluten strength and protein content of the kernel. Barley ( Hordeum vulgare ) is a main dietary component of human beings and is also known as the poor man’s crop as it necessities low input and has better adaptability to marginal lands, alkalinity, salinity, and drought. Barley is largely sown as livestock feed but also utilized for human consumption as beer and malt or for cooking and baking purposes. In novel years, there has been a growing interest in H. vulgare for food because of recent research confirming its health benefits in human diets. It is planted over the spring season in almost all parts of the world with arid or semi-arid environments, particularly in the driest areas (15 million ha annually) of the Mediterranean basin including Syrian fields (FAOSTAT, 2023). Biotic constraints, i.e., pests and pathogens, are most serious stresses to Triticum and Hordeum production and would continue to be a main challenge in achieving the goal sustainable food security. Bread wheat, durum wheat, and barley as other gramineous crops, suffers from susceptibility to Fusarium head blight (FHB), a noxious fungal disease that affects small-grain cereals in many growing areas of the world (Parry et al., 1995; McMullen et al., 2012). The abundance of climatic conditions, especially moisture, during and after anthesis detects the intensity of FHB disease (Ma et al., 2019; Buerstmayr et al., 2020).
Fungi of the genus Fusarium infest gramineous crops planted all over the world (Fernando et al., 2021), but the occurrence of individual Fusarium pathogen depends on weather conditions, principally on temperature and humidity (Dahl and Wilson, 2018). Thus, observations about the most conductive metrological parameters, especially those linked to humidity, which were proven to influence the infestation with Fusarium species, are required to predict the occurrence in a particular field (Cowger et al., 2009; Shah et al., 2014). Epidemic occurrence of FHB disease is the cause of significant economic damages due to infections of panicles and heads by Fusarium pathogens leads to a significant decrease in the quality and size of kernel yield (Sakr, 2022). The level of contamination of wheat/barley kernel with Fusarium mycotoxins, deoxynivalenol (DON), accounts on several factors, among others climatic conditions, method and date of grain harvest, cultivation system, as well as the level of resistance of cultivated cultivars to Fusarium pathogen infection (Mesterhazy, 2024).
FHB outbreak are strongly dependent on the efficient environmental parameters; a moist and warm climate with high relative humidity (above 90%) can stimulate conidial ascosporic and conidial dispersal by rain, wind or insects to healthy wheat/barley heads to trigger infestations during anthesis (Moonjely et al., 2023). FHB is provoked, principally, by pathogens within the Fusarium graminearum species complex, with F. graminearum sensu stricto being the most-recorded causative agent (McMullen et al., 2012; Ma et al., 2019). Some other Fusarium pathogens such as F. culmorum, F. avenaceum, F. cerealis, and F. poae can also provoke the FHB disease (Mesterhazy, 2020). DON is a main mycotoxin released by Fusarium species (Toth et al., 2020) and is a pathogenicity factor in FHB (Miedaner et al., 2021). In the field, the Fusarium inoculum remains on crop debris as macroconidia (nonsexual structures) or ascospores within perithecia (sexual structures), and there it can resist the weather variables (Parry et al., 1995). The spore deposition on wheat and barley heads was highly linked with rain duraions in fields with residues (Dahl and Wilson, 2018). Under conductive climatic conditions (high relative humidity and warm temperatures) over wheat/barley anthesis, the inoculum is able of dispersing by rain or wind to reach the anther and start the plant colonization and infection (Mesterhazy, 2024). First, the spores germinate; then the hyphae grow on the lemma, pale, and ovary; and, after that, they start mycotoxin production (Fernando et al., 2021).
One of the most efficient ways to control FHB disease is to grow cultivars that include Fusarium resistance genes (Ma et al., 2019). Resistance to the primary infestation of the head and resistance to fungal development and spread from the infection site along the head are called Type I and Type II resistance, respectively, as well as Type III, resistance to grain infection (Fernando et al., 2021). Nevertheless, the challenge with Fusarium -resistance breeding is that it is quantitative and includes a combination of several genes (Mesterhazy, 2024). Till now, strategies to control FHB are restricted since commercial wheat/barley cultivars with high degress of resistance are not available to growers (Buerstmayr et al., 2020). In the gramineous crop- Fusarium pathosystem, a main factor that determines the parasitic fitness of a strain is aggressiveness (Xue et al., 2019), which is a quantitative measurement of the degree of disease provoked by the pathogen (Lannou, 2012). It reflects different basic quantitative features of the fungal life cycle, such as spore production rate, sizes of the lesion, infection efficiency and for some cases, also toxin accumulation (Lannou 2012). Numerous works have been highlighted the highest significant diversities in aggressiveness of diverse FHB pathogens and even among strains within the same pathogen (Xue et al., 2019; Toth et al., 2020; Miedaner et al., 2021; Sakr, 2022, 2023; Mesterhazy, 2020, 2024).
Fusarium species infect wheat-barley heads at different times (Parry et al., 1995), but the most susceptible to FHB infestation by these fungi is in the flowering stage and immediately after anthesis (McMullen et al., 2012), practically in humid and warm weather conditions (Dahl and Wilson, 2018), and abundant prolonged rainfall and dew over this period (Fernando et al., 2021). Heavy dew, high humidity, and frequent rainfall that coincide with the period of gramineous crop susceptibility, which extends from anthesi to the soft dough stage of grain development, favor FHB infection (Moonjely et al., 2023). Moisture in the form of relative humidity or rainfall, during or shortly after flowering, has been associated to higher Fusarium incidences, Type I and Fusarium severities, Type II (Rohacik and Hudec 2005). Climatic variables, especially humidity, play a crucial role in FHB development and DON accumulation in infected grains. Several works exhibited that moisture in late stages after flowering, i.e., 10, 20, and 30 days after inoculation enhanced disease incidence, disease severity, and DON level of F. graminearum in bread wheat (Lemmens et al., 2004; Nita et al., 2005; Culler et al., 2007; Cowger et al., 2009; Gautam and Dill-Macky 2012; Andersen et al., 2015). Cowger and Arellano (2010) reported that asymptomatic bread wheat field with low infested grain might also constitute higher FHB disease damage due to rainfall and late infection immediately after flowering. It is well reported that conductive moisture variables lead to more aggressive strains (Sakr, 2022) that will cause more damage and therefore greater economic loss (Xue et al., 2019; Miedaner et al., 2021).
Recently, Sakr et al., (unpublished data) reported that humidity durations for 14, 21 and 28 days after inoculation enhances susceptibility of wheat bread, durum wheat and barley heads to FHB infection and aggressiveness of Fusarium pathogens, i.e., F. culmorum , F. solani , F. verticillioides , and F. equiseti ; but treatments receiving the least amount of sprayirrigation (0 and 7 days of spray) did not affect both: aggressiveness and resistance; showing that extended moisture promotes Fusarium development and enhances grain fungus colonization in head tissues. Nevertheless, whether the humidity effect on pathogenicity in fungi and resistance in host plants is specifically or not is unknown. In the present investigation, our goal was to determine the nature of post-anthesis moisture influence on quantitative traits, pathogenicity in fungi and resistance in host plants, in FHB-wheat/barley interaction. Thus a clearer epidemiological picture will be emerged on the complex relations among moisture, resistances of cultivars, and aggressiveness of Fusarium pathogens.
MATERIALS AND METHODS
Plant materials and growth conditions
Throughout this study, a set of widely grown high-yielding eight cereal cultivars of Syrian origin with favorable agronomic and quality traits (high harvest index, earliness, shorter plants and improved biomass partitioning to the grain) and resistance to different fungal diseases and covering a wide genetic and resistant variability including six T. aestivum and T. durum cultivars and two H. vulgare cultivars: Arabi Abiad (AB) and Arabi Aswad (AS) was chosen from previous in vitro , growth chamber and field experiments (Sakr, 2023) to represent a range of quantitative resistance types to FHB infection. Wheat and barley cultivars AS and Bohoth10 (released in 2014, bread) moderately resistant, AB, Cham4 and Douma4 (released in 1986, 2007, respectively, bread) moderately susceptible, Cham7 and Cham9 (released in 2004, 2010, respectively, durum) susceptible to moderately susceptible, and Acsad65 (released in 1984, durum, susceptible) were used.
Eight surface-sterilized wheat and barley seeds were sown in plastic pots (20 × 15 cm) containing of thoroughly mixed and pasteurized soil (2kg) collected from the field at the depth layer of 0 to 0.2m. Soil was air dried before being ground and sieved to remove small rocks and plant litter. Using a gamma irradiator (ROBO, Russia), the pasteurization of the soil was carried out at 5 k Gy of Gamma Ray with Co60 source. The experimental soil was clay in texture (57% clay, 39% loam, 2% sand) with organic matter of 1.25%; K, Na, Ca, Mg = 1.81, 2.99, 33.1, 14 mg/100 g soil respectively; P = 13.4 mM and pH 7.8. One week after germination, plants were thinned to five plants per pot, and nitrogen, in the form of urea, was applied at 0.173 g/pot at two dates: emergence and tillering. Each pot was used as one treatment. The pots were placed outdoor under natural climatic conditions. To decrease year impacts on results, it appeared to be important in these arid conditions to help the growth development at roughly weekly intervals afterward by irrigation of bread wheat pots.
Fungal isolates and inoculum preparation
Sixteen single-spored cultures of four Fusarium species causing head blight, i.e. ( F. culmorum (5 isolates), F. solani (6 isolates), F. verticillioides (synonym F . moniliforme ) (4 isolates), and F. equiseti (one isolate)), representative of the range in aggressiveness capacity were used. These isolates were used in previous studies and proved their aggressiveness ability. The isolates were collected through the 2015 growth season from naturally infected wheat heads over 9 locations in Ghab Plain with a FHB history, one of the principal Syrian wheat production areas. By using the keys of Leslie and Summerell (2006), single spore cultures on Petri-dishes with potato dextrose agar (PDA) with 13 mg/l kanamycin sulphate added after autoclaving, were classified morphologically to species level. By using random amplified polymorphic DNA markers, the 16 Fusarium species causing head blight isolates were recently analyzed (Sakr, 2023). The isolates were stored by freezing at-16°C or in sterile distilled water at 4°C until used for inoculation (Sakr, 2020).
To produce conidia for artificial inoculations, isolates were cultured in Petri dishes on PDA under continuous darkness at 22ºC for 10 days to allow sporulation and fungal development. The macroconidia were harvested from 7-day-old cultures following covering of fungal cultures with 10 ml of sterile distilled water and filtrating through 2 layers of sterile cheesecloth to remove the pieces of mycelia and agar as described by Sakr (2023). Inoculum was directly adjusted to desirable concentrations as inoculum sources with a Neubauer chamber under an optical microscope.
Experimental Design and Treatments
A split-split-plot experiment with five replications was conducted at the research field of Deir AL-Hajar Station located south east of Damascus, Syria (36° 280E, 33° 210 N; altitude 617m) in the growing season of 2022/2023. The site is located within a dry Mediterranean semiarid area with hot-dry summer and cold winter. The total annual rainfall is about 120 mm, and most precipitations occur between November and early April. For the last ten years, the average minimum temperature in winter was 1.3°C in January, while it increases to the average maximum temperature of 38.3 °C in July. Some climatic data of the experimental site during the growing period are shown in Table 1.
Experiments were split-split-plots with five replications. Main plots were spray-irrigation treatments with four levels [spray irrigation from inoculation until 7, 14, 21, or 28 days after inoculation (DAI) which corresponds to the wheat and barley growth stages of 67, 75, 83 and 85, respectively]. Sub-plots were the eight bread wheat, durum wheat and barley cultivars [Acsad65, Cham7, Cham9, Cham4, Douma4, Bohoth10, AB and AS] and the sub-sub-plots were the sixteen isolates of four Fusarium species with contrasting aggressiveness (Figure 1). Replications within each spray-irrigation main plot, cultivars within replications and isolates within the cultivar sub-plots were assigned randomly. Five replicate pots of each cultivar were subjected to each duration of post-anthesis spraying, and the experiment was repeated twice.
Inoculation and spraying
For inoculation of pots placed under natural climatic conditions, bread wheat, durum wheat and barley cultivars were separately inoculated with the 16 Fusarium cultures to evaluate the incidence of FHB (DI, Type I resistance), severity of FHB (DS, Type II) and Fusarium- damaged kernels (FDK, Type III) as indicators of the cultivar’s resistance and aggressiveness of isolates. At full flowering (GS=65) in the early morning, inoculation of wheat and barley plants was conducted by foliar spraying of the spore suspension or SDW for noninoculated wheat and barley plants. Uniformly spraying of the inoculum onto wheat and barley heads was carried out on one date. The inoculated wheat heads were kept inside plastic bags the inner surfaces of which had been sprayed with SDW. Acsad65, Cham7, Cham9, Cham4, Douma4, Bohoth10, AB and AS plants were maintained under these conditions for 48 h in order to provide humid conditions favorable for the initial phase of pathogenesis.
Spraying was provided using a sprinkler heads. The different durations of post-anthesis spray were provided by opening or closing individual lateral irrigation lines. By means of a programmable timer, spraying was provided for 2 min of each 20-min period for 3 h each in the morning and afternoon, or a total of 36 min per day in order to avoid drying of inoculum. All periods of postanthesis spraying (0, 7, 14, 21 or 28 DAI) started on in April, 2023. Spraying treatments were initiated at the first inoculation date.
Disease assessment
Under natural climatic conditions, disease assessments were conducted on each cultivar approximately 21 days after it had been inoculated. DI, DS and FDK were determined to decide the degree of Fusarium infection in light of visual damages in head tissues under changeable watered conditions. DI was determined as the percentage of heads with diseased spikelets and DS as the mean percentage of diseased spikelets in the infected heads. After harvesting, mature infected heads from each replication were taken for further evaluations; the heads were threshed to save infected and shrivelled grains. The percentage of scabby (tombstone) kernels was rated visually on one hundred kernels for each replication to assess FDK for Type III (Mesterhazy et al., 1999). Pathogenic reactions of all cultivars infected with Fusarium fungi under changeable watered conditions were previously evaluated according to methods described by Sakr et al., (unpublished data).
Statistical analyses
The experimental data were subjected to analysis of variances (ANO A) using DSAASTAT add-in version 2011. Before statistical analysis, the percentages were transformed using the angular transformation to achieve homogeneity of variances. DI, DS and FDK data from 0, 7, 14, 21, and 28 DAI treatments were combined, as all three treatments received equal spraying until the time when FHB damage was assessed. ANO A incorporating the Fisher’s LSD test at p<0.05 was used to compare the resistance of cultivars and aggressiveness of fungi. Comparison for aggressiveness of FHB isolates under changeable watered conditions was made by the contrast procedure.
RESULTS
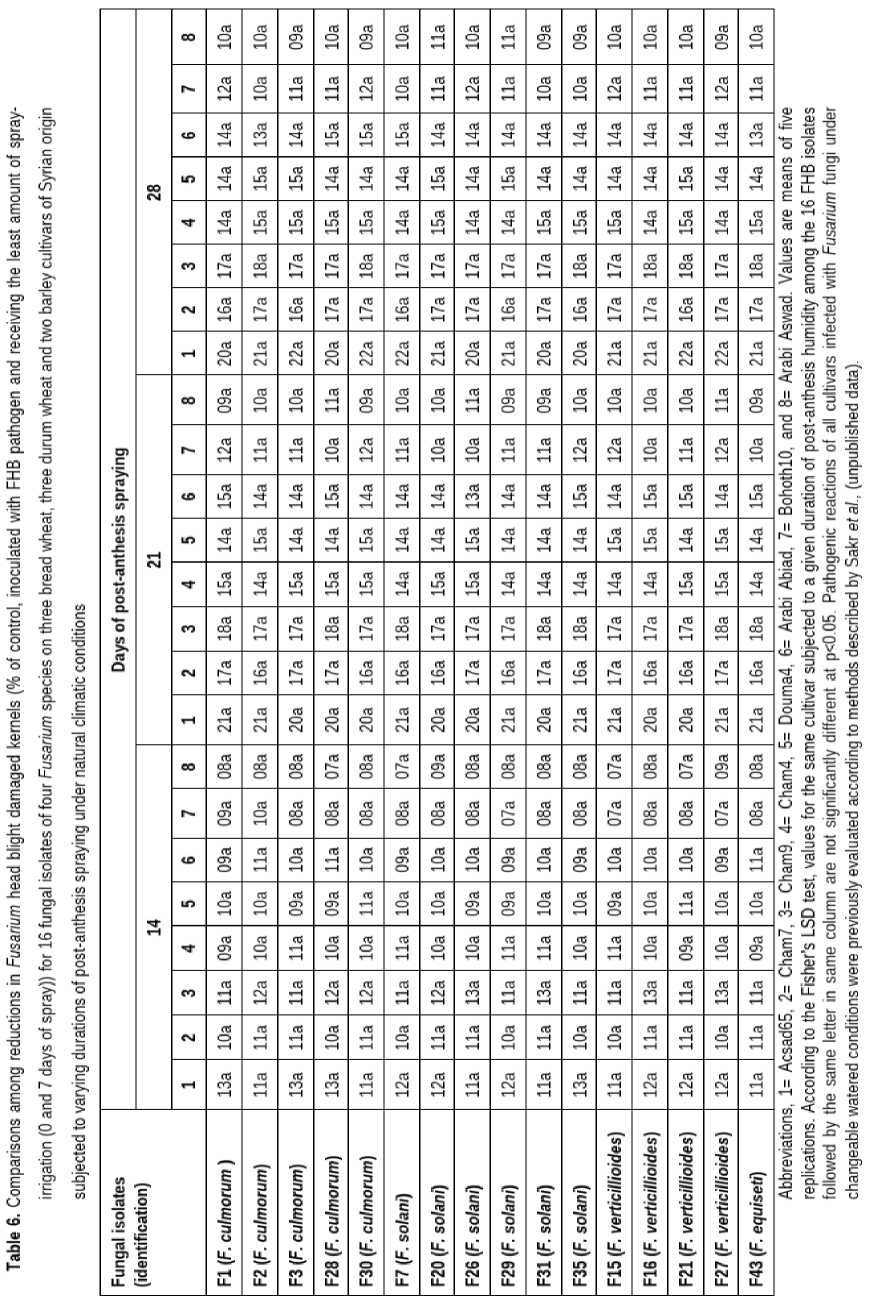
Temperature and rainfall (Table 1) during the bread wheat cycle were favorable to FHB disease development in small-grain cereals (Figure 1) over the growing season of 2022/2023. ANO A showed that moisture, isolate, cultivar, isolate × cultivar, isolate × moisture interaction, and cultivar × moisture interaction had highly significant effects (p<0.05) on DI and DS (data not shown). However, significant interactions (p<0.05) were observed for isolate × moisture interaction and cultivar × moisture interaction regarding FDK. The four susceptible responses, i.e., susceptible, susceptible to moderately susceptible, moderately susceptible and moderately resistant, did not vary also in their relative rankings in the eight tested cereal plants (Tables 2 and 3). Overall, changeable watered conditions applied on plant materials of different resistance levels nonspecifically affected aggressiveness, DI, DS and FDK, of the tested Fusarium isolates whatever their pathogenic backgrounds (low, medium and high aggressiveness) and genetic origin (i.e., F. culmorum, F. solani, F. verticillioides, and F. equiseti) (Tables 4, 5 and 6).
None of the eight tested wheat and barley cultivars: Acsad65, Cham7, Cham9, Cham4, Douma4, Bohoth10, AB and AS was immune from FHB disease under changeable watered conditions (Tables 2 and 3). On host plants, duration of post-anthesis spraying irrigation resulted in higher disease intensity based on the percentage of diseased florets and smaller necrotic patches, and more bleaching of the florets and discoloured kernels as compared to treatments receiving no moisture excess (Figure 2). Average disease intensities, when combined across spray-irrigation, eight wheat and barley cultivars and Fusarium isolate treatments, were 12.9%, 13.6% and 16.5%, respectively, for DI, DS, and FDK, respectively. Overall, the disease DI and DS for Acsad65, Cham7, Cham9, Cham4, Douma4, and AB cultivars were significantly higher than in moderately resistant cultivars Bohoth10 and AS (Tables 2 and 3). However, the disease FDK for Acsad65, Cham7, Cham9, Cham4, Douma4, Bohoth10, AB and AS was not statistically different because FDK criterion did not differentiate FHB isolates and the eight tested bread wheat, durum wheat and barley cultivars following artificial infection
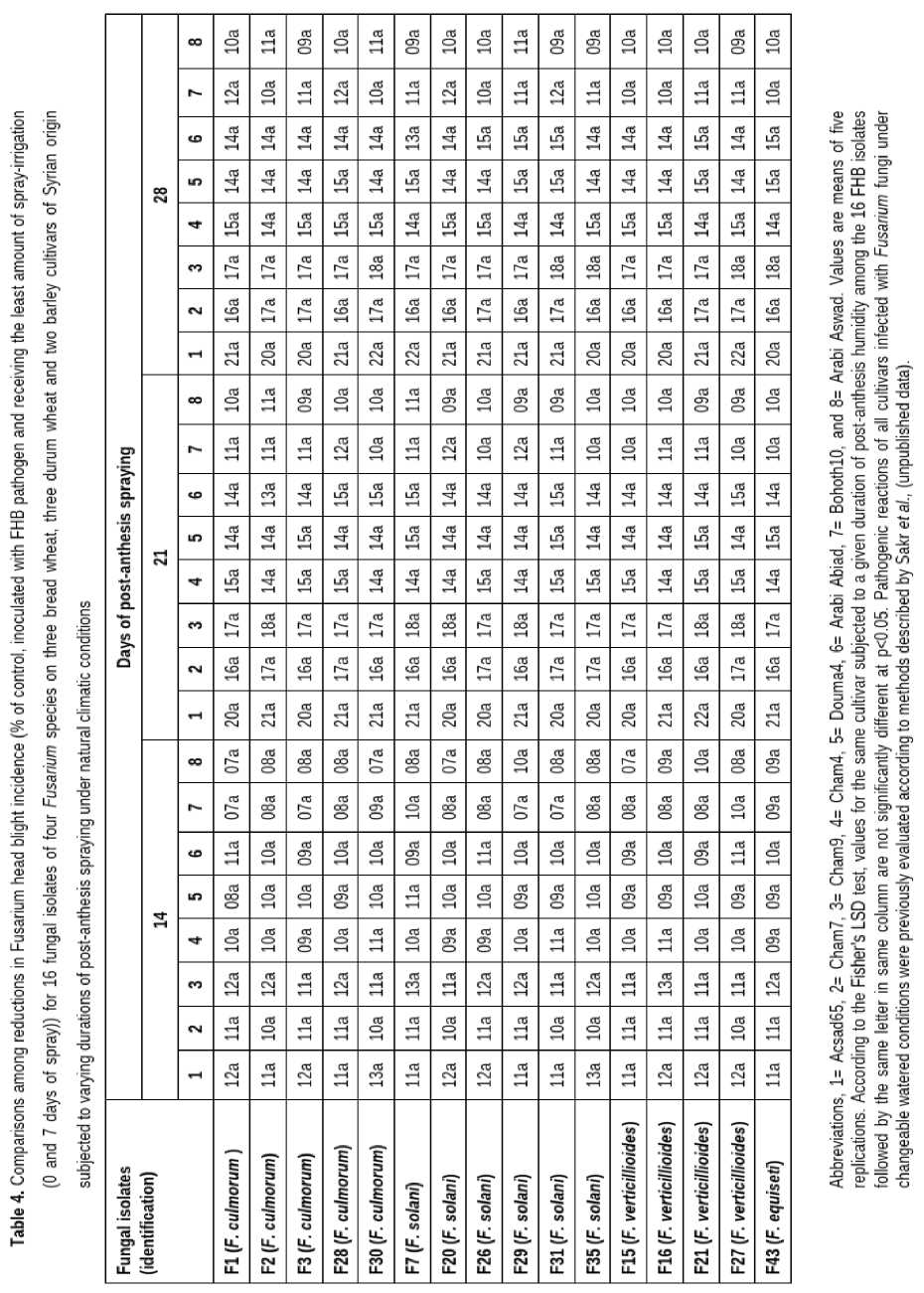
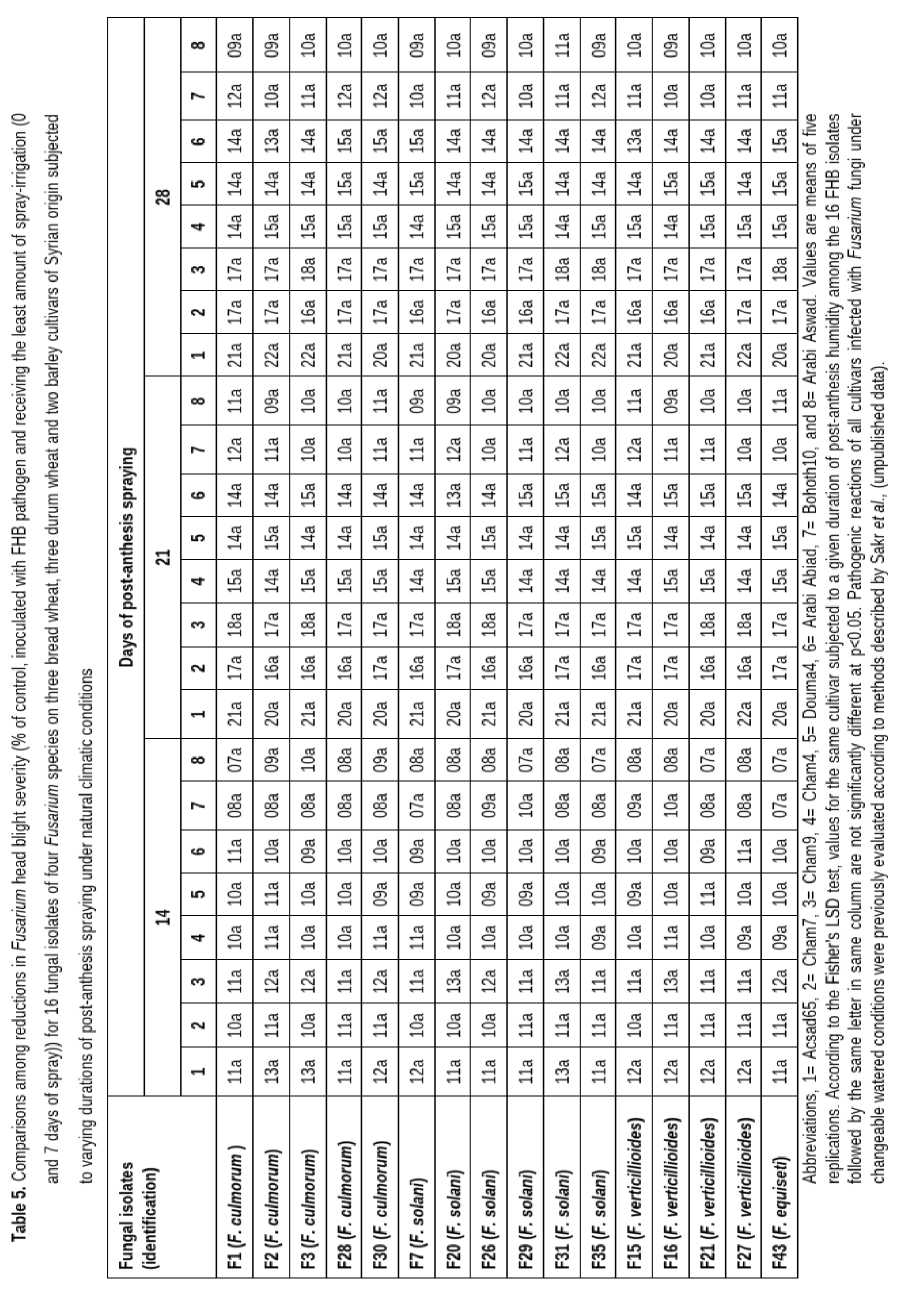
All the 16 tested FHB isolates were pathogenic and induced clear and typical FHB symptoms which are easy to score in the inoculated spikes and spikelets across durations of spray-irrigation of 0, 7, 14, 21, and 28 days (Tables 4, 5 and 6), suggesting a strong effect of duration of moisture on Fusarium growth in head tissues of the three wheat cultivars, while no symptoms were present in the control with no fungal infection. Isolates did significantly differ for DI and DS in all tested cultivars. However, The FHB DI and DS did not vary in their relative rankings under changeable watered conditions. Regarding FHB FDK, fungal isolates did not vary in Acsad65, Cham7, Cham9, Cham4, Douma4, Bohoth10, AB and AS resulting in constancy of aggressiveness order among Fusarium isolates.
Spray irrigation duration treatments had a significant impact on all pathogenic components evaluated in the current investigation. Longer periods of spray-irrigation resulted in increased DI, DS and FDK values. Generally, no significant differences were observed for DI, DS and FDK between treatments receiving the least amount of spray-irrigation (0 and 7 days of spray), suggesting that extended moisture promotes disease development and increases grain fungus colonization. 21 or 28 days of spray had the same effect and were associated with an increase in mean DI by 14.9% and 15.4%, respectively and DS by 15.0% and 14.4%, respectively compared with 0 or 7 days of spray, and 14 days of spray was also associated with an increase by 9.8% and 10.5%, respectively in mean of these pathogenic criteria (Tables 4, 5 and 6). Mean FDK percentages at 0 and 7 days of spray were the same and significantly lower than FDK percentages under 21 days by 17.5% or 28 days by 17.8% of spray, and 14 days of spray was also associated with an increase by 14.4% in mean of FDK (Tables 4, 5 and 6).
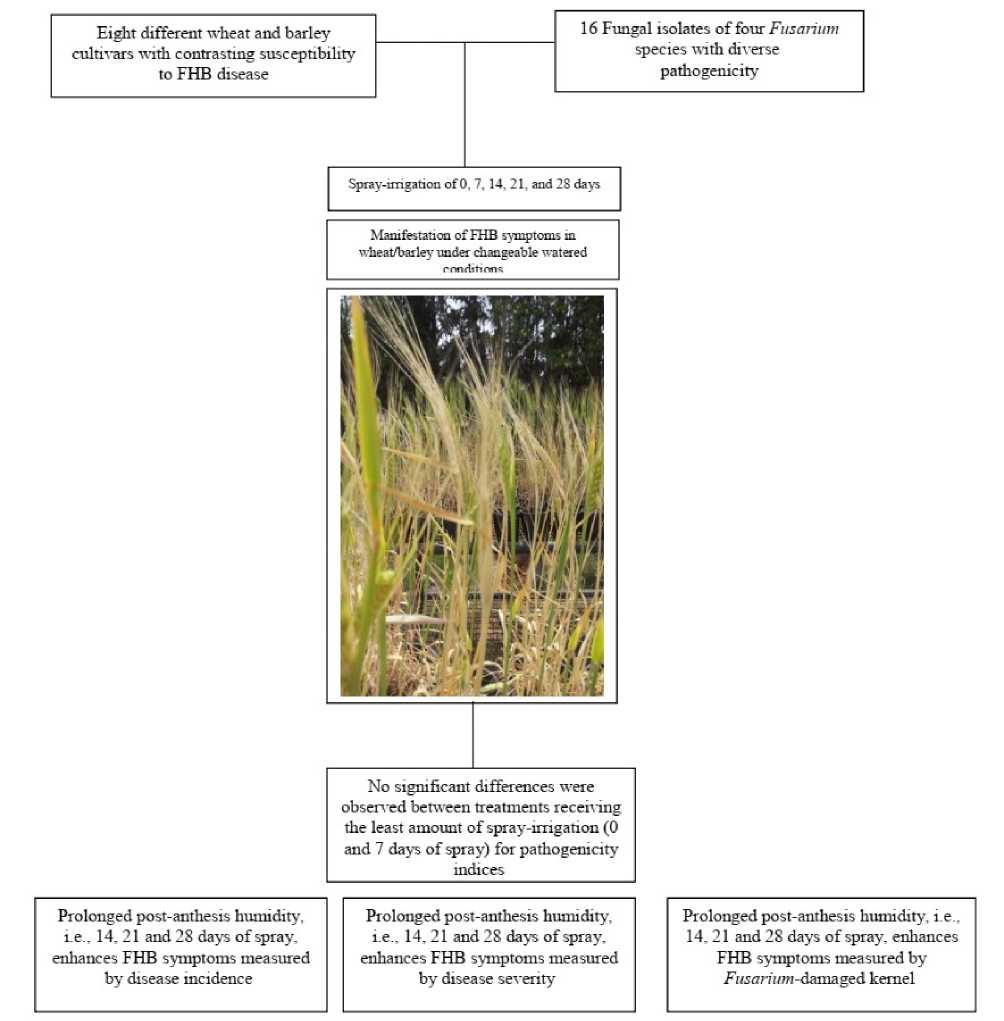
Figure 1. A schema of the experimental design in the study
Table 1 : Some climatic data collected over the growing seasons 2022/23 at the experimental station
|
Growing season |
Variable |
November |
December |
January |
February |
March |
April |
May |
|
2022/23 |
T min (oC) |
8.4 |
4.7 |
3.8 |
3.9 |
7.2 |
9.9 |
13.2 |
|
T max (oC) |
21.0 |
16.1 |
14.2 |
16.5 |
20.3 |
30.2 |
31.3 |
|
|
RH (%) |
72.0 |
74.0 |
83.5 |
75.5 |
73.5 |
54.5 |
53.5 |
|
|
Rainfall (mm) |
47.5 |
42.0 |
26.9 |
18.1 |
23.7 |
6.4 |
4.0 |
Abbreviations: T min – minimum temperature; T max – maximum temperature; RH – relative air humidity.

Figure 2. Fusarium head blight symptoms on heads of bread wheat cultivar Bohoth10 inoculated with Fusarium pathogen subjected to varying durations of post-anthesis spray, i.e., 0, 7, 14, 21, and 28 days of spray, under natural climatic conditions compared with negative water control
Table 2. Mean values (%) for Fusarium head blight incidence (Type I) of three bread wheat, three durum wheat and two barley cultivars infected with 16 fungal isolates of four Fusarium species and subjected to varying durations of post-anthesis spray under natural climatic conditions
|
Cultivars |
Days of post-anthesis spraying |
|||||||
|
0/7 days |
14 days |
21 days |
28 days |
|||||
|
% |
Rank |
% |
Rank |
% |
Rank |
% |
Rank |
|
|
Acsad65 |
54a |
1 |
62a |
1 |
68a |
1 |
69a |
1 |
|
Cham7 |
49b |
2 |
55b |
2 |
58b |
2 |
59b |
2 |
|
Cham9 |
46b |
2 |
52b |
2 |
56b |
2 |
58b |
2 |
|
Cham4 |
41c |
3 |
44c |
3 |
51c |
3 |
49c |
3 |
|
Douma4 |
42c |
3 |
45c |
3 |
52c |
3 |
47c |
3 |
|
Arabi Abiad |
42c |
3 |
46c |
3 |
49c |
3 |
50c |
3 |
|
Bohoth10 |
39d |
4 |
42d |
4 |
44d |
4 |
43d |
4 |
|
Arabi Aswad |
38d |
4 |
41d |
4 |
42d |
4 |
42d |
4 |
Abbreviations, 1= susceptible, 2= susceptible to moderately susceptible, 3= moderately susceptible and 4= moderately resistant. alues are means of five replications. According to the Fisher’s LSD test, values for the same cultivar subjected to a given duration of post-anthesis humidity followed by the same letter in same column are not significantly different at p<0.05. Pathogenic reactions of all cultivars infected with Fusarium fungi under changeable watered conditions were previously evaluated according to methods described by Sakr et al., (unpublished data).
Table 3. Mean values (%) for Fusarium head blight severity (Type II) of three bread wheat, three durum wheat and two barley cultivars infected with 16 fungal isolates of four Fusarium species and subjected to varying durations of post-anthesis spray under natural climatic conditions
|
Cultivars |
Days of post-anthesis spraying |
|||||||
|
0/7 days |
14 days |
21 days |
28 days |
|||||
|
% |
Rank |
% |
Rank |
% |
Rank |
% |
Rank |
|
|
Acsad65 |
41a |
1 |
48a |
1 |
51a |
1 |
52a |
1 |
|
Cham7 |
38b |
2 |
44b |
2 |
46b |
2 |
47b |
2 |
|
Cham9 |
37b |
2 |
42b |
2 |
45b |
2 |
46b |
2 |
|
Cham4 |
34c |
3 |
36c |
3 |
39c |
3 |
41c |
3 |
|
Douma4 |
33c |
3 |
35c |
3 |
37c |
3 |
38c |
3 |
|
Arabi Abiad |
32c |
3 |
37c |
3 |
38c |
3 |
37c |
3 |
|
Bohoth10 |
30d |
4 |
31d |
4 |
33d |
4 |
34d |
4 |
|
Arabi Aswad |
29d |
4 |
31d |
4 |
33d |
4 |
33d |
4 |
Abbreviations as in Table 2.
DISCUSSION
Fusarium head blight in Triticum and Hordeum is one of the noxious diseases worldwide and caused high losses of yield and kernel quality. It is well documented that when humidity is conductive (Ma et al., 2019), Fusarium development and spread can occur any time after commencement of anthesis in wheat and barley (Mesterhazy, 2020, 2024), but anthesis is the growth stage most vulnerable to infection (Miedaner et al., 2021). Thus, major outbreaks of FHB were principally associated to humid, wet weather conditions during flowering and maturity stages (Fernando et al., 2021). A better understanding of the function of moisture on this disease infection in wheat and barley head tissues is crucial for refining and developing prediction models to help guide FHB control and kernel marketing decisions (Moonjely et al., 2023). It is very obvious from designed experiments and empirical observations that prolonged durations of high moisture, especially around flowering, are critical for F. graminearum development on T. aestivum (Lemmens et al., 2004; Nita et al., 2005; Culler et al., 2007; Cowger et al., 2009; Gautam and Dill-Macky 2012; Andersen et al., 2015) and F. culmorum, F. solani, F. verticillioides, and F. equiseti on bread wheat, durum wheat and barley (Sakr et al., unpublished data). However, whether its effect on pathogenicity in fungi and resistance in hosts is specifically or not is unknown. To fill this gap, we reported for the first time the nature of post-anthesis moisture at and ⁄ or shortly after anthesis non-specific influence on quantitative traits in FHB-wheat/barley interaction. Our data have clear implications for FHB disease epidemiology in complex relationship involving humidity, resistances of cultivars, and aggressiveness of Fusarium pathogens. From a grower’s perspective, extended rainy durations following wheat/braley flowering should be a signal to scout Triticum and Hordeum crops for FHB symptoms and mycotoxin accumulation, even if pre-flowering conditions were not suitable to FHB.
Isolate diversity for pathogenicity and toxin production has been well documented in Fusarium pathogens (Toth et al., 2020; Sakr, 2022). In this study, although the sixteen isolates tested changed in their ability to produce FHB disease symptoms in inoculated heads under natural climatic conditions, moisture in late stages after anthesis, i.e., 14, 21, and 28 days after infection did not change the relative rankings of FHB DI and DS for the tested isolates on bread cultivars, durum cultivars and barley differed in FHB susceptibilities, showing that extended moisture does not influence aggressiveness order among FHB isolates whatever the level of resistance of materials plants, and suggesting the non-specific nature of humidity on pathogenicity of Fusarium fungi. Gautam and Dill-Macky (2012) reported identical results for F. graminearum strains on T. aestivum bread showing diverse resistance levels. Therefore, it is hypothesized that there must be other factors besides toxin production which contribute to isolate aggressiveness (Mesterhazy, 2020, 2024).
The exclusively incomplete basal resistance of Triticum and Hordeum to Fusarium, the lack of major resistance genes and the dependence of intensity of FHB on gramineous crops on climatic conditions point out the necessity to select different cultivars with quantitative resistance crucial to enhance resistance breeding (Fernando et al., 2021; Moonjely et al., 2023). The resistance of wheat and barley to Fusarium can influence the development of FHB species in head tissues (Dahl and Wilson, 2018; Ma et al., 2019). Culler et al., (2007) also observed that the resistance in moderately resistant cultivars can be overwhelmed by high inoculum pressure under highly conductive weather conditions. In the present study, susceptible cultivar, i.e., Acsad65, susceptible to moderately susceptible, i.e., Cham7, Cham9, and moderately susceptible cultivars, i.e., Cham4 and Douma4, had consistently, and significantly, higher FHB severities, compared to the cultivars with moderately resistance, Bohoth10 and AS in treatments receiving spraying after 14, 21 and 28 days after inoculation. Gautam and Dill-Macky (2012) reported very similar findings when analyzing F. graminearum and T. aestivum cultivars with diverse degrees of FHB resistance. The four susceptible responses, i.e., susceptible, susceptible to moderately susceptible, moderately susceptible and moderately resistant, did not vary also in their relative rankings in the eight tested cereal plants, showing the non-specific nature of humidity on resistance of plant materials. Our findings seemed to apply equally to moderately resistant cultivars with either Type I or Type II resistance, or both, and to susceptible cultivars. Thus, our data are likely applicable to breeding programs globally. Our results propose that the possible leaching of mycotoxin, toxins may function as aggressiveness factors and enhance the pathogenicity of Fusarium fungi in bread wheat (Buerstmayr et al., 2020; Mesterhazy, 2020, 2024), is also higher in susceptible cultivars than resistant ones. We suggest that a higher rate of lignin accumulation in the resistant line in reaction to Fusarium invasion (Kang and Buchenauer 2000) might in turn have some effect on decreased leaching of mycotoxin from the spike tissues (Toth et al., 2020; Miedaner et al., 2021). However, additional research on this issue is warranted to derive a definitive conclusion.
The most crucial environmental variables such as precipitation, air humidity, and temperature, which directly affect the cereal-host plant, Fusarium growth and spread in the field (Fernando et al., 2021; Moonjely et al., 2023). Possibly, temperature in combination with relative air humidity and sufficient rain before anthesis supported growth and sporulation of our tested pathogens, i.e., F. culmorum, F. solani, F. verticillioides, and F. equiseti. It is well known that rain events before flowering may also participate to Fusarium spore emergence, growth of mycelia, and dispersal of sufficient amounts of spores to in the area of the emerging head and upper leaves (Buerstmayr et al., 2020). We reported in the present investigation links for weather conditions through the reproductive stage of wheat and barley, showing complexity of Fusarium epidemiology in small-grain cereals. In this work, the moisture nonspecifically affects quantitative traits in FHB-wheat/barley pathosystem; however, temperature specifically affects occurrence of individual Fusarium pathogens (Mesterhazy, 2020, 2024). F. graminearum generally occurs in hot and warm climate locations, with an average annual temperature above 15°C, but it is also commonly present in temperate climate countries in the growing seasons distinguished by high humidity and higher temperatures (Ma et al., 2019). F. graminearum is characterized as the principal cause of wheat/barley head blight and maize ear rot in several countries of Southern Europe, as well as Asia and North and South America (Andersen et al., 2015). F. fujikuroi is also found to be a thermophilic pathogen, and high degrees of maize ear infestation by this fungus and accumulation of fumonisin are often reported in dry and hot vegetation seasons (Moonjely et al., 2023). F. culmorum, F. poae and F. avenaceum pathogens generally infect gramineous crops in colder zones. F. culmorum is also tolerant to changing thermal conditions, although the harmfulness of this fungus to cereals is greater at higher temperatures (McMullen et al., 2012). According to various authors (reviewed in Fernando et al., (2021), F. poae can colonize small-grain cereal heads even in dry weather conditions. F. avenaceum usually occurs in locations with an average annual air temperature of 5– 15°C and moderate rainfall from 500 to 1000 mm per year or high, above 1000 mm, however, this pathogen exhibits significant tolerance to humidity and temperature (Mesterhazy et al., 2020).
The longer moisture duration possibly enhanced FHB germination rate and time frame to infect the cereal plant which led to more disease damage, as reported in T. aestivum infected with F. graminearum (Lemmens et al. 2004; Nita et al. 2005; Culler et al. 2007; Cowger et al. 2009; Gautam and Dill-Macky 2012; Andersen et al., 2015). Hence, prolonged durations of spraying during anthesis in the tested wheat and barley cultivars can enhance the risk of FHB infections (Sakr et al., unpublished data) In the present investigation, we used an artificial infection-environment experiments designed to better understand the function of variable postanthesis humidity patterns on Fusarium development. Our observation showed that weather conditions, particularly moisture, over the growing seasons 2022/23 at the experimental station played an crucial role in FHB development in infected grains in harmony with previous studies (Lemmens et al. 2004; Nita et al. 2005; Culler et al. 2007; Cowger et al. 2009; Gautam and Dill-Macky 2012; Andersen et al. 2015) These experiments carried out under natural climatic conditions provided conditions conductive to Fusarium development in head tissues; the pathogens can easily penetrate wheat/braley heads through extruded anthers and open florets as reported in previous investigations (Lemmens et al. 2004; Nita et al. 2005; Culler et al. 2007; Cowger et al. 2009; Gautam and Dill-Macky 2012; Andersen et al. 2015). In addition, extruded anthers in our tested bread wheat, durum wheat and barley cultivars trapped Fusarium spores and stimulate FHB development and growth by supplying nutrients required for fungal penetration and germination as observed earlier (Lemmens et al. 2004 Nita et al. 2005; Culler et al. 2007; Cowger et al. 2009; Gautam and Dill-Macky 2012; Andersen et al. 2015). Fusarium head blight is greatly influenced by climatic conditions (Dahl and Wilson, 2018). Fusarium infections over the anthesis period are favored by prolonged periods (2–3 days) of >90% relative humidity (Fernando et al. 2021). These climatic conditions are optimal for Fusarium specie development (Xue et al. 2019), as demonstrated in our study for the four tested pathogen species. Unlike other studies that use the misting treatment to create favorable condition to fungal colonization during and /or after the flowering stage (Lemmens et al. 2004; Nita et al. 2005; Culler et al., 2007; Cowger et al. 2009; Gautam and Dill-Macky 2012; Andersen et al. 2015); we used herein a spraying irrigation and found that this system is effective as misting irrigation to track the effect of prolonged durations of humidity on Fusarium development in wheat/barley spikes under changeable watered conditions.
CONCLUSION
Our work yielded useful information that, along with the results of other studies, helps to improve our understanding of the interaction of factors involving cereal host genetics and Fusarium fungus aggressiveness capacity, and the impact of climatic conditions, especially humidity, on the development of FHB in head blight-infested bread wheat, durum wheat and barley. We showed for the first time that moisture in late stages after anthesis, i.e., 14, 21, and 28 days after infection in bread wheat, durum wheat and barley cultivars showing different resistance levels nonspecifically affect development of Fusarium in terms of aggressiveness of fungi and resistance of host plants in the head tissues. Changeable watered conditions applied on plant materials of different resistance levels nonspecifically affected aggressiveness, DI, DS and FDK, of the tested Fusarium isolates whatever their pathogenic backgrounds (low, medium and high aggressiveness) and genetic origin (i.e., F. culmorum, F. solani, F. verticillioides, and F. equiseti). The four susceptible responses, i.e., susceptible, susceptible to moderately susceptible, moderately susceptible and moderately resistant, did not vary also in their relative rankings in the eight tested cereal plants. A clearer epidemiological picture emerged based on the complex relations among moisture, resistances of cultivars, and aggressiveness of Fusarium pathogens. Knowledge of the influence of humidity should be of value to barley breeders and pathologists, particularly by improving our understanding of the conditions that result in discrepancies between visual assessments of FHB, post-harvest visual evaluation of FHB damage to kernel that are frequently observed in both spray-irrigated and dry-land germplasm screening nurseries.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
ACKNOWLEDGEMENTS
The author would like to thank Director General of the Syrian Atomic Energy Commission (Dr. I. Othman) and the Head of the Department of Agriculture (Dr. M. Zarkawi) for supporting this project. The technical assistance of Samer Ammar, Haidara Mahmoud, Maher Alhouraney, and Yassine Kareym at the department of Agriculture is greatly acknowledged.


