Changes in blood monocyte functional profile in breast cancer
Автор: Fedorov A.A., Prostakishina E.A., Patysheva M.R., Frolova A.A., Iamshchikov P.S., Larionova I.V., Stakheyeva M.N., Dorofeeva M.S., Bragina O.D., Choynzonov E.L., Kzhyshkowska J.G., Cherdyntseva N.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 6 т.21, 2022 года.
Бесплатный доступ
The purpose of the study was to identify functional features of circulation monocytes in patients with non-metastatic breast cancer. Material and Methods. The study cohort consisted of 10 breast cancer patients treated at Tomsk Cancer Research institute. 7 healthy female volunteers were enrolled as a control group. CD14+16-, CD14+16+ and CD14-16+ monocytes subsets were obtained from blood by sorting. Whole transcriptome profiling was provided in monocytes from patients and healthy females. Macrophages were differentiated from the obtained monocytes under in vitro conditions. The ability of conditioned media obtained from macrophages to influence apoptosis and proliferation of MDA-MB 231 cell line was evaluated. Results. Transcriptomic profiling revealed significant changes in monocytes of breast cancer patients. CD14+16- subset showed higher expression of transporters ABCA1 and ABCG1; chemokines CCR1, CRRL2, CXCR4; maturation and differentiation factors Mafb and Jun; endocytosis mediating factors CD163 and Siglec1; proteases and tetrasponins ADAM9, CD151, CD82, and growth factor HBEGF in patient group. Macrophages derived from monocytes of breast cancer patients produced factors that supported proliferation of the MDA-MB 231 cell line, which was not observed for monocytes from healthy volunteers. Conclusion. Thus, breast carcinoma has a systemic effect on peripheral blood monocytes, programming them to differentiate into macrophages with tumor supporting capacity.
Monocytes, tumor-associated macrophages, breast cancer, transcriptome, rna sequencing
Короткий адрес: https://sciup.org/140296696
IDR: 140296696 | УДК: 618.19-006.6:612.1 | DOI: 10.21294/1814-4861-2022-21-6-68-80
Текст научной статьи Changes in blood monocyte functional profile in breast cancer
The immune response to cancer differs from the immune response to viruses and pathogenic microorganisms, due to the fact that tumor cells are not appear to be alien to the patient’s body [1]. Cancer cells possess mechanisms that allow them, in addition to evading immune surveillance, to use cells of the immune system to support its growth and development. The tumor microenvironment is able to attract circulating monocytes, which can differentiate into tumor-associated macrophages (TAM) and form an inflammatory infiltrate [2]. Monocyte differentiation into a specific cell type with antitumor or protumor activity is a critical event for the clinical course of disease and increasing therapy effectiveness [3, 4].
Monocytes and macrophages are important components of innate immunity, as they are involved in maintaining tissue homeostasis [3, 5]. Monocytes are a population of circulating white blood cells that enter the blood from the bone marrow. They have the ability to phagocytize, present antigens, secrete chemokines or cytokines, and differentiate depending on tissue microenvironment stimuli [2]. Despite their wide range of functions, monocytes are considered immature immune cells [5]. When ingested into tissues, monocytes are able to differentiate into macrophages, dendritic cells, and suppressor cells of myeloid origin. According to the classification adopted in 2010, the blood monocyte population is divided into three subpopulations depending on the expression of CD14 (one of the components of bacterial wall lipopolysaccharide receptor complex), and CD16 (FcyRIII, a membrane protein of the immunoglobulin superfamily) [6]. There are 3 monocyte subsets: CD14+16-classical, CD14+16+ intermediate, and CD14-16+ non-classical. The percentage of classical, intermediate and non-classical monocytes was 80–90 %, 10–15 % and 2–5 %, respectively [6]. The contribution of each subpopulation to cancer pathogenesis has not yet been elucidated and needs to be studied. The tumor can influence both the cell differentiation and changes in the phenotype of monocytes. The acquisition of immunosuppressive activity is the most common cancer-induced phenotypic change in human peripheral blood monocytes [7]. Typically, this phenomenon coincides with suppression of the MHC class II surface protein HLA-DR, a key mediator of antigen presentation, which is also highly expressed on monocytes of healthy individuals [8, 9].
Among the approaches that are available to resolve questions concerning phenotypic and functional monocyte heterogeneity, gene expression studies are may be particularly valuable [10, 11]. Microarray analysis and next-generation RNA sequencing allow the relative quantification of gene expression. The development of genetic sequencing technology has provided opportunities for a comprehensive study of phenotypic and functional differences between recognized immune cell subtypes, as well as the possibility of identifying new cell subpopulations [11]. The identification of differentially expressed genes in the studied immune cells and monocytes allows comparisons between different cell populations or within the same cell population isolated under different conditions [10, 12]. Microarray analysis is a powerful tool for predicting the functional cell profile. In vitro experiments are also important to validate functional predictions based on gene expression data [10]. Thus, the study of circulating monocytes in cancer patients using high-tech research methods is of great importance.
The purpose of the study was to identify functional features of circulation monocytes in patients with non-metastatic breast cancer.
Material and Methods
The study group included 10 female patients aged 35 to 64 years with stage II–III and T1–3N0–2M0 breast cancer (BC). The diagnosis was verified morphologically. All patients had an invasive breast carcinoma of no special type. The molecular subtype of the tumor was determined as part of standard pathology protocols. The distribution of molecular subtypes among patients with breast cancer was as follows: 6 patients with luminal breast cancer type B, and 4 patients with triple-negative breast cancer (TNBC). Patients received 6–8 courses of neoadjuvant chemotherapy doxorubicin and cyclophosphamide followed by docetaxel or taxotere in accordance to the Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up 2015”. All patients were undergoing surgical treatment, radiotherapy, and an adjuvant chemotherapy after neoadjuvant chemotherapy. Whole blood samples were obtained from the healthy volunteers and patients before any treatment procedures. The control group included 7 healthy females aged 37 to 56 years. The study was approved by the ethics committee of the Cancer Research Institute of the Tomsk National Research Medical Center and complied with the Helsinki Declaration of 1964. All patients signed an informed consent to participate in the study.
Assessment of the monocyte functional potential in vitro
The stimulation system of primary human macrophages derived from peripheral blood monocytes was used as a model of tumor-associated macrophages (TAMs) [13]. Monocytes were isolated from venous blood using CD14-positive magnetic separation according to the protocol of Myltenyi Biotec (Germany) with an isolation purity of at least 90 % of CD14+ cells. Monocytes obtained from BC patients and healthy donors were cultured for 6 days at the concentration of 1x106 cells/ml in X-Vivo-10 serum-free media (Lonza, Germany) in an optimally selected concentration of the macrophage colony-stimulating factor M-CSF (10 ng/ml) at 37 °C and 7,5 % CO2. After 6 days of cultivation, conditioned media from the obtained macrophages were selected, filtered through filters with a low protein binding ability. The resulting conditioned media samples were stored at -80°C for no more than 6 months. Before use, the conditioned media were removed from the storage system and thawed at +40°C for 4 hours.
The BC cell line MDA-MB 231 (Russian Collection of Vertebrate Cell Cultures, Institute of Scientific Center of the Russian Academy of Sciences, St. Petersburg) was used as targets. MDA-MB 231 cells were cultured in DMEM medium (PanEco, Russia)
with 10 % FCS (BioSera, France), 1 % penicillin, and gentamicin at 37°C and 5.0 % CO2 until confluence was reached.
Assessment of monocyte conditioned media influence on apoptosis and proliferation of breast cancer cells
To detect the apotosis-inducing ability, confluent MDA-MB 231 cells were supplemented with conditioned media in a volume of 20 % of culture medium total volume. Next, the cells were incubated for 48 hours at 37 °C and 5.0 % CO2. For the apoptosis analysis, cells were removed with trypsin (PanEco, Russia) and stained for 10 min with FITC-labeled annexin-V and propidium iodide (Biolegend, USA). Fluorescently labeled annexin-V and propidium iodide were evaluated using a CytoFLEX flow cytometer (Beckman Coulter, USA) using standard protocols. The stage of early apoptosis, late apoptosis and necrosis was assessed based on the combination of annexin-V gates and propidium iodide. Data were visualized and analyzed using CytExpert Software 2.0.
To study the conditioned media effect on proliferation, tumor cells were transplanted onto specialized chips of the RTCA iCELLigence system (ACEA Biosciences, Japan). Conditioned media were 20 % of culture medium total volume. A well with MDA-MB 231 cells supplemented with 20 % X-Vivo-10 culture medium (Lonza, Germany) was used as a control. The proliferation of MDA-MB 231 cells of each sample was continuously detected and displayed as graphs in real time for 72 hours. The proliferation index (PI) was calculated and the data were visualized using the RTCA Software Lite software (ACEA Biosciences, Japan).
Statistical analysis
Statistical analysis was performed using Statistica version 10 for Windows (StatSoft Inc). Variable distribution was presented as mean ± Standard Deviation (SD). Normal distribution was confirmed using the Kolmogorov Smirnov test for in vitro studies. The Student t test was used to detect significant differences between two groups to apoptosis and proliferation analysis via vitro tests.
Next generation sequencingand bioinformatics data analysis
To analyze the transcriptome of monocytes, the peripheral blood of patients with breast cancer and healthy women was used. The fraction of peripheral blood mononuclear cells was obtained from the blood on the Ficoll density gradient (1.077 g/cm3). Next, monocytes of the classical (CD14+16-), intermediate (CD14+16+), and non-classical (CD14-16+) subpopulations were isolated for the sequencing using flow cytometric sorting. The panel of conjugated monoclonal antibodies against CD45, CD56, CD14, CD16, and 7-AAD markers was used (Table 1).
table 1/Таблица 1 panel of antibodies used to sort monocytes from peripheral blood
Панель антител, используемых для сортировки моноцитов из периферической крови
|
Antibodies, dyes/ Антитела, красители |
Clone/ Клон |
Isotype/ Изотип |
Manufacturer/ Производитель |
|
CD45 – APC-Cy7 |
2D1 |
Mouse IgG1, κ |
BD Bioscience |
|
CD56 – PE-Cy7 |
CMSSB |
Mouse / IgG1, κ |
eBioscience, Thermo Fisher Scientific |
|
CD14 – FITC |
M5E2 |
Mouse IgG2a, κ |
BD Bioscience |
|
7-AAD |
BD Bioscience |
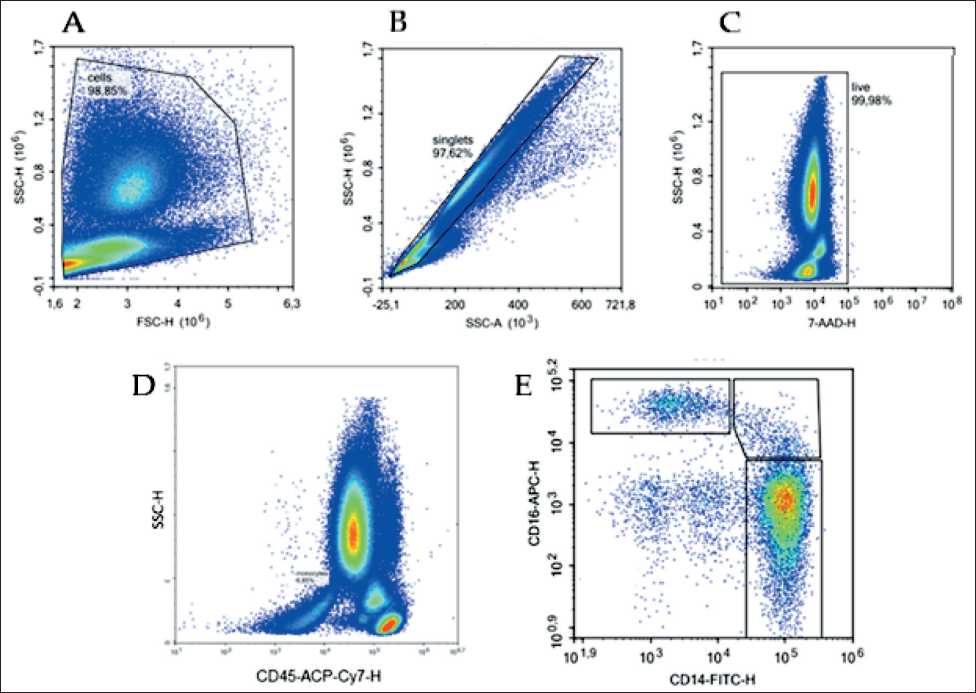
Fig. 1. Flow cytometry gating strategy for identifying monocyte subpopulations: A – leukocyte gate isolation based on forward and side light scatter; B – singlet cell isolation; C – living cells isolation based on 7-AAD staining; D – isolation of monocyte pool of among CD45+ cells; E – isolation of the gate of classical (CD14+16-), intermediate (CD14+16+), and non-classical (CD14-16+) monocytes. Sorting of subpopulations of interest was based on these gates
Рис. 1. Цитофлуориметрическая стратегия гейтирования для идентификации субпопуляций моноцитов: А – выделение гейта лейкоцитов на основе прямого и бокового светорассеивания; B – выделение синглетных клеток;
C – выделение живых клеток на основе окрашивания 7-AAD; D – выделение пула моноцитов среди клеток CD45+; E – выделение гейта классических (CD14+16-), промежуточных (CD14+16+) и неклассических (CD14-16+) моноцитов. Сортировка субпопуляций интереса проходила на основе этих гейтов
Samples were analyzed on a MoFlo XDP cell sorter (Beckman Coulter, USA). The target subpopulation for sorting was characterized by the parameters 7-ADD-CD45+CD56-CD14+16- (Fig. 1).
Monocyte sorting was carried out in the Purify 1–2 mode. The sorting efficiency in this mode was 70 %, and the purity of the target population was 98–99 %. At least 600 thousand cells of the target subpopulation were sorted into RLT lysis buffer (Qagen, Germany). Then, total RNA was isolated from the resulting lysate in no more than 60 min using RNAeasy mini kit plus (Qagen, Germany) according to the manufacturer’s protocol. The quality of the obtained RNA was assessed using a TapeStation 4150 automated capillary electrophoresis station (Agilent Technology, USA). The RNA integrity index – RIN was 8.5–9.9. Samples of total RNA were stored at -80°C until the next work stage, excluding freezing and thawing stages.
The full transcriptome profile of monocytes was determined with massively parallel sequencing. Libraries for mass parallel sequencing were prepared using the NEXT flex Rapid Directional qRNA-SeqKit kit and the NEXTflex-qRNA-8nt-Barcodes single-ended index kit according to the standard protocol (Perkin Elmer, USA). Ribosomal RNA depletion was performed using the NEBNext® rRNA Depletion Kit (Human/Mouse/ Rat) (New England Bilab, USA). The prepared libraries were pooled, and sequencing was performed on the
NextSeq500 platform (Illumina, USA) with a set of reagents for single-ended reading 1x75 (single read). The number of cycles was 50. As a result, each library had an average of about 5 million reads.
Reads were mapped onto the genome using the STAR 2.5 program [14], The GRCh38 genomic assembly and GENCODE.R34 annotations were used as a reference. After mapping, data obtaining on the number of mapped reads to individual genes was performed using the QoRTs program [15]. After this stage, using the DESeq2 software package, which is part of the R medium, data were obtained on the differential gene expression between the control and experimental groups. Principal component analysis (PCA) also was applied to dimension reduction and assess which patient samples differed from the healthy group. To describe the differential gene expression in monocytes of BC patients compared to monocytes of healthy persons, was used the log2 of the gene expression difference between comparison groups (2logFoldChange). Using the Enri-chr software package [16], enrichment was carried out for biochemical and regulatory pathways using lists of genes ranked by expression level (log2FoldChange) and p-value. The Hallmark gene sets, Reactome, KEGG, and GO databases were used for the experiment. The associations of differentially expressed genes were determined with the STRING database [17]. The data were visualized using the Enrichr and Phantasus programs [18], as well as the R environment tools.
Results
Influence of conditioned media obtained in vitro by monocyte cultivation on proliferation and apoptosis of breast cancer cells
The study of monocyte functional potential was carried out using a model system in which monocytes isolated from peripheral blood were transformed into macrophages under in vitro conditions under the influence of M-CSF. The conditioned media obtained by macrophage culturing were added to MDA-MB 231 breast cancer cells line. Then their effect on the MDA-MB 231 BC cells proliferation and apoptosis was evaluated.
Apoptosis evaluation of target cells cultured with conditioned macrophage media using the flow cytometry method did not reveal a significant effect of factors produced by macrophages on the death of tumor cells (Table 2). This fact was noted both for a group of macrophages obtained from healthy individuals and from patients with breast cancer (Table 2).
However, the effects of the conditioned media of induced macrophages on the proliferative activity of the MDA-MB 231 tumor line were different for patients with breast cancer and for the group of healthy individuals (Fig. 2). To detect the proliferation ability of target cells, the proliferation index (PI) was calculated. PI is the ratio of indicators of the cell proliferation level in wells with conditioned media to the well indicators. Cell cultivation was carried out under standard conditions.
In the group of healthy women, PI was below sample proliferation baseline and amounted to 3.81 ± 0.047 conventional units (Fig. 2A, B). Conditioned media was not added. PI during tumor cells co-cultivation with macrophage conditioned media of BC patients did not differ from the baseline and was equal to 4.28 ± 0.45 conventional units, which exceeded the PI index of the healthy group with p=0.008 (Fig. 2A, B).
No difference in proliferative activity between macrophages of patients with triple-negative breast cancer and macrophages of patients with type B luminal breast cancer was found (proliferation indexes were 4.0 ± 0.17 and 4.0 ± 0.43, respectively, p=0.642). Thus, the ability of conditioned media obtained from BC patient macrophages to support tumor cells proliferation under in vitro conditions was observed. It is notable that the molecular subtype has no pronounced effect on circulating monocytes in the blood. It was suggested that luminal B and triple-negative BC patient monocytes could have transcriptional programs caused by the breast tumor presence and activated upon differentiation into macrophages. To test the hypothesis, transcriptome study of BC patient monocytes was carried out in comparison with healthy women monocytes.
Influence of breast cancer on the transcriptome landscape of monocytes
A transcriptome profile is a set of genes that are expressed in cells at specific times and characterize the biological processes occurring in them. To characterize peripheral blood monocytes, the complete transcriptomic profile of these cells was assessed using massively parallel sequencing in a group of BC patients and healthy women. The transcriptome of CD14+16- breast cancer monocytes was shown to dif- table 2/Таблица 2
Results of MDa-MB 231 cell line apoptosis under the influence of macrophage conditioned media in BC patients and healthy individuals
Результаты апоптоза клеток линии MDa-MB 231 под влиянием кондиционных сред макрофагов больных РМЖ и здоровых лиц
|
Apoptosis phase/ Фаза апоптоза |
Group of BC patients/ Группа больных РМЖ |
Control group/ Группа контроля |
p-value |
|
Initial phase/Начальная |
1,5 ± 0,3 % |
2,5 ± 0,6 % |
0,341 |
|
Final phase/Финальная |
1,8 ± 0,6 % |
2,1 ± 0,4 % |
0,763 |
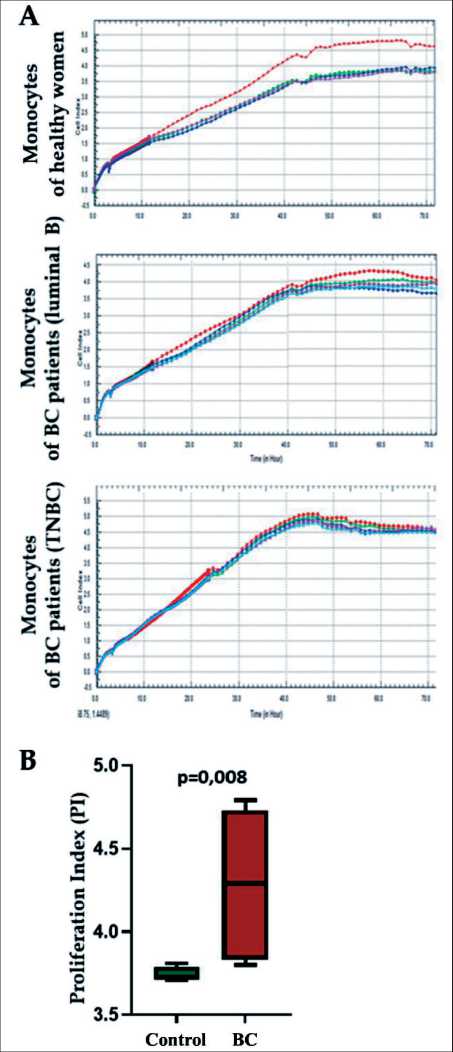
Fig. 2. Influence of macrophage conditioned media of healthy women and breast cancer (BC) patients on MDA-MB 231 cell line proliferation: A – dynamics of changes within 72 hours of MDA-MB 231 cells proliferative activity under the influence of conditioned media obtained from the studied macrophages (red line – sample without the addition of conditioned media, blue, green and crimson line – samples with the addition of conditioned media); B – proliferative index of target cells in BC patient group compared with the control group
Рис. 2. Влияние кондиционных сред макрофагов здоровых женщин и пациенток с РМЖ на пролиферацию клеток линии MDA-MB 231: А – динамика изменения в течение 72 ч пролиферативной активности клеток линии MDA-MB 231 под влиянием кондиционных сред, полученных от макрофагов исследуемых (красная линия – образец без добавления кондиционных сред, синяя, зеленая и малиновая линия – образцы с добавлением кондиционных сред); В – пролиферативный индекс клеток-мишеней в группе больных по сравнению с группой контроля fer from that of healthy women monocytes. The group of BC patient samples was located separately from a group of healthy volunteer’s samples on a PCA plot (Fig. 3A). PCA reveals that the top 2 principal components (PCs) account for 63 % of the total variance, with the first component (PC1) alone accounting for 53,5 % of the variance and showing clear separation between the BC and healthy samples (Fig. 3A).
In breast cancer patients, there was a significant difference in the transcriptome profile between CD14+16-and CD14+16+/CD14-16+monocyte subpopulations. However, no significant difference in the transcriptome profile between CD14+16+ and CD14-16+monocyte subpopulations was found. (Fig. 4 A). In healthy women, no differences between monocyte subpopulations were observed (Fig. 4 B). For breast cancer patients, it is noteworthy that a comparison of the expression of individual genes in each subpopulation revealed a decrease in the level of transcripts of the S100A8 and S100A9 genes in CD14+16- (Fig. 4C).
Verification of tissue specificity according to the set of differentially expressed genes using the Jensen tissues database (18) showed that the profile of the detected genes matched the signature of blood monocytes (Fig. 3B). Further gene expression difference analysis with p-value≤0.01 and log2FoldChange≥0.75 showed that patient monocytes were characterized by an increased expression of genes for ABCA1 , ABCG1 transporter proteins, CCR1 , CRRL2 , CXCR4 chemok-ines, HBEGF growth factor, MAFB and JUN factors of maturation and monocyte differentiation, factors mediating endocytosis CD163 , Siglec1, proteases and tetrasponins ADAM9 , CD151 , CD82 , receptors (Fig. 3C). A comprehensive assessment of transcript functional interactions using the STRING database revealed that the most likely factors that ensure tumor cells proliferation can be heparin-binding epidermal growth factor ( HBEGF ), proteases, and ADAM9 , CD151 , CD82 tetrasponins (Fig. 3D) (16). The set of activated signaling pathways typical for patient monocytes was represented by processes characteristic of the immune response, signaling pathways associated with interferons, and the process of neutrophil degranulation. (Fig. 3E).
Discussion
The main function of monocytes in malignant neoplasms is to replenish the pool of tumor-associated macrophages, dendritic cells, and myeloid suppressor cells [2]. It is known that monocytes differentiate into tissues under the influence of autocrine stimuli [19]. Currently, it is assumed that monocytes can have certain transcriptome programs mediated by the tumor presence at the stage of bloodstream circulation [9, 20, 21]. Cancer, as a systemic disease, can affect monocytes at the progenitor level, in circulation, and also during the monocyte migration into tissue [7, 22]. In general, an increased number of peripheral blood monocytes in cancer has been described for
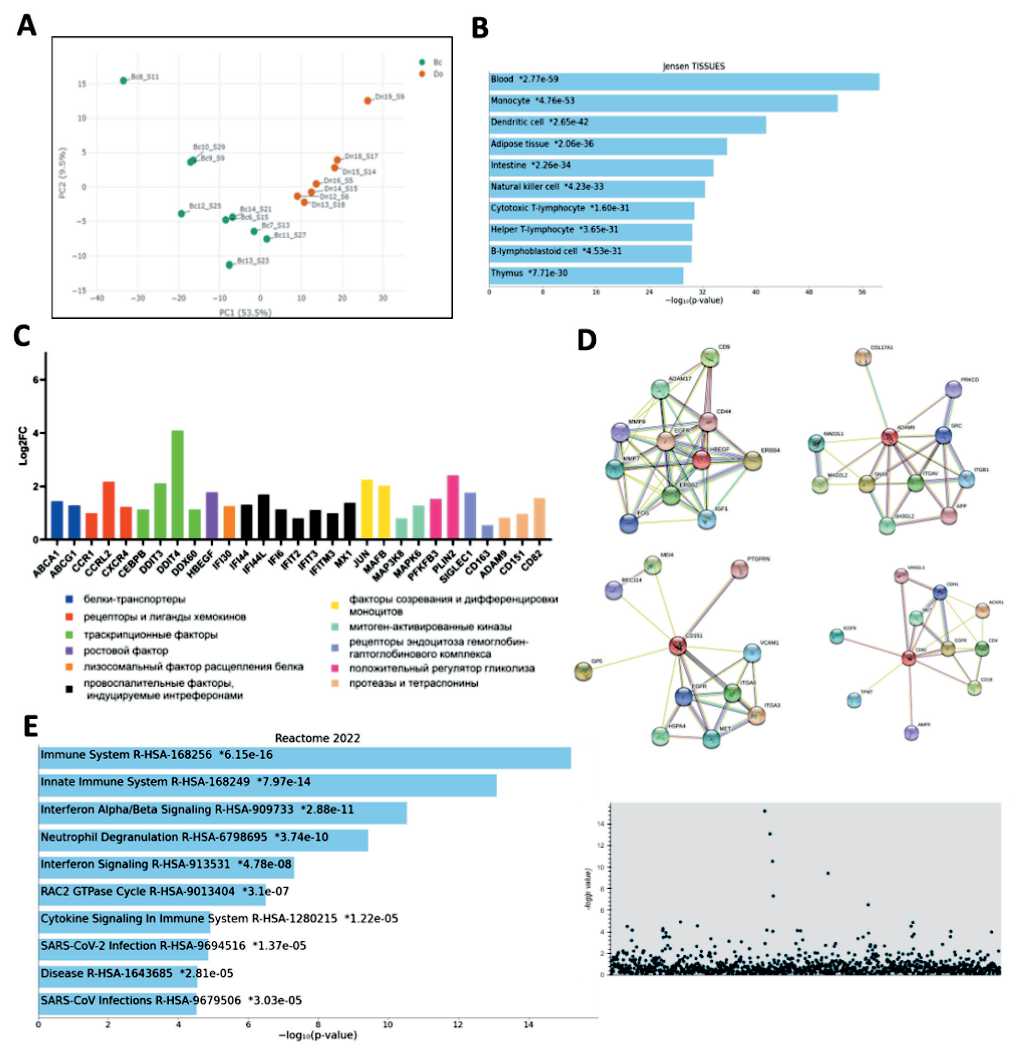
Fig. 3. Transcriptome features of CD14+16- monocytes of BC patients (n=9) in comparison with a group of healthy women (n=7): A – comparison of the transcriptome of monocytes of BC patients and healthy ones using the method of principal components; B – assessment of the tissue affiliation of study objects; C – groups of genes with increased expression in patient monocytes;
D – functional associations characteristic of the HBEGF , ADAM9 , CD151 , and CD82 genes with increased expression in monocytes of patients; E – signaling pathways characterizing the biological processes of monocytes in BC patients are presented in the form of a histogram and a Manhattan plot
Рис. 3. Особенности транскриптома CD14+16- моноцитов больных РМЖ (n=9) в сравнении с группой здоровых женщин (n=7): А – сравнение транскриптома моноцитов больных РМЖ и здоровых с помощью метода главных компонент; B – оценка тканевой принадлежности исследуемых объектов; C – группы генов с повышенной экспрессией в моноцитах больных; D – функциональные ассоциации, характерные для генов HBEGF, ADAM9, CD151, CD82 с повышенной экспрессией для моноцитов больных;
E – сигнальные пути, характеризующие биологические процессы моноцитов больных РМЖ, представлены в виде гистограммы и манхеттен-плота
both humans and laboratory animals [7, 23]. In most carcinomas, monocyte activity is most likely aimed at the tumor support. Thus, patients with the higher monocyte levels in blood have a worse prognosis for pancreatic cancer, gastric cancer, ovarian cancer, and prostate cancer [23–26]. The search for mechanisms of switching the functional activity of monocytes towards antitumor immune response activation is a promising task of cancer immunology.
It is known that TAMs are essential components of the tumor microenvironment [19, 27]. It has been shown that the functional polarization of TAM is aimed at the tumor supporting in the overwhelming majority of BC cases [4, 28]. Thus, the typical M2-type TAM markers are associated with poor prognosis [22, 29–31]. Like all tissue macrophages, TAM have a certain level of heterogeneity and the ability to adapt their phenotype under the influence of growth
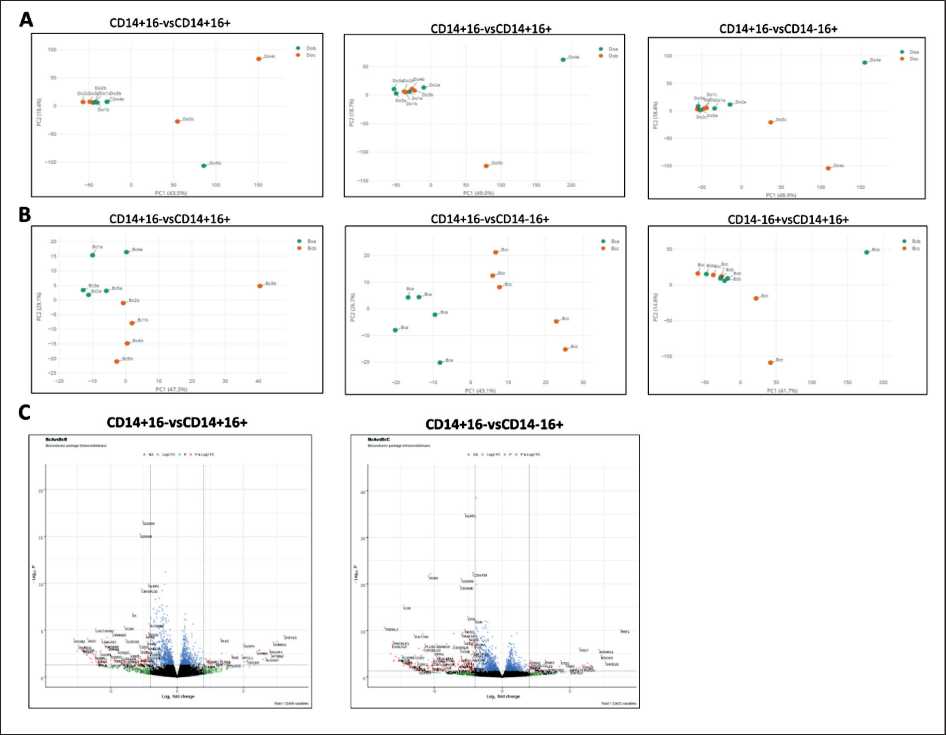
Fig. 4. Differences in transcriptome between CD14+16- (DoA), CD14+16+ (DoB), and CD14-16+ (DoC) monocyte subpopulations in healthy women, n=5 (A) and CD14+16- (Bca), CD14-16+ (Bcc), and CD14+16+ (Bcb) monocyte subpopulations in patients with breast cancer, n=5 (B); C – differential gene expression in CD14+16- (BcA) subpopulation compared with CD14-16+ (BcB) and CD14+16+ (BcC) monocyte subpopulations in BC patients
Рис. 4. Различия в транскриптоме между субпопуляциями моноцитов СD14+16- (DoA), СD14+16+ (DoB) и СD14-16+ (DoC) у здоровых женщин, n=5 (А) и СD14+16- (Bca), СD14-16+ (Bcс) и СD14+16+ (Bcb) у больных РМЖ, n=5 (B); C – дифференциальная экспрессия генов в субпопуляции СD14+16- (BcА) по сравнению с СD14-16+ (BcВ) и СD14+16+ (BcС) у больных РМЖ
factors and cytokines produced by tumor cells [32]. TAMs, in turn, secrete growth factors, cytokines, and extracellular matrix components that support tumor progression and increase its malignant potential [20, 33]. Since monocytes are the main plastic resource of tissue OAM, it is important in which direction and how quickly monocytes will differentiate into OAM. In this work, it was shown that monocytes from breast cancer patients differentiated into macrophages, which produced inducers capable of supporting the BC cell line growth. However, the components of conditioned macrophage media obtained from healthy women monocytes reduced the proliferation rate of the breast cancer cell line. Thus, it was noted that monocytes of BC patients, in contrast to healthy individuals, differentiated into macrophages with possible protumor functional activity. A similar observation was shown in vitro modeling of triple-negative breast cancer. In that situation, the macrophage introduction into the model increased the tumor cell proliferation [34]. In our study, we found no differences for groups with luminal B and triple negative molecular subtypes. There were no differences in the effect of these two subtypes on the ability of monocyte-derived macrophages to produce proliferation inductors to the MDA-MB 231 cell line. Circulating monocytes are likely susceptible to breast carcinoma without a pronounced effect of the molecular subtype.
To clarify the possible mechanisms of breast tumor influence on circulating monocytes, we studied the transcriptome profile of CD14+16-, CD14+16+, and CD14-16+ monocytes in breast cancer. It should be noted that only in the group with breast cancer, CD14+16- monocytes significantly differed from CD14+16+ and CD14-16+ subpopulations, and were characterized by a decrease in the expression of S100A8 and S100A9 genes. Previously, it was shown that a decrease in S100A8 and S100A9 production corresponded to extravasation phase of monocytes and neutrophils [35, 36]. A similar phenomenon is associated with the monocyte differentiation into tissue macrophages and dendritic cells [36–38]. Consequently, the monocytes circulating in the peripheral blood of ВС patients are prepared for the differentiation phase. It distinguishes them from healthy people monocytes. Thus, the CD14+16- subpopulation may represent a resource for replenishing the TAM pool in the BC group. According to the obtained results, the presence of breast cancer affects the CD14+16 cell transcriptome. In breast cancer, the expression of CXCR4, CCR1, and CCRL2 components of the receptor-ligand complex, which are specific to the stages of monocyte migration into tissues, is increased [39, 40]. It should be noted that patient monocytes contain an increased content of CD163 and SIGLEC1 transcripts which indicate the differentiation into M2-like macrophages [19, 20, 41]. In addition, the expression of MAFB and JUN factors increased in monocytes of the BC group. These factors indicate the monocyte maturation and differentiation into macrophages [42, 43]. The tran-scriptomic profile of BC patient monocytes indicated a more pronounced readiness for differentiation into macrophages, compared with the normal physiological state of monocytes.
Due to the fact that conditioned media derived from differentiated monocytes supported tumor growth, the research focused on finding monocytic factors that could support or stimulate malignant cell proliferation. According to the obtained data, monocytes have an increased level of expression of HBEGF in breast cancer. HBEGF is an important ligand of the epidermal growth factor receptor ( EGFR) . It is noted that HBEGF is a key progression factor in liver, colon, skin, prostate, bladder, and breast tumors [44–46]. It is known that M2-type macrophages secrete higher levels of HBEGF compared to M1-type macrophages. By releasing HBEGF, M2 macrophages induce the proliferation of ovarian cancer cells [47]. It has been shown that HBEGF is co-expressed with oncostatin M in TAM of breast carcinoma patients, and both ligand plasma levels are strongly correlated [45]. An increase
Список литературы Changes in blood monocyte functional profile in breast cancer
- Goldszmid R.S., Dzutsev A., Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe. 2014; 15(3): 295-305. https://doi.org/10.1016/j.chom.2014.02.003.
- Olingy C.E., Dinh H.Q., Hedrick C.C. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019; 106(2): 309-22. https://doi.org/10.1002/JLB.4RI0818-311R.
- Saqib U., Sarkar S., Suk K., Mohammad O., Baig M.S., Savai R. Phytochemicals as modulators of M1-M2 macrophages in infammation. Oncotarget. 2018; 9(25): 17937-50. https://doi.org/10.18632/oncotarget.24788.
- Larionova I., Tuguzbaeva G., Ponomaryova A., Stakheyeva M., Cherdyntseva N., Pavlov V., Choinzonov E., Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front Oncol. 2020; 10. https://doi.org/10.3389/fonc.2020.566511.
- Ma W.T., Gao F., Gu K., Chen D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front Immunol. 2019; 10: 1140. https://doi.org/10.3389/fmmu.2019.01140.
- Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N., Leenen P.J., Liu Y.J., MacPherson G., Randolph G.J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J.M., Lutz M.B. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010; 116(16): 74-80. https://doi.org/10.1182/blood-2010-02-258558.
- Kiss M., Caro A.A., Raes G., Laoui D. Systemic Reprogramming of Monocytes in Cancer. Front Oncol. 2020; 10: 1399. https://doi.org/10.3389/fonc.2020.01399.
- Poschke I., Mougiakakos D., Hansson J., Masucci G.V., Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010; 70(11): 4335-45. https://doi.org/10.1158/0008-5472.CAN-09-3767.
- Hamm A., Prenen H., Van Delm W., Di Matteo M., Wenes M., Delamarre E., Schmidt T., Weitz J., Sarmiento R., Dezi A., Gasparini G., Rothé F., Schmitz R., D’Hoore A., Iserentant H., Hendlisz A., Mazzone M. Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut. 2016; 65(6): 990-1000. https://doi.org/10.1136/gutjnl-2014-308988.
- Cormican S., Griffn M.D. Human Monocyte Subset Distinctions and Function: Insights From Gene Expression Analysis. Front Immunol. 2020; 11: 1070. https://doi.org/10.3389/fmmu.2020.01070.
- Reuter J.A., Spacek D.V., Snyder M.P. High-throughput sequencing technologies. Mol Cell. 2015; 58(4): 586-97. https://doi.org/10.1016/j.molcel.2015.05.004.
- Chen S., Chai X., Wu X. Bioinformatical analysis of the key differentially expressed genes and associations with immune cell infltration in development of endometriosis. BMC Genom Data. 2022; 23(1): 20. https://doi.org/10.1186/s12863-022-01036-y.
- Kzhyshkowska J., Gudima A., Moganti K., Gratchev A., Orekhov A. Perspectives for Monocyte/Macrophage-Based Diagnostics of Chronic Inflammation. Transfus Med Hemother. 2016; 43(2): 66-77. https://doi.org/10.1159/000444943.
- Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29(1): 15-21. https://doi.org/10.1093/bioinformatics/bts635.
- Hartley S.W., Mullikin J.C. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics. 2015; 16(1): 224. https://doi.org/10.1186/s12859-015-0670-5.
- Xie Z., Bailey A., Kuleshov M.V., Clarke D.J.B., Evangelista J.E., Jenkins S.L., Lachmann A., Wojciechowicz M.L., Kropiwnicki E., Jagodnik K.M., Jeon M., Ma’ayan A. Gene Set Knowledge Discovery with Enrichr Curr Protoc. 2021; 1(3): 90. https://doi.org/10.1002/cpz1.90.
- Szklarczyk D., Gable A.L., Nastou K.C., Lyon D., Kirsch R., Pyysalo S., Doncheva N.T., Legeay M., Fang T., Bork P., Jensen L.J., von Mering C. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measure ment sets. Nucleic Acids Res. 2021; 49(D1): 605-12. https://doi.org/10.1093/nar/gkaa1074. Erratum in: Nucleic Acids Res. 2021; 49(18): 10800.
- Zenkova D. K.V., Sablina R., Artyomov M., Sergushichev A. Phantasus: visual and interactive gene expression analysis. 2018. https://doi.org/10.18129/B9.bioc.phantasus.
- Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014; 41(1): 49-61. https://doi.org/10.1016/j.immuni.2014.06.010. Erratum in: Immunity. 2014; 41(5): 866.
- Cassetta L., Fragkogianni S., Sims A.H., Swierczak A., Forrester L.M., Zhang H., Soong D.Y.H., Cotechini T., Anur P., Lin E.Y., Fidanza A., LopezYrigoyen M., Millar M.R., Urman A., Ai Z., Spellman P.T., Hwang E.S., Dixon J.M., Wiechmann L., Coussens L.M., Smith H.O., Pollard J.W. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specifc Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019; 35(4): 588-602. https://doi.org/10.1016/j.ccell.2019.02.009.
- Ramos R.N., Rodriguez C., Hubert M., Ardin M., Treilleux I., Ries C.H., Lavergne E., Chabaud S., Colombe A., Trédan O., Guedes H.G., Laginha F., Richer W., Piaggio E., Barbuto J.A.M., Caux C., MénétrierCaux C., Bendriss-Vermare N. CD163+ tumor-associated macrophage accumulation in breast cancer patients refects both local diferentiation signals and systemic skewing of monocytes. Clin Transl Immunology. 2020; 9(2): 1108. https://doi.org/10.1002/cti2.1108.
- Patysheva M., Larionova I., Stakheyeva M., Grigoryeva E., Iamshchikov P., Tarabanovskaya N., Weiss C., Kardashova J., Frolova A., Rakina M., Prostakishina E., Zhuikova L., Cherdyntseva N., Kzhyshkowska J. Efect of Early-Stage Human Breast Carcinoma on Monocyte Programming. Front Oncol. 2022; 11. https://doi.org/10.3389/fonc.2021.800235.
- Sanford D.E., Belt B.A., Panni R.Z., Mayer A., Deshpande A.D., Carpenter D., Mitchem J.B., Plambeck-Suess S.M., Worley L.A., Goetz B.D., Wang-Gillam A., Eberlein T.J., Denardo D.G., Goedegebuure S.P., Linehan D.C. Infammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013; 19(13): 3404-15. https://doi.org/10.1158/1078-0432.CCR-13-0525.
- Pan Y.C., Jia Z.F., Cao D.H., Wu Y.H., Jiang J., Wen S.M., Zhao D., Zhang S.L., Cao X.Y. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine (Baltimore). 2018; 97(52). https://doi.org/10.1097/MD.0000000000013896.
- Lu C., Zhou L., Ouyang J., Yang H. Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: A meta-analysis. Medicine (Baltimore). 2019; 98(24). https://doi.org/10.1097/MD.0000000000015876.
- Hayashi T., Fujita K., Tanigawa G., Kawashima A., Nagahara A., Ujike T., Uemura M., Takao T., Yamaguchi S., Nonomura N. Serum monocyte fraction of white blood cells is increased in patients with high Gleason score prostate cancer. Oncotarget. 2017; 8(21): 35255-61. https://doi.org/10.18632/oncotarget.13052.
- Rakina M.A. Kazakova E.O., Sudarskikh T.S., Bezgodova N.V., Villert A.B., Kolomiets L.A., Larionova I.V. Giant foam-like macrophages in advanced ovarian cancer. Siberian Journal of Oncology. 2022; 21(2): 45-54. https://doi.org/10.21294/1814-4861-2022-21-2-45-54.
- Fedorov A.A., Ermak N.A., Gerashchenko T.S., Topolnitskii E.B., Shefer N.A., Rodionov E.O., Stakheyeva M.N. Polarization of macrophages: mechanisms, markers and factors of induction. Siberian Journal of Oncology. 2022; 21(4): 124-36. https://doi.org/10.21294/1814-4861-2022-21-4-124-136.
- Jeong H., Hwang I., Kang S.H., Shin H.C., Kwon S.Y. TumorAssociated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J Breast Cancer. 2019; 22(1): 38-51. https://doi.org/10.4048/jbc.2019.22.e5.
- Tiainen S., Tumelius R., Rilla K., Hämäläinen K., Tammi M., Tammi R., Kosma V.M., Oikari S., Auvinen P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015; 66(6): 873-83. https://doi.org/10.1111/his.12607.
- Miyasato Y., Shiota T., Ohnishi K., Pan C., Yano H., Horlad H., Yamamoto Y., Yamamoto-Ibusuki M., Iwase H., Takeya M., Komohara Y. High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci. 2017; 108(8): 1693-700. https://doi.org/10.1111/cas.13287.
- Ge Z., Ding S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front Oncol. 2020; 10. https://doi.org/10.3389/fonc.2020.590941.
- Chen Y., Song Y., Du W., Gong L., Chang H., Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019; 26(1): 78. https://doi.org/10.1186/s12929-019-0568-z.
- Norton K.A., Jin K., Popel A.S. Modeling triple-negative breast cancer heterogeneity: Efects of stromal macrophages, fbroblasts and tumor vasculature. J Theor Biol. 2018; 452: 56-68. https://doi.org/10.1016/j.jtbi.2018.05.003.
- Eue I., Pietz B., Storck J., Klempt M., Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000; 12(11): 1593-604. https://doi.org/10.1093/intimm/12.11.1593.
- Viemann D., Strey A., Janning A., Jurk K., Klimmek K., Vogl T., Hirono K., Ichida F., Foell D., Kehrel B., Gerke V., Sorg C., Roth J. Myeloid-related proteins 8 and 14 induce a specifc infammatory response in human microvascular endothelial cells. Blood. 2005; 105(7): 2955-62. https://doi.org/10.1182/blood-2004-07-2520.
- Simkhes Yu.V., Karpov S.M., Baturin V.A., Vyshlova A. Role of s100 protein in the pathogenesis of pain syndromes. Neurology, Neuropsychiatry, Psychosomatics. 2016; 8(4): 62-4. https://doi.org/doi.org/10.14412/2074-2711-2016-4-62-64.
- Kim J.H., Oh S.H., Kim E.J., Park S.J., Hong S.P., Cheon J.H., Kim T.I., Kim W.H. The role of myofbroblasts in upregulation of S100A8 and S100A9 and the diferentiation of myeloid cells in the colorectal cancer microenvironment. Biochem Biophys Res Commun. 2012; 423(1): 60-6. https://doi.org/10.1016/j.bbrc.2012.05.081.
- Fox J.M., Kausar F., Day A., Osborne M., Hussain K., Mueller A., Lin J., Tsuchiya T., Kanegasaki S., Pease J.E. CXCL4/Platelet Factor 4 is an agonist of CCR1 and drives human monocyte migration. Scientifc reports. 2018; 8(1): 9466. https://doi.org/10.1038/s41598-018-27710-9.
- Schioppa T., Sozio F., Barbazza I., Scutera S., Bosisio D., Sozzani S., Del Prete A. Molecular Basis for CCRL2 Regulation of Leukocyte Migration. Front Cell Dev Biol. 2020; 8. https://doi.org/10.3389/fcell.2020.615031.
- Jayasingam S.D., Citartan M., Thang T.H., Mat Zin A.A., Ang K.C., Ch’ng E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol. 2020; 9: 1512. https://doi.org/10.3389/fonc.2019.01512.
- Fontana M.F., Baccarella A., Pancholi N., Pufall M.A., Herbert D.R., Kim C.C. JUNB is a key transcriptional modulator of macrophage activation. J Immunol. 2015; 194(1): 177-86. https://doi.org/10.4049/jimmunol.1401595.
- Hamada M., Tsunakawa Y., Jeon H., Yadav M.K., Takahashi S. Role of MafB in macrophages. Exp Anim. 2020; 69(1): 1-10. https://doi.org/10.1538/expanim.19-0076.
- Rigo A., Gottardi M., Zamò A., Mauri P., Bonifacio M., Krampera M., Damiani E., Pizzolo G., Vinante F. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol Cancer. 2010; 9: 273. https://doi.org/10.1186/1476-4598-9-273.
- Vlaicu P., Mertins P., Mayr T., Widschwendter P., Ataseven B., Högel B., Eiermann W., Knyazev P., Ullrich A. Monocytes/macrophages support mammary tumor invasivity by co-secreting lineage-specifc EGFR ligands and a STAT3 activator. BMC Cancer. 2013; 13: 197. https://doi.org/10.1186/1471-2407-13-197.
- Ongusaha P.P., Kwak J.C., Zwible A.J., Macip S., Higashiyama S., Taniguchi N., Fang L., Lee S.W. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004; 64(15): 5283-90. https://doi.org/10.1158/0008-5472.CAN-04-0925.
- Carroll M.J., Kapur A., Felder M., Patankar M.S., Kreeger P.K. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget. 2016; 7(52): 86608-20. https://doi.org/10.18632/oncotarget.13474.
- Yonemitsu K., Miyasato Y., Shiota T., Shinchi Y., Fujiwara Y., Hosaka S., Yamamoto Y., Komohara Y. Soluble Factors Involved in Cancer Cell-Macrophage Interaction Promote Breast Cancer Growth. Anticancer Res. 2021; 41(9): 4249-58. https://doi.org/10.21873/anticanres.15229.
- Yu X., Zhang Q., Zhang X., Han Q., Li H., Mao Y., Wang X., Guo H., Irwin D.M., Niu G., Tan H. Exosomes from Macrophages Exposed to Apoptotic Breast Cancer Cells Promote Breast Cancer Proliferation and Metastasis. J Cancer. 2019; 10(13): 2892-2906. https://doi.org/10.7150/jca.31241.
- Wu D.M., Wen X., Han X.R., Wang S., Wang Y.J., Shen M., Fan S.H., Zhang Z.F., Shan Q., Li M.Q., Hu B., Lu J., Chen G.Q., Zheng Y.L. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MicroRNA-126 -3p Inhibits Pancreatic Cancer Development by Targeting ADAM9. Mol Ther Nucleic Acids. 2019; 16: 229-45. https://doi.org/10.1016/j.omtn.2019.02.022. Retraction in: Mol Ther Nucleic Acids. 2022; 29: 617.
- Zhao K., Wang Z., Hackert T., Pitzer C., Zöller M. Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression. J Exp Clin Cancer Res. 2018; 37(1): 312. https://doi.org/10.1186/s13046-018-0961-6.
- Xiao D., Dong Z., Zhen L., Xia G., Huang X., Wang T., Guo H., Yang B., Xu C., Wu W., Zhao X., Xu H. Combined Exosomal GPC1, CD82, and Serum CA19-9 as Multiplex Targets: A Specifc, Sensitive, and Reproducible Detection Panel for the Diagnosis of Pancreatic Cancer. Mol Cancer Res. 2020; 18(2): 300-10. https://doi.org/10.1158/1541-7786.MCR-19-0588.
- Yunusova N.V., Zambalova E.A., Patysheva M.R., Kolegova E.S., Afanas’ev S.G., Cheremisina O.V., Grigor’eva A.E., Tamkovich S.N., Kondakova I.V. Exosomal Protease Cargo as Prognostic Biomarker in Colorectal Cancer. Asian Pac J Cancer Prev. 2021; 22(3): 861-9. https://doi.org/10.31557/APJCP.2021.22.3.861.


