Changes in fatty acid composition in leaf lipids of canola biotech plants under short-time heat stress
Автор: Sakhno Liudmyla O., Slyvets Mariia S., Korol Nataliia A., Karbovska Nataliia V., Ostapchuk Andriy M., Sheludko Yuriy V., Kuchuk Mycola V.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.10, 2014 года.
Бесплатный доступ
In order to study the influence of expression of heterologous genes of different origin ( cyp 11A1 and des C) on canola thermotolerance improvement on leaf membrane level the fatty acid composition was analyzed under short-time heat test. C yp 11A1 gene encodes cytochrome P450 SCC from bovine adrenal cortex mitochondria and was shown to affect the biosynthesis of steroid compounds. DesC gene encodes ∆9-acyl-lipid desaturase of cyanobacterium Synechococcus vulcanus. Decrease in palmitlinolenic acid content and index unsaturation as well as increase in total fatty acid and palmitic acid content were identified in cyp 11A1 canola in comparison with wild-type plants in stressfull conditions. But control and des C plants demonstrated similar changes in saturated (16:0), trienoic (16:3 and 18:3) fatty acid quantity, total fatty acid content and index unsaturation. Heterologous des C gene expression did not influence fatty acid composition and did not give advantages for plant under heat. Integration of cyp 11A1 gene in canola led to thermotolerance improvement on membrane level.
Brassica napus, cyp11a1, desc, fatty acids, heat stress
Короткий адрес: https://sciup.org/14323863
IDR: 14323863
Текст научной статьи Changes in fatty acid composition in leaf lipids of canola biotech plants under short-time heat stress
Increasing of the oil content, quality and yield remains the major aim of oilseed rape ( Brassica napus L.) breeding. Resistance to abiotic stresses becomes essential characteristic of plants because of environment changes.
Transgenic approaches are successfully used for both understanding plant abiotic stress tolerance (Bhatnagar-Mathur et al., 2008) and applying for rapeseed creation with different stress resistance (Basu et al., 2001). Transgenic canola plants overexpressing a vacuolar Na+/H+ antiport from Arabidopsis thaliana were able to produce seeds in the presence of 200 mM NaCl. Seed yields and seed quality were not affected by the high salt concentration (Zang et al., 2001). Overexpression of wheat mitochondrial Mn superoxide dismutase enhanced transgenic canola heat, drought and cold tolerance both under artificial stress conditions and in the field trial (Gusta et al., 2009). Biotech canola plants with heterologous expression of rice Osmyb4 transcriptional factor demonstrated an increased resistance to low positive temperature due to proline and phenolic compounds accumulation (Gomaa et al., 2012). Transgenic B.napus plants carrying either b-subunit of Arabidopsis farnesyltransferase (ERA1) antisense construct driven by a drought-inducible rd29A promoter or α-subunit of Arabidopsis farnesyltransferase (FTA) antisense construct driven by a drought-inducible Arabidopsis hydroxypyruvate reductase (AtHPR1) promoter were more resistant to seed abortion induced by water deficit during flowering. Field trial studies suggested that with adequate water, transgenic canola produced the same amount of seed as the parental control. Under moderate drought stress conditions at flowering, the seed yield of transgenic plants was significantly (up 16%) higher than the control (Wang et al., 2005; Wang et al., 2009). Early flowering and maturation, enhanced drought tolerance due to significant changes in gene expression and phytohormone distribution patterns were documented for canola overexpressing phosphatidylinositolphospholipase C2 (BnPtdIns-PLC2) (Georges et al., 2009). Overexpression of the RNA-binding domain of the flowering control locus A protein led to increase plant size, organ size, cell size, plant productivity and oil content in transgenic rape plants by downregulating the cell-cycle-related cyclin-B2-1 gene, an activator of cyclin-dependent kinase 1 (Qi et al.,
We have obtained canola lines bearing either bovine cyp 11A1 (Sakhno et al., 2010a) or bifunctional hybrid desC::licBM3 (Sakhno et al., 2012) genes in their nuclear genome using Agrobacterium tumefaciens- mediated leaf disk transformation (Sakhno et al., 2008).
Cytochrome P450 SCC from bovine adrenal cortex mitochondria is encoded by cyp 11A1 gene and was shown to affect the biosynthesis of steroid compounds in transgenic Nicotiana tabacum L. (Spivak et al., 2010). Obtained cyp 11A1 canola plants were resistant to BASTA herbicide treatment in greenhouse conditions due to expression of bar gene which was used in transformation cassette as a selective marker. Some of biotech plants accumulated an increased amount of total soluble proteins in leaves and seeds. They have enhanced antioxidant activity in leaf tissues. Some of them flowered 5-7 days earlier than the control plants (Sakhno et al., 2010a). We have shown that the integration of cyp 11A1 gene of animal origin under constitutive (35S) promoter also affected canola oil composition Sakhno et al., 2011). Increase in oleic acid (from 66 mole % to 73 mole %) was accompanied by decrease in linolenic (from 6 mol% to 3 mole %) acid. The total fatty acid content in canola seeds remained at the level of control plants.
Glyphosate- and BASTA resistant canola expressing bifunctional hybrid desC::licBM3 gene was another group of obtained transgenic plants (Sakhno et al., 2012). In the hybrid gene the sequence of desC desaturase of cyanobacterium Synechococcus vulcanus without plastid targeting was fused with the sequence of thermostable lichenase reporter licBM3 gene. Activity of the licBM3 gene product as a part of hybrid protein allowed quantitative and qualitative estimation of the desaturase gene expression. Fatty acid desaturases catalyze the transformation of a single bond between carbon atoms in acyl chains into the double bond (Murata, Los, 1997). Changes in the unsaturation of fatty acid residues in cellular membranes determine the membrane properties providing tolerance to low and high temperatures Wahid et al., 2007), and fungal pathogens (Madi et al., 2003). Plants are needed cell membrane stability under stress conditions (Blum, Ebercon, 1981).
Analysis of seed germination under high temperature revealed differences between control and cyp 11A1 seedlings in fresh weight, hypocotyl and root length, SOD activity Sakhno , 2011). Changes in fatty acid composition of leaf lipids of two canola transgenic groups were detected by using gas chromatography during growth under favourable conditions (Sakhno et al., 2010b, Sakhno et al., 2013). The aim of the present work was the study of fatty acid composition changes in leaf lipids of cyp 11A1 and des C canola plants under shorttime heat stress because it was documented that results of the heat tolerance test (short-time growth under 42°С in growth chamber) were positively correlated with improvement of high temperature resistance in field trial (Gusta et al., 2009).
MATERIALS AND METHODS
Plant materials. Spring canola plants (Brassica napus L.) cv Mariia (National Agrarian University of the Ukrainian Academy of Agrarian Sciences selection) micropropagating in vitro were used as the control material. Primary transformants expressing cyp11A1 gene were obtained using explants from aseptic cv Mariia plants (Sakhno et al., 2010). Transgenic homozygous Т21а and Т22с lines which were obtained by successive self pollination of T0 and T1 transformants under greenhouse conditions and in vitro phosphinothricin selection were also analysed. Cyp11A1 canola plants as well as control ones were micropropagated in aseptic conditions.
The initial plants in the experiments with ∆9-acyl-lipid ( desC ) desaturase of S. vulcanus were spring canola cv Obreey and they were used as controls. Canola lines 18b (primary transformant) and 18b/25 (T 1 generation) with selective nptII and bar genes, and target epsps and desC::licBM3 genes, which were produced by simultaneous cocultivation of canola explants with two agrobacterial vectors (Sakhno et al., 2012) were also tested. Control canola plants as well as desC ones were micropropagated under in vitro conditions.
Aseptic plants both controls and transgenic were transplanted into soil in greenhouse (12/12 photoperiod, +22°С). After two week adaptation they were transferred into Programmable Plant Growth Chamber, model WGC-P9 (WiseCube®WGC, Korea) for 14 day growth under the following conditions: 16 h (light)/8 h (dark) photoperiod, temperature +22°С (day)/+18°С (night), 70% humidity, 480-550 µmol/m2s light intensity).
Heat tolerance tests. Humidity, photoperiod, and light intensity in growth chamber were maintained without changes. Temperature was increased with 2°С/h gradient to 42°С. Plants were taken isothermal at 42°С for 16 h (Gusta et al., 2009).
Gas chromatography - mass spectrometry of fatty acid methyl esters (FAMEs). One-step lipid extraction and FAMEs preparation method (Garces,
Mancha, 1993) was used for canola leaf fatty acid composition evaluation. Tissue samples (200 mg) together with heptadecanoic acid (C 17:0) as internal standard were placed in tubes with Teflon-lined caps. Methylating mixture containing methanol:benzene:H 2 SO 4 (44:20:2, by vol) was used. All reagents were analytical grade. Mixture (3.3 ml) and heptane (1.7 ml) were added to the sample. The tubes were placed in a water bath at 80°C for 2 h. Vigorous shaking after some 2-3 min heating was necessary to mix all the components into a single phase. After heating, the tubes were cooled at room temperature and shaken again. The upper phase contained the FAMEs. They were analyzed by using Agilent 6890N/5973inert GC/MS with DB-FFAP capillary column (30m ×0.25mm ×0.25µm) (J&W Scientific). Temperature program was from 150°С to 220°С with 2°/min gradient, injector temperature was 250°С. Helium was applied as a carrier gas with flow rate 1 ml/min.
Index unsaturation which characterized fatty acid lipid unsaturation in membrane lipids was calculated by the formula:
I=(Σ С n:1 +2Σ C n:2 +3Σ C n:3 )/100
С n:1 – content (% by weight) of the corresponding unsaturated fatty acids.
Statistical analysis was performed according to Duncan multiple range test. Differences from control values were significant at p≤0.05. Three independent experiments were conducted in five replications.
RESULTS
Gas chromatography-mass spectrometry of fatty acid esters
Qualitative composition of fatty acids from leaves of analyzed biotech lines did not differ from control plants (Fig.1). There are (in descending order by quantity) linolenic (18:3), palmitic (16:0), palmitlinolenic (16:3), linoleic (18:2), and palmitoleic (16:1) acids. Plants of parental cultivars differed in the palmitoleic acid content. It was less than 3 mole % in leaves of Mariia variety (Fig.1B), and it was not detected in ones of Obreey (Fig.1A).
Linolenic acid content was similar in control and des C canola leaves both in normal and high temperatures (Fig.1A). It declined under stressful conditions analyzed. Linolenic acid content in Т 2 1а leaves was slight significantly lower (56.28±1.47 mole %) than in control (59.07±1.04 mole %) at 22°C (Fig.1B). It was the same in Т 2 2c (58.48±1.61 mole %) and control leaves. High temperature influence led to decrease in linolenic acid content in control plants on 2.43 mole % (0.04%), but no changes were detected in transgenic ones.
There were not detected differences in palmitic acid between initial and transgenic plants under favourable temperature (Fig.1). Under heat C16:0 content increased in control (up 1.12-fold) as well as in transgenic des C (up 1.18-1.21-fold) canola leaves (Fig.1A). High temperature growth also affected increment in palmitic acid content in cyp 11A1 ones by 12% (Т 2 1а line) – 19% (Т 2 2c line). But it did not change in cv Mariia untransformed plants (Fig.1B).
In primary des C transformant (18b line) palmitlinolenic acid content was higher than in control under 22°С (by 36 %) and 42°С (by 26 %). It did not significantly differ in non-transformed (10.07 and 9.12 mole %) and 18b/25 (11.4 and 10.01 mole %) plants in two temperature regimes tested. C16:3 content was the same (~13 mole %) in both control and cyp 11A1 canola leaves at 22°С. Under heat stress it did not change in control plants and decreased in transgenic ones by 31-33% (Fig.1B).
Linoleic acid content was higher in untransformed cv Obreey leaves both in normal temperature (7.75 mole %) and heat (9.29 mole %) than in des C ones (Fig.1A). It retained unchanged in 42°С in biotech des C plants (7.08 and 7.72, respectively) and increased in control by 20%. Under higher temperature C18:2 content rose by ~24% in cv Mariia control and cyp 11A1 canola leaves (Fig.1B). Any differences between the control and transgenic plants in without stress conditions were not found. Under heat the linoleic acid content slight significantly declined in Т 2 2c in comparison with control and Т 2 1а leaves.
Palmitoleic acid content was 31% lower in control plants in comparison with cyp 11A1 at 22°C. Under heat stress it increased to transgenic plant level. Palmitoleic acid content remained unchanged in Т 2 1а and Т 2 2c leaves under heat temperature test (~4 mole %).
Total fatty acid content was similar in control and desC transgenic leaves (Fig. 2A) under temperature regimes tested except 15% increase in 18b/25 line in stress. Total fatty acid content was significantly lower by ~27% in cyp11A1 canola leaves than in control ones under normal temperature conditions in growth chamber (Fig. 2B). It decreased up 33% in control as a result of heat temperature influence, while it remained unchanged in cyp11A1 canola in both conditions. At 42°С the excess of total fatty acid content was 2025% in transgenic plants under in control.
Index unsaturation was the same for control and transgenic plants at normal temperature (Fig. 3) except increase up 1.04% in 18b line. It did not change for control leaves, but slight significantly decreased (by 0.05%) from 2.39±0.02 to 2.27±0.03 in cyp 11A1 plants at heat stress (Fig. 3B). Index unsaturation declined in both control cv Obreey and des C transgenic plants under 42°С by 0.05% (Fig. 3A).
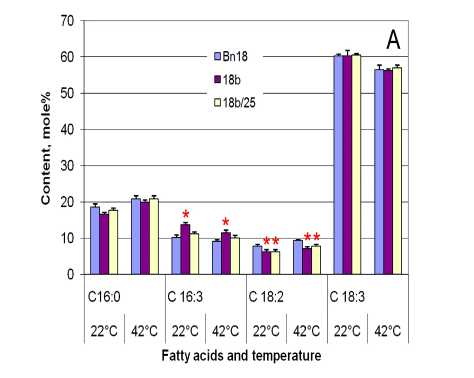
Figure 1. Fatty acid composition of canola leaf lipids in des C ( А ) and cyp 11A1 ( B ) canola leaves before (growth temperature 22°С) and after (temperature 42°С) heat stress: Bn18 and Bn12 are control plants (cv Mariia and Obreey, respectively); 18b and 18b/25 are transgenic plants (T 0 and T 1 generation, respectively) expressing ∆9-acyl-lipid desaturase from S.vulcanus ; Т 2 1а and Т 2 2c are homozygous cyp 11A1 lines of second generation. Fatty acids are palmitic (С16:0), palmitoleic (С16:1), palmitlinolenic (С16:3), linoleic (С18:2), linolenic (С18:3).
Error bars represent mean ± one standard deviation and asterisk * indicates significant differences between experimental values compared with the control ones (p≤0.05).
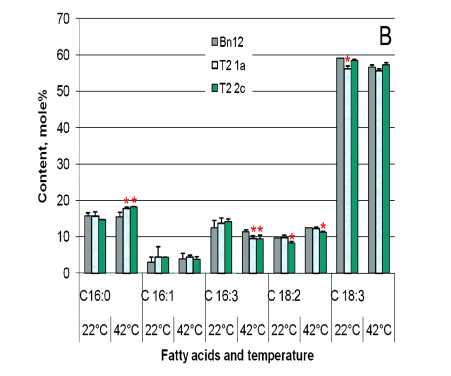
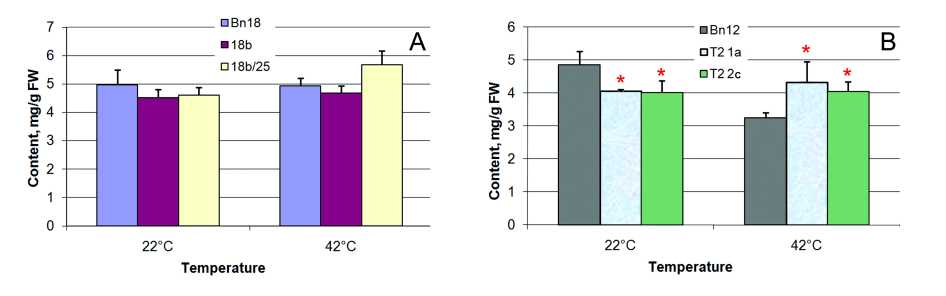
Figure 2. Total fatty acid content in des C ( А ) and cyp 11A1 ( B ) canola leaves before (growth temperature 22°С) and after (temperature 42°С) heat stress: Bn18 and Bn12 are control plants (cv Mariia and Obreey, respectively); 18b (T 0 generation) and 18b/25 (T 1 generation) are transgenic plants expressing ∆9-acyl-lipid desaturase from S.vulcanus ; Т 2 1а and Т 2 2c are homozygous cyp 11A1 lines of second generation.
Error bars represent mean ± one standard deviation and asterisk * indicates significant differences between experimental values compared with the control ones (p≤0.05).
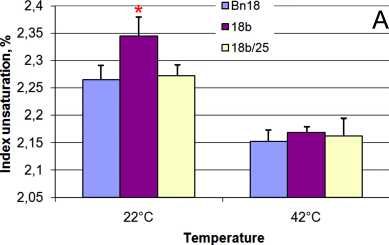
Figure 3. Index desaturation of membrane lipids in des C ( А ) and cyp 11A1 ( B ) canola leaves before (growth temperature 22°С) and after (temperature 42°С) heat stress: Bn18 and Bn12 are control plants (cv Mariia and Obreey, respectively); 18b and 18b/25 are T 0 and T 1 generation of transgenic plants expressing ∆9-acyl-lipid desaturase from S.vulcanus ; Т 2 1а and Т 2 2c are homozygous cyp 11A1 lines of second generation.
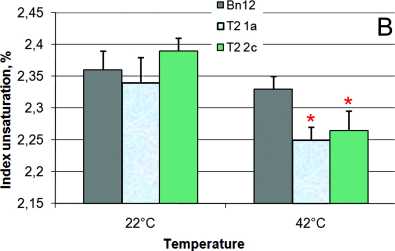
Error bars represent mean ± one standard deviation and asterisk * indicates significant differences between experimental values compared with the control ones (p≤0.05).
DISCUSSION
Heat stress during vegetative growth causes many physiological and metabolic changes, including alterations in hormone homeostasis, reductions in the activities of antioxidant enzymes, modifications in membrane functions mainly because of the alteration of membrane fluidity (Barnabás et al., 2008). High leaf temperatures reduce plant growth and limit crop yields.
The control and transgenic plants expressing heterologous cyp11A1 or desC genes differed in leaf quantitative composition of fatty acids during growth under favourable and high temperatures in growth chamber. Under heat stress trienoic fatty acid (linolenic and palmitlinolenic) content was lower in cyp11A1 canola leaves in comparison with control and desC ones because C16:3 content decrease by 31-33%. Reduction in trienoic fatty acid content is a precondition for the countering the damaging effects of high temperature (Iba, 2002). It was shown that thermotolerance across the 13 different lines of Arabidopsis thaliana triple mutants fad3-2 fad7-2 fad8 was more strongly correlated with leaf 16:3 content than to 18:3 content or to the sum of 16:3 + 18:3, the average number of double bonds per glycerolipid molecule, or other measures of membrane unsaturation (Routaboul et al., 2012). The most resistant mutants contained less than 2% 16:3 compared with 12% in wild-type Arabidopsis. By contrast, the levels of 18:3 among these mutants varied from 0 to 54%. The most thermosensitive lines contained 12–13% 16:3 in their leaf lipids together with 37–48% 18:3. The degree of linear correlation (r2) between PSII inactivation and 16:3 content was 0.88, while the r2 for 18:3 content was only 0.37. Also three Arabidopsis mutants deficient in 16:3 had enhanced thermotolerance, relative to the wild type, when plants were grown at 17°C. Thermotolerance of these mutant lines did not change under 29°C but it increased in the wild type to be equal to that of the mutants due to 67% decrease in the proportion of 16:3 in the total leaf lipids of wild-type plants (Falcone et al., 2004).
Under normal temperature palmitic acid content was similar in leaves of control and transgenic plants. It did not change in control cv Mariia plants but increased in cv Obreey at 42°C. It indicates a better adaptability of the latter to short-time heat stress. Under heat increase in unsaturated (C16:0) fatty acid content was the same in control (cv Obreey) and desC leaves. But in cyp11A1 canola leaves it was higher in comparison with control cv Mariia by 12-19% indicating thermotolerance improvement (Murata, Los, 1997). In Arabidopsis thaliana triple mutants fad3-2 fad7-2 fad8 which did not synthesize trienoic fatty acids increase in palmitic acid under heat stress was documented (Routaboul et al., 2012).
Total leaf fatty acid content of cyp 11A1 canola was not influenced by heat stress and was reduced in control plants up 25%. In des C and control cv Obreey leaves total fatty acid content was similar in 22°C and retained unchanged under 42°C.
It is known that the desert plants have reductions in overall unsaturation of leaf lipids at high temperatures (Pearcy, 1978). In our experiments index unsaturation was the same for control and transgenic plants at the normal temperature. It did not change for control Mariia leaves, but slight significantly decreased in cyp 11A1 at heat stress. The index unsaturation decline is one of the distinguishing features of the thermotolerant plants (Iba, 2002). Index unsaturation decreased in both control cv Obreey and des C transgenic plants under 42°С, thus difference in this parameter between these plants in heat was not documented.
Some characteristics of cyp 11A1 canola plants such as decrease of 16:3 content and index unsaturation, increase in total fatty acid and palmitic acid content under short-time heat stress allow us to consider these lines more thermotolerant than untransformed control. Heterologous des C gene expression did not influence fatty acid composition and did not give advantages for plant under heat.
CONCLUSIONS
Study of fatty acid composition of canola leaf lipids made it possible to reveal differences between initial and transgenic plants under short time heat test. Decrease in palmitlinolenic acid content and index unsaturation as well as increase in total fatty acid and palmitic acid content were identified in cyp11A1 canola in comparison with wild-type plants in stressfull conditions. But control and desC plants demonstrated similar changes in saturated (16:0), trienoic (16:3 and 18:3) fatty acid quantity, total fatty acid content and index unsaturation. Heterologous desC gene expression did not give advantages for plant under heat. Integration of cyp11A1 gene in canola led to thermotolerance improvement on membrane level.
ACKNOWLEDGMENTS
Experiments were partially supported by National Academy of Sciences of Ukraine grant №0110U006062.
Список литературы Changes in fatty acid composition in leaf lipids of canola biotech plants under short-time heat stress
- Barnabás, B., Jäger, K., Fehér A. (2008) The effect of drought and heat stress on reproductive processes in cereals Plant, Cell and Environment. 31: 11-38
- Bhatnagar-Mathur, P., Vadez, V., Sharma, K.K. (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep. 27: 411-424
- Blum, A., Ebercon, A. (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Science. 21: 43-47
- Falcone, D.L., Ogas, J.P., Somerville, C.R. (2004) Regulation of membrance fatty acid composition by temperature in mutants of Arabidopsis with alternations in membrane lipid composition. BMC Plant Biology. 4: 17
- Garces, R., Mancha, M. (1993) One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 211: 139 -143
- Georges, F., Das, S., Ray, H., Bock, C., Nokhrina, K., Kolla, V. A., Keller, W. (2009) Over-expression of Brassica napus phosphatidylinositolphospholipase C2 in canola induces significant changes in gene expression and phytohormone distribution patterns, enhances drought tolerance and promotes early flowering and maturation. Plant, Cell and Environment. 32: 1664-1681
- Gomaa, A.M., Raldugina, G.N., Burmistrova, N.A., Radionov, N.V., Kuznetsov, Vl.V. (2012) Response of Transgenic Rape Plants Bearing the Osmyb4 Gene from Rice Encoding a Trans-Factor to Low Above-Zero Temperature. Rus. J. Plant Physiol. 59: 105-114
- Gusta, L.V., Benning, N.T., Wu, G., Luo, X., Liu, X., Gusta, M.L., McHughen, A. (2009) Superoxide dismutase: an all-purpose gene for agri-biotechnology. Mol. Breeding. 24: 103-115
- Iba, K. (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 53: 225-245
- Murata, N., Los, D. A. (1997) Membrane Fluidity and Temperature Perception. Plant Physiol. 11: 875-879
- Pearcy, R. (1978) Effect of growth temperature on the fatty acid composition of the leaf lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiology. 61: 484-486
- Qi, W., Zhang, F., Sun, F., Huang, Y., Guan, R., Yang, J., Luo, X. (2012) Over-expression of a conserved RNA-binding motif (RRM) domain (csRRM2) improves components of Brassica napus yield by regulating cell size. Plant Breed. 131: 614-619
- Routaboul, J.-M., Skidmore, C., Wallis, J. G., Browse, J. (2012) Arabidopsis mutants reveal that short-and long-term thermotolerance have different requirements for trienoic fatty acids. J. Experim. Botany. 63: 1435-1443
- Sakhno, L. A., Gocheva, E. A., Komarnitskii, I. K., Kuchuk, N. V. (2008) Stable expression of the promoterless bar gene in transformed rapeseed plants. Cytology and Genetics. 42: 21-28
- Sakhno, L.O., Моrgun, B.V., Кvasko, O.Y., Кuchuk, M.V. (2010a) Transformed canola plants expressing mammalian cyp11A1 gene of cytochrome P450SCC. Biotechnology (Kyiv). 3: 74-82 (in Russian)
- Sakhno, L.O. (2011) Seed germination features of canola plants expressing mammalian cytochrome P450SCC cyp11A1 gene. The Bulletin of Vavilov Society of Geneticists and Breeders of Ukraine. 9: 253-259 (in Russian)
- Sakhno, L.O., Ostapchuk, A.M., Klochko, V.V., Kuchuk, M.V. (2011) Fatty acid oil composition of canola plants expressing mammalian cytochrome P450SCC cyp11A1 gene. In Szłyk, E. (ed.) Advances in research and technology of rapeseed oil. Monograph -part III. Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika, Toruń, pp. 55-59
- Sakhno, L. O., Gerasymenko, I. M., Komarnitsskii, I. K., Sheludko, Y. V., Goldenkova-Pavlova, I. V. (2012) Creation of glyphosate-resistant Brassica napus L. plants expressing DesC desaturase of cyanobacterium Synechococcus vulcanus. Biopolymers and Cell. 28: 449-455
- Sakhno, L.O., Slyvets, M.S., Ostapchuk, A.M., Korol, N.A., Sheludko, Y.V., Goldenkova-Pavlova, I.V. (2013) Canola with cyanobacterium Synecococcus vulcanus DesC transgene: leaf fatty acid composition. Abstracts of the X International Conference “Plant Cell Biology In Vitro and Biotechnology”, Kazan, p.255
- Spivak, S. G., Berdichevets, I. N., Litvinovskaya, R. P., Drach, S. V., Kartel, N. A., Shpakovski, G. V. (2010) Some Peculiarities of Steroid Metabolism in Transgenic Nicotiana tabacum Plants Bearing the CYP11A1 cDNA of Cytochrome P450SCC from the Bovine Adrenal Cortex. Rus. J. Bioorganic Chem. 36: 224 -232
- Trehub, M.S., Sakhno, L.O. (2012) Transgenic Brassica napus plants expressing cytochrome Р450SCC cyp11A1 gene under in vitro osmotic stress conditions. In International Conference “Biotechnology and Plant Breeding. Perspective Towards Food Security and Sustainability”. Radzikow, Poland, p. 156
- Wahid, A., Gelani, S., Ashraf, M., Foolad, M.R. (2007) Heat tolerance in plants: an overview. Environ. Exp. Bot. 61: 199-233
- Wang, Y., Beaith, M., Chalifoux, M., Ying, J., Uchacz, T., Sarvas, C., Griffiths, R., Kuzma, M., Wan, J., and Huang, Y. (2009) Shoot-Specific Down-Regulation of Protein Farnesyltransferase (α-Subunit) for Yield Protection against Drought in Canola. Molecular Plant. 2: 191-200
- Wang, Y., Ying, J., Kuzma, M., Chalifoux, M., Sample, A., McArthur, C., Uchacz, T., Sarvas, C., Wan, J., Dennis, D.T., McCourt, P., Huang, Y. (2005) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J. 43: 413-424
- Zang, H.X., Hodson, J.N., Williams, J.P., Blumwald, E. (2001) Engineering salt-tolerant Brassica plants: Characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc. Natl. Acad. Sci. USA. 98: 12832-12836


