Changes in osmolites contents, lipid peroxidation and photosynthetic pigment of Aeluropus lagopoides under potassium deficiency and salinity
Автор: Alikhani Fatemeh, Saboora Azra, Razavi Khadija
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.7, 2011 года.
Бесплатный доступ
Potassium, the most abundant cation in plant cells, is responsible for numerous physiological functions. In saline environment, similarity of Na+ and K+ causes an unbalance in K+ uptake and disorder in the its functions. In the present research, changes of four biochemical parameters (proline, glycinebetaine, photosynthetic pigments and malondialdehyde) have been investigated in Aeluropus lagopoides seedling under salinity and potassium deficiency. Sterile seeds had been cultured on modified Murashige-Skoog containing 0, 1.75 or 100 mM potassium, with or without 600 mM NaCl for 30 days. The results showed that maximum proline content was observed in root and shoot by 600 mM NaCl + 1.75 mM K+ treatment. Also in this treatment, amount of carotenoids and chlorophyll a was more decreased. Potassium deficiency caused to reduced MDA and chlorophyll b content. The highest amount of glycinebetaine was measured in the presence of 600 mM NaCl in the company of 100 mM K+. It can conclude that chlorophyll oxidation was occurred in K+ deficiency because of increasing lipid peroxidation and disruption of protein-pigment complexes. The accumulation rates of two osmolite in different organ was shown that in A. lagopoides proline and glycinebetaine play more important role in osmotic adjustment of the shoot and root, respectively.
Aeluropus lagopoides, proline, glycinebetaine, mda, potassium deficiency, salinity
Короткий адрес: https://sciup.org/14323523
IDR: 14323523
Текст научной статьи Changes in osmolites contents, lipid peroxidation and photosynthetic pigment of Aeluropus lagopoides under potassium deficiency and salinity
Effect of salinity on plants is complex; there is evidence that high levels of salt cause an unbalance in the ion uptakes that is reason of both ion toxicity and osmotic stress (Ashraf and Harris, 2004). Accumulation of salts in the root environment induces nutrient deficiency (Maathuis and
Amtmann, 1999; Tester and Davenport, 2003). Changes in the carrier functions are responsible for ion toxicity and reduced plant growth in this condition. For example, potassium is the most abundant cation in plant cell (more than 1% dry weight) which affected by salinity (Very and
Sentenace, 2003; Leigh and Yones, 1984). Potassium plays several biochemical roles including: maintain integrity of photosynthetic structure, CO 2 fixation, photosynthate transport, regulating of chlorophyll content and adjusting of turgor pressure (Maathuis and Sanders, 1996; Kochian and Luccas , 1988; Pier and Benkowitz, 1987; Zhao et al., 2001).
In terms of chemical properties, K+ and Na+ are similar. So in high concentration of Na+, this ion replaces K+ and consequently appears K+ deficiency syndrome. Homeostasis of Na+/K+ ratio is very important specially for functional regulation of membrane carriers and channels that are associated in the plant cells for K+ influx and Na+ efflux (Amtmann et al., 2004). Under salt stress, competition between uptake of K+ and Na+ through common carriers may have generates disorder in the activities of K+ channels especially in root cells. In Arabidopsis taliana , Eucalyptus and wheat, K+ channels with low affinity act as carriers of Na+ under salinity (Chinnusamy et al., 2005).
Salt tolerance in higher plants is regulated by a number of different physiological and biochemical processes. Therefore, study and identification of some mechanisms that involve in the plant resistance under salinity and nutrient deficiency will be useful. The results can be relevant for genetic breeding techniques and for improvement of the crop tolerance to salinity. Aeluropus lagopoides (Poacae), is a monocotyledon halophytic plant that grows in poor soils and dry lands. This pasture plant is a C4 photosyntetic type with underground durable stems which use as forage and it prevent soil erosion due to a very extensive root network. Vegetative reproduction is occurring by seeds and stolen division. The developed root systems, waxy epicotyle and small leaves are the main characters that adapted the plant to environment stress such as drought and salinity. A. lagopoides has outstanding physiological and molecular properties that can be used for grassland restore and increasing of crop tolerance. Some information has been reported about transferring desirable traits from wild relatives to crop varieties. For example, Wei et al. (2001) was reported transgenic wheat which had the salt tolerance trait of A. littoralis sinensis via asymmetric somatic hybridization. There is a number research related to tolerance of Aeluropus genus under stress condition (Mohsenzadeh et al., 2006; Jannesar et al., 2009).
In the present study seeds of A. lagopoides were cultured as hydroponic condition in the presence of sufficient quantity and deprived of K+ that supplemented by 0 or 600 mM NaCl. Then, physiological responses of the treated seedlings were investigated through measurement of changes in proline, glycinebetaine, photosynthetic pigments and MDA content. The results can identify how the osmotic homeostasis takes place in this plant and how existence of Na+ and K+ concentration influence on leakage of the cell membrane.
MATERIALS AND METHODS
Plant material and treatments: The A. lagopoides seeds were collected from river margin of Ghandi-Abad (Kashan, Esfehan provines, Iran). They were sterilized by solution containing sodium hypochlorite 3% and Triton X-100 1% for 7 minutes. The seeds after washing with sterile distilled water were cultured on the mesh located in modified hydroponic 1/ 2 MS media (Murashige and Skoog, 1962). The cultures were kept at 4 ˚C in dark for four days. Germinated seeds were transferred to growth chamber (23± 2 ˚C, 16/8 h light/dark). After 8 days the seedlings were transferred to new culture medium containing: 1.25 mM KNO 3 , 1.5 mM
Ca(NO 3 ) 2 , 0.75 mM MgSO 4 , 0.5 mM KH 2 PO 4 , 75 μM FeEDTA, 50 μM H 3 BO 3 , 10μM MnCl 2 , 2 μM ZnSO 4 , 1.5 μM CuSO 4 and 0.075 μM (NH 4 ) 6 Mo 7 O 24 for 72 h, following treatment of the seedlings were performed as table 1. For each treatment, three replicates were considered and the nutrient solution (pH 5.8) was renewed every 3days. At the end of the thirteenth day, root and shoot of the seedling were separated and kept at -20 ˚C.
Malondialdehyde content : For the MDA assay, 0.1 g fresh tissue (root and shoot) were homogenized with 1 ml trichloroacetic acid (TCA) 1% and centrifuged at 10000 ×g for 5 min. For measurement of MDA concentration, 4 ml of 20% trichloroacetic acid containing 0.5% thiobarbituric acid were added to a 1 ml aliquot of the supernatant. The mixture was heated at 95 ˚C for 30 min, quickly cooled in an ice bath and then centrifuged at 10000 ×g for 10 min. The absorbance of the supernatant was read at 532 and 600 nm. The concentration of MDA was calculated using the MDA extinction coefficient of 155 mM-1 cm-1 (Heath and Packer, 1986). The result of MDA was expressed as μg mg-1 FW
Proline Assay: Fresh tissue (50 mg) was powdered in liquid nitrogen and homogenized by 1.5 ml solphosalisylic acid 3% , and was centrifuged at 12000 ×g for 7 minutes. Then, 2 ml ninhydrin 1% in acetic acid 60% and 500 μl distilled water was added to 500 μl supernatant. Mixture was incubated in boiling water batch, after 1 hour in order to stop the reaction test tubes were placed on ice and 2 ml toluene were added to each test tube and solutions as well mixed (Bates, 1973). Absorbance of toluene phase was determined in 518 nm and proline content was estimated as nmol mg-1 FW.
Glycinebetaine assay: Dry powder (25 mg) was extracted in 2 ml double distilled water for 24h at room temperature. After centrifugation (10000 g , 10 min) , 250 μl supernatant was mixed with 250 μl sulfuric acid 1 M and incubated at 4˚C for 1h. Following addition of 200 μl KI-I2 reagent to the solution, mixture reaction was maintained 16h in a cold room and centrifuged at 10000 ×g for 15 min in a refrigerated centrifuge .Deposit was dissolved in 4 ml 1,2- dichloroethanol and was kept at room temperature for two hours. The absorbance of the solution was read at 365 nm. Glycinebetaine content was expressed as μg g-1 DW (Grieve and Grattan, 1983).
Measurement of pigment contents: the content of photosynthetic pigments were determined according to the method of Lichtenthaler and Wellburn (1985), 0.2 g fresh leaves were homogenized in 4 ml acetone 80% and centrifuged at 3000 Чg for 10 min. Absorbance of the clear supernatant was read at 663.2, 646.8 and 470 nm and pigment concentrations were calculated using the following formula:
Chl a (μg / ml) = 12.25 A 663.2 - 2.79 A 646.8
Chl b (μg / ml) = 21.50 A 646.8 - 5.10 A 663.2
Chl T (μg / ml) = Chl a + Chl b
C X+C = (1000 A 470 -1.8 Chl a-85.02 Chl b) / 198
In this formula Chl a, Chl b, Chl T and C X+C are, respectively, chlorophyll a, chlorophyll b, total chlorophyll and carotenoids concentrations. Amount of the pigments was calculated as mg g-1 FW leaf.
Statistical analysis: All treatments were carried out based on completely randomized block design in three replicates. The data related to proline, glycinebetaine and MDA content (in root and shoot) were analyzed using SPSS software (version 11.5)
by two-way variance analysis (GLM) and a simple variance analysis (ANOVA) was performed for photosynthetic pigments and then, differences between the means were compared by Duncan multiple range test at P<0.05.
RESULTS
Statistical analysis based on the interaction of two factors (different treatments and organs) were showed a significant difference (P <0.05) between the means of proline, glycinebetaine and MDA in root and shoot after different treatments. Also, the difference between averages of photosynthetic pigments and ratio of chlorophyll a/b were significant (P<0.05).
Lipid peroxidation
The MDA concentration was measured in leaves and roots as an indicator of oxidative stress in plants. Our results were showed that in natural condition, lipid peroxidation of the shoot was about 2.5 fold of the root in A. lagopoides (Fig. 1) but in media containing low concentration of K+ (K0 and K1.75 treatments), lipid peroxidation in the shoot were 22.4 and 8.1 times greater than the root, respectively. On the contrary, by increasing of K+ concentration in the media (100 mM K+), peroxide production decreased significantly in shoot compared with the root (Fig. 1). Producing of the lipid peroxids in the seedling were markedly increased under salt stress because of Na+ accumulation (15-16 μg against to 0.07 μg per mg FW in the control). In this regard, presence of the high concentrations of K+ was not significant effect on the root MDA content. The produced MDA in the roots by 1.75 and 100 mM K+ treatments under saline condition were 0.08 and 0.04 μg mg-1 FW, about 6.5 and 3 fold of the control, respectively. But in the shoot tissues, low concentration of K+ was dramatically effected on degradation of the lipids (Fig. 1).
Lipid peroxidation stimulates degradation of membrane structures (Lester and Stein, 1993) and because of electrolyte leakage (Tiburcio et al., 1994) alters selective permeability of the membrane (Weckx et al., 1993). So, in different stress, stability of the cell membranes in sensitive and tolerate plants is widely depending on integrity of these structures (Aziz and Larher, 1998; Liang et al., 2003). In our result, minor increasing of the MDA in both organ tissues of A. lagopoides take placed in the presence of 600 mM NaCl that exhibited resistance of the membrane of this halophytic plant to salinity and the more sensitivity to the K+ deficiency. Plant physiological response to oxidative stress is depending on plant species. Abscisic acid (ABA) is a plant hormone that its level will be increased after biotic and abiotic stress. Jannesar et al. (2009) were showed that evidence of ABA function on reducing MDA production in the root of A. lagopoides. MDA concentration was reduced in barley leaves under salinity stress (Liang, 1999). Among wheat cultivars, level of this compound was increased under salinity in Alvand (salt sensitive) but it was constant in the Sardari, a resistant cultivar (Esfandiari et al., 2007). In glycophytic plants, unusual high ratio of Na+/K+ is caused inactivity of the many enzymes and inhibition of protein biosynthesis. In the same condition, Na+ was replaced to Ca2+ in plasma membrane of tobacco hairy roots and thus creating a plasma membrane permeability changes which followed by K+ leakage from the cells (Cramer et al., 1985). Besides, antioxidant enzymes reduce MDA production, Some researchers had been showed that under salinity stress, there is a strong relationship between increasing of antioxidant enzymes activity and decreasing of the lipid peroxidation in salt tolerate tomatoes (Lycopersicum pennellii) and wild beets (Beta maritime) (Shalata and Tal, 1998; Bol et al., 2003).
Proline content
Proline content of the shoot and root in treated seedling which was grown under potassium deficiency (K 0 ) were 0.3 and 0.04 μmol mg-1 FW, respectively. Under control and non-saline conditions (K 1.75 ), the means of proline content in the shoots were not significantly different but the means of root proline had substantial difference; comparison of two treatments showed that ratio of the shoot/ root proline were 2.3 in control seedling and 0.25 in seedling treated by 1.75 mM K+. Compared with the control, proline content was reduced about 20% in the shoot seedlings that were grown in the presence of 1.75 mM K+. While its value was increased 660% higher than the root control (Fig. 2). On the other hand, by increasing of K+ concentration in the media (up to 100 mM), measured proline in the roots was near to the control but accumulation of this osmolite was 100 times higher than the control (Fig. 2).
It is possible that under K+ deficiency, proline was transported from root to shoot and in sufficient K+ biosynthesis of proline and proteins was induced in the shoot. By increasing of sodium content in the media, accumulation of proline was elevated in the both shoot and root. As it is shows in Figure 2, proline content were variously raised in the shoot tissues at the presence of 600 mM NaCl depending on K+ concentrations. For example, when seedlings have been treated by low concentration of K+, proline content was at maximum level in both shoot and root (19.1 and 21.1 μmol mg-1 FW, respectively), but it found less increased after treatment of the seedling by salinity and high concentration of K+. it is interesting that ratio of the shoot/root proline content was altered from 2.8 to 5.5 in the saline media when K+ concentration was changed from 1.75 to 100 mM.
To maintain osmotic homeostasis, biosynthesis and accumulation of the compatible osmolites such as glycinebetaine and proline increase in plant cells. Halophytes are able to regulate strongly osmotic adjustment by mineral ion accumulation in the vacuole and compatible solute accumulation in the cytosol (Wang et al, 2004, Cuin and Shabales., 2008). Accumulation of Proline, as a non-toxic osmoprotectant, is general in numerous monocotyledons under saline conditions (Ahsraf and Harris, 2004; Lee and Liu, 1999). Proline involves in protection of enzymes and cellular structures and also removing of the free radicals (Soloman et al., 1994; Van Rensburg et al., 1993). It suggests that accumulation of the osmolites in glycophytes will not increase osmotic pressure, but these compounds are trap of the activated oxygen species (Cuin and shabales, 2007).
In the present study, proline accumulation in the A. lagopoides root and shoot was minimum in natural and non-saline conditions, but treatment of the seedling by 100 mM potassium promoted proline accumulation in shoot compared with the control, it was may be due to role of K+ in protein biosynthesis. According to our result, it was reported that proline accumulation and sodium content had been increased in both root and shoot of A. lagopoides under salt stress (Jannesar et al., 2009). Unbalance in K+ and Na+ uptake was main reason of this occurrence that created a new water balance by adjustment of water potential in the cells. Although, Na+ could be useful to maintain turgor but in long term it cannot be considered as a suitable alternate for K+. Correlation between proline accumulation and Na+ is more than it and Cl- or K+. It is more likely a critical level of Na+ is necessary for proline biosynthesis (Rout and Shaw, 1998). Proline accumulation in plant tissues under osmotic stress can be resulted by: 1) increase proline biosynthesis, 2) reduction in the Proline degradation, 3) increased protein hydrolysis, and 4) reduce the consumption of proline (Hsu et al., 2003). It seems that each four reasons can be help to increasing proline accumulation in A. lagopoides after interaction effects of low concentration of K+ and salinity but in high concentration of K+ protein hydrolysis is less effective. Tissue specific accumulation of proline under biotic and abiotic stress has also been reported in other halophytes such as: Thellugiella halophila and Lepidiumcrassifolium. In barley, proline accumulation was caused adaptation of the plant to salinity and osmotic stress (Delauney and Verma, 1993).
Glycinebetaine content
Amount of this osmolite was showed no significant difference between shoot and root of the control plants (64-67 μg g-1 DW). Also, content of the glycinebetaine was approximately equal in plant roots which had grown in low or high concentration of K+ (K0 or K100 treatment). In the absence of potassium, glycinebetaine content of the root and shoot were about 71 and 82 μg g-1 DW, respectively (Fig. 3). But by addition of 1.75 mM K+ to culture medium, amount of this compound raised in the root up to 1.4 fold of the Shoot. While in the presence of 100 mM potassium, accumulation of glycinebetaine in the root was lesser than the shoot (about 84 and 105 μg g-1 DW, respectively). It is represented that high concentrations of K+ had a positive role on the glycin-betaine biosynthesis in the shoot (Fig. 3). The results were showed that maximum amount of this osmolite was observed in the shoot of seedlings that were treated by 600 mM NaCl combined with 1.75 or 100 mM potassium (233 and 214 μg g-1 DW, respectively). In these treatments, the accumulation of glycinebetaine was significantly increased in the root too (163 and 206 μg g-1 DW) which were 2.4 and 3 times more than the control. In salinity and high concentrations of K+, content of this compound was not showed significantly different between root and shoot; but in low concentrations of K+, glycinebetaine accumulation in root was less than the shoot.
Changes of glycinebetaine content in root and shoot of A. lagopoides at different treatment were revealed that biosynthesis of this compound increased considerably in both organs under salinity. Availability of K+ alone had no important effect but in the presence of high concentration of Na+ and K+ accumulation of this compound was more promoted. Solute compatible is derivatives of intermediate metabolites that protect enzymes against additional anions and cations. Subarrao et al (2001) were showed that created osmotic stress by high concentration of Na+ can be inducing glycinebetaine biosynthesis pathway. Research on Arabidopsis thaliana and barley had been demonstrated that compatible osmolites had been reduced potassium efflux from cell root (Cuin and Shabala, 2005 and 2007) but another report has been mentioned that glycinebetaine was not active as trap free radicals (Smirnoff and Cubes, 1989). Therefore, it is suggested that this osmolite in order to decrease salt injury, reduced toxic effects of the ROS on the K+ channels (Cuin and Shabala, 2008). Alizadeh et al (2010) reported that A. lagopoides seedling had been accumulated glycinebetaine in the roots after 5 days treatment with 600 mM NaCl while only one hour after salinity treatment could be measured a dramatic increase in glycinebetaine content of the shoot. Also, Murta et al., (2008) have shown that glycinebetaine protects both PSII and ATP synthase from inhibitory effects of NaCl. Thus this compound play important role in the shoot plant under osmotic stress.
Content of photosynthesis pigments
Among applied treatments, chlorophyll contents of the seedling that treated by 1.75 or 100 mM K+ was similar to the control (Fig. 4). In the presence of 100mM K+ and other treatments, amount of carotenoids clearly increased. Maximum amount of the carotenoids was observed in treatments that were supplemented with 1.75 or 100 mM K+ accompanied with 600 mM NaCl (0.97 and 1.15 mg g-1 FW, respectively, 5.6 and 7.5 times more than the control). These pigments were raised only to 3.5 and 2.75 times more than the control by supplemented media without potassium or with high concentration of K+ up to 100 mM (Fig. 4).
By comparing changes in chlorophyll a and b in different treatments have been represented a brief reduction in chlorophyll a content and increased in chlorophyll b in the presence of 600 mM NaCl. Amount of the chlorophyll a was reduced to 0.05 mg g-1 FW by salt stress in low concentration of K+, against 0.08 mg g-1 FW in the control. Comparing means of the chlorophyll b content were revealed that there is a significant difference among treatments. So that maximum ratio of the chlorophyll a/b was obtained about 6 by potassium deficiency treatment and this ratio was reduced to 2.6 - 2.7 by 600 mM NaCl treatment (accompanied with 1.75 or 100 mM K+).
Decrease in the chlorophyll a content which happens under salinity could be due to stimulation of proline biosynthesis. It is demonstrated that glutamate is chlorophyll precursor in higher plants and during salinity a part of glutamate converts to proline that cause to decline input of this compound to chlorophyll biosynthesis pathway (Hand et al., 1986). On the other hand, it seems that reduce in chlorophyll a and chlorophyll a/b ratio under salinity could be related to decreasing K+ concentration in the leaf (Sotiropoulos et al, 2006). Our result showed that by supplementing of the media with high concentration of potassium decreased negative effect of salinity stress on chlorophyll content. In this regard, it found that chlorophyll degradation was improved when plant exposed to extreme salinity treatments while when salt stress applied in gradual chlorophyll degradation did not accrued (Luna et al., 2002; Yildirim et al, 2008). Increased osmotic potential and Na+ access into organelles creates damage in respiratory and photosynthetic electron transport (Allakhverdiev et al, 1999). Thus, plants cannot use effectively from absorbed light energy under stress and it is suggested that reducing photosynthetic pigments is a stress adaptation response (Demmig et al 1996, Poormohmmad Kiani et al., 2008).
In addition, obtained result in this research was confirmed that carotenoid contents as antioxidant compounds had increased considerably under saline conditions and in the absence of K+. Carotenoids are pigments that protect plant against light inhibitory induced by activated oxygen species (AOS) during oxidative stress, also they contribute to stability of lipid membranes (Kitahata et al, 2006; Lobato et al, 2009). This phenomenon take placed via two ways: function of xanthophyll cycle which turn away absorbed light energy to heat and fluorescence quenching of triplet chlorophyll molecules that reduces peroxidation of the thylakoid lipids and chlorophyll oxidation; therefore reduce photosystem injury and photosynthetic devices will be protects against AOS (Pogson and Rissler, 2000; Tardu and Havaux, 1996).
Table 1 Treatment program of A. lagopoides seedling by NaCl and KCl in modified Murashige-Skoog
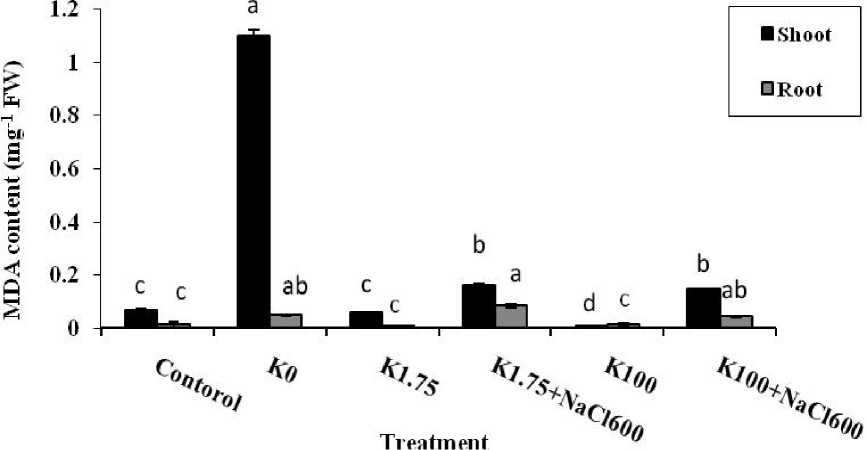
Figure 1 Comparison of MDA content in shoots and roots of A. lagopoides treated by :
K0 (0 mM K+), K1.75 (1.75 mM K+) , K1.75N600 (1.75 mM K+ +600 mM NaCl), K100 (100 mM K+) and K100 N600 (100 mM K+ +600 mM NaCl).
Different letters on top of bars indicate differences (Dancan ʼ s test, p < 0.05 at each organ).
Vertical bars indicate the mean ± SE.
media (MS)
|
Treatment Days Treatment |
4 Days |
8 Days |
3 Days |
3 Days |
13 Days |
|
control |
1/ 2 Ms, 4˚C |
1/ 2 Ms |
MS |
MS |
MS |
|
K 0 |
1/ 2 Ms, 4˚C |
1/ 2 Ms |
MS |
0 mM KCl |
0 mM KCl |
|
K 100 |
1/ 2 Ms, 4˚C |
1/ 2 Ms |
MS |
0 mM KCl |
100 mM KCl |
|
K 100 N 600 |
1/ 2 Ms, 4˚C |
1/ 2 Ms |
MS |
0 mM KCl |
100 mM KCl + 600 mM NaCl |
|
K 1.75 |
1/ 2 Ms, 4˚C |
1/ 2 Ms |
MS |
1.75 mM KCl |
1.75 mM KCl |
|
K 1.75N600 |
1/ 2 Ms, 4˚C |
1/ 2 Ms |
MS |
1.75 mM KCl+ 600 mM NaCl |
1.75 mM KCl + 600 mM NaCl |
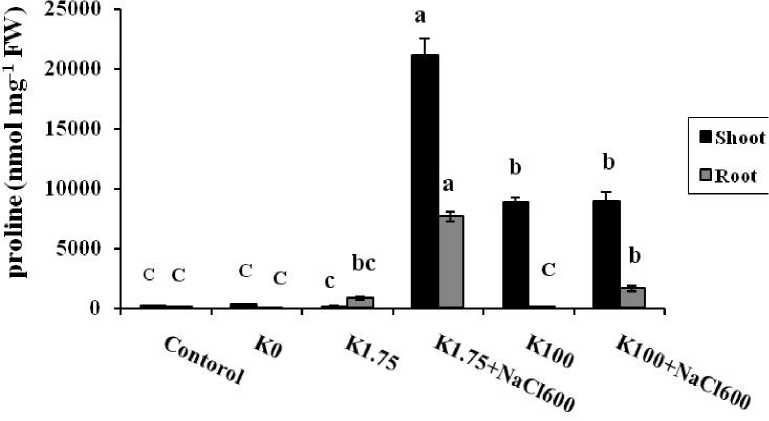
Treatment
Figure 2 Comparison of proline content in shoots and roots of A. lagopoides treated by :
K0 (0 mM K+), K1.75 (1.75 mM K+) , K1.75N600 (1.75 mM K+ +600 mM NaCl), K100 (100 mM K+) and K100 N600 (100 mM K+ +600 mM NaCl).
Different letters on top of bars indicate differences (Dancan ʼ s test, p < 0.05 at each organ).
Vertical bars indicate the mean ± SE.
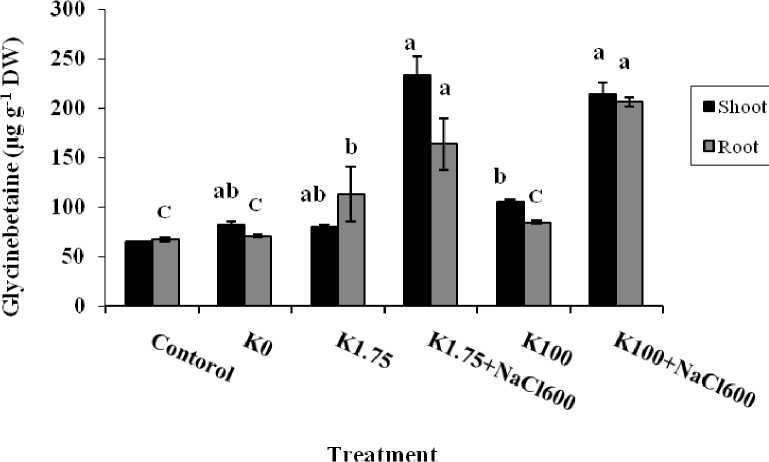
Figure 3 Comparison of glycinebetaine content in shoots and roots of A. lagopoides treated by : K0 (0 mM K+), K1.75 (1.75 mM K+) , K1.75N600 (1.75 mM K+ +600 mM NaCl), K100 (100 mM K+) and K100 N600 (100 mM K+ +600 mM NaCl).
Different letters on top of bars indicate differences (Dancan ʼ s test, p < 0.05 at each organ).
Vertical bars indicate the mean ± SE.
■ Chia Chib eCar
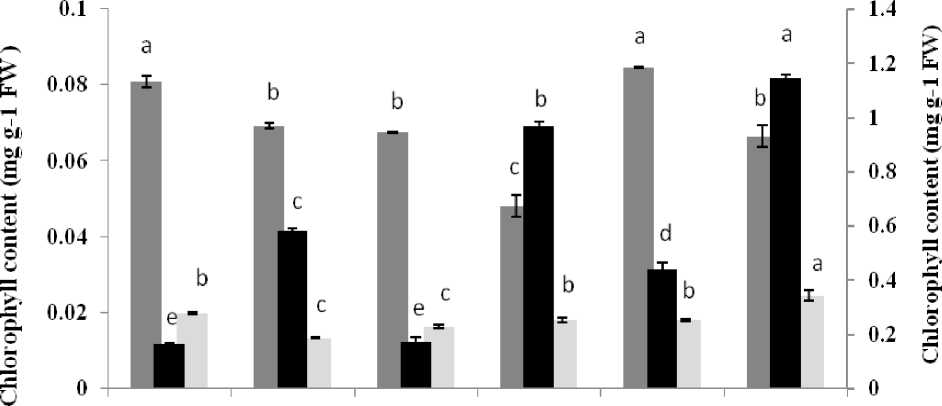
Treatment
Figure 4 Comparison of photosynthetic pigment content in leaf of A. lagopoides treated by : K0 (0 mM K+), K1.75 (1.75 mM K+) , K1.75N600 (1.75 mM K+ +600 mM NaCl), K100 (100 mM K+) and K100 N600 (100 mM K+ +600 mM NaCl).
Different letters on top of bars indicate differences (Dancanʼs test, p < 0.05 at each pigment).
Vertical bars indicate the mean ± SE.
CONCLUSION
Potassium is one of the essential elements that plays important roles in sustain of stability and function of the cell membranes; while Na+ reduced growth of the most higher plants. Under non-saline conditions, cytosol of the higher plants is containing 100 to 200 mM K+ and 1 to 10 mM Na+. Our result presented that K+ deficiency was the most effective factor which promoted noticeably lipid peroxidation in A. lagopoides shoot (about 16 times more than the control). While the most MDA content was obtained in the root by salinity treatment under low concentration of potassium but it was very lesser than the previous treatment. It seems that existence of the photosynthetic pigments in leaves was main reason for higher sensitivity of the shoot against the root. It is demonstrated that existence of sodium in the rhizospher not only is not harmful for this halophytic plant but also by adjustment of the osmotic pressure and increasing of carotenoids, lipid peroxidation decreased in the shoots. Establishment of great pools of carotenoids and glycinebetaine in the shoot of A. lagopoides alleviated membrane damage in this plant under salt stress. On the other hand, increasing of the proline content in the shoots by salinity and also addition of K+ concentration in the culture media, membrane damage was approximately inhibited in the shoot and root of A. lagopoides. The accumulation rates of two osmolite in different organ was shown that in A. lagopoides proline and glycinebetaine play more important role in osmotic adjustment of the shoot and root, respectively.
ACKNOWLEDGEMENT
This study was supported by National Institute of Genetic Engineering and Biotechnology and also Research Councils of the Graduate studies of Alzahra University. The authors thank for their generous funding and support.
Список литературы Changes in osmolites contents, lipid peroxidation and photosynthetic pigment of Aeluropus lagopoides under potassium deficiency and salinity
- Allakhverdiev S.I., Nishiyama Y., Suzuki I., Sakamoto Y.A., and Murata N., (1999). Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc. Natl. Acad. Sci., 96: 5862-5867.
- Amtmann A., Armengaud P., Volkov V., (2004). Potassium nutrition and salt stress. In: Blatt, M.R. (Ed), Membrance transport in plants. Blackwell, Oxford, 293-339.
- Ashraf M. and Harris P.J.C., (2004). Potential biochemical indicators of salt tolerance in plants. Plant Sci. 166: 3-16.
- Aziz A. and Larher F., (1998). Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brasica napus L. J Plant Physiol 153: 754-762
- Bates L.S., (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39: 205-207.
- Bol R., J. Moering J., Kuzyakov Y. and Amelung W. (2003). Quantification of priming and CO2 respiration sources following slurry-C incorporation into grassland soils with different C content. Rapid Communications in Mass Spectrometry 17: 2585-2590.
- Chinnusamy V., Jagendorf A. and Zhu J.K. (2005). Understanding and Improving Salt Tolerance in Plants. Crop Sci., 45: 437-448.
- Cramer G.R., Lauchli A., and Polito V.S., (1985). Displacement of Ca2+ by Na+ from the plasmalemma of root cells. A primary response to salt stress? Plant Physiol. 79: 207-211.
- Cuin T.A. and Shabala S., (2005). Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol. 46: 1924-1933.
- Cuin T.A. and Shabala S., (2007). Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 30: 875-885.
- Cuin T.A. and Shabala S. (2008). Compatible solutes mitigate damaging effects of salt stress by reducing the impact of stressinduced reactive oxygen species. Plant Signal Behav. 3: 207-208
- Delauney A.J., Verma D.P.S., (1993). Proline biosynthesis and osmoregulation in plants. Plant J. 4: 215-223.
- Demmig-Adams B., Gilmore A., and Adams W.W., (1996). In vivo functions of carotenoids in higher plants. FASEB Journal 10: 403-412.
- Esfandiari E., Shekari F., Shekari F. and Esfandiari M., (2007). The effect of salt stress on antioxidant enzymes activity and lipid peroxidation on the Wheat seedling. Not. Bot. Hort. Agrobot. Cluj. 35 (1): 48-55.
- Grieve C.M. and Grattan S.R., (1983). Rapid assay for determination of water-soluble quaternary ammonium-compounds. Plant Soil 70: 303-30.
- Heath R.L. and Packer L., (1968). Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys.,. 125: 189.
- Hsu S.Y., Hsu Y.T., and Kao C.H., (2003). The effect of polyethylene glycol on proline accumulation in rice leaves. Bil. Plant, 46: 73-78.
- Jannesar M., Saboora A., and Razavi K. (2009). Effects of ABA and Ca on the changes of some biochemical compounds during adaptation to salinity in Aeluropus lagopoides Iranian Journal of Rangelands and Forests Plant Breeding and Genetic Research, 17(1): 15-28.
- Kitahata N., Han S.Y., Noji N., Saito T., Kobayashi M., Nakano T., (2006). A 9-cisepoxycarotenoid dioxygenase inhibitor for use in the elucidation of abscisic acid action mechanisms. Bioorg. Med. Chem. 14: 5555-556
- Kochian L.V., and Luccas W.J., (1988). Potassium transport in roots. Adv. Bot. Res., 15: 93-178
- Laster G., and Stein E, (1993). Plasma membrane physiochemical changes during maturation and pastharvest strorage of muskmelon fruit. J.Am.Soc.Hort.Sci. 118: 223-227.
- Lee T.M., and Liu C.H., (1999). Correlation of decreased calcium contents with proline accumulation in the marine green macroalga Ulva fasciata exposed to elevated NaCl contents in seawater, J. Exp. Bot. 50: 1855-1862.
- Leigh R.A., and Jones R.G.W. (1984). A hypothesis relating critical Potassium concentrations for growth to the distribution and functions of this ion in the plant-cell. New phytol., 97: 1-13.
- Liang Y.C., Chen Q., Liu Q., Zhang W. and Ding R. (2003). Exogenous silicon (Si) increases antioxidant enzyme activity and reduced lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol. 160: 1157-11
- Liang Y.C. (1999). Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant. Soil. 209: 217-224.
- Lichtenthaler H.K. and Wellburn A.R., (1985). Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biol. Soc. Trans., 11: 591-592.
- Lobato A.K.S., Santos F.B.G., Costa R.C.L., Oliveira, N.C.F., Meirelles A.C.S., Cruz F.J.R., Alves G.A.R., Neves H.K.B., Pita J.D., Lopes M.J.S., Freitas J.M.N., (2009). Plant Soil Environ., 55(2): 58-61
- Luna C, Luca M.D. and Taleisnik E. (2002). Physiological causes for decreased productivity under high salinity in bom tetraploid Chloris gayana cultivar. II. Oxidative stress. Aust J Agric Res,. 53(6): 669-674.
- Maathuis F.J.M. and Amtmann A. (1999). K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann. Bot., 84: 123-133.
- Maathuis F.J.M. and Sanders D. (1996). Mechanisms of potassium absorption by higher plant roots. Physiol. Plant, 96: 158-168.
- Mohsenzadeh S., Malboobi M.A., Razavi K. and Farrahi-Aschtiani S. (2006). Physiological and molecular responses of Aeluropus Lagopoides (Poacae) to water deficit. Environ. Experiment. Bot., 56: 314-322.
- Murashige T., and Skoog F. (1962). A revised medium for rapid growth and bioassys with tobacco tissue culture. Physiol. Plant 15 437-493.
- Murta T.H.H., and Murata N. (2008). Glycinebetaine: an effective protectant against abiotic stress in plants. Trends in Plant Science.13 (9) 499-505.
- Pier P.A. and Benkowitz G.A., (1987). Modulation of water-stress effects on photosynthesis by altered leaf. Plant Physiol., 85: 655-61.
- Pogson B.J., and Rissler R (2000). Genetic manipulation of carotenoid biosynthesis and photoprotection. Phil Trans R. Soc. B: Biol. Sci. 355: 1395-1403.
- Poormohammad Kiani S., Maury P., Sarrafi A., and Grieu P (2008). QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well watered and water-stressed conditions. Plant science, 175, 565-573.
- Rout N.P. and Shaw B.P. (1998). Salt tolerance in aquatic macrophytes: probable role of proline, the enzymes involved in its synthesis and C4 type of metabolism. Plant Sci. 136: 121-130.
- Shalata A. and Tal M. (1998). The effect of salt stress on lipid peroxidatiopn and antioxidants in the cultivated tomato and its wild salttolerant relative Lycopersicon pennellii. Physiol Plant 104: 169-174
- Smirnoff N., and Cubes Q.J. (1989). Hydroxyl radical scavengering activity of compatible solutes. Phytochemistry, 28: 1057-1060.
- Solomon A., Beer S., Waisel Y., Jones G.P., and Paleg L.G. (1994) Effects of NaCl on the carboxylating activity of Rubisco from Tamarix jordains in the presence and absence proline-related compatible solutes. Physiol Plant 90: 198-204.
- Subarro G.V., Wheeler R.M., Levine L.H., and Stutte G.W., (.2001) Glycinebetaine accumulation, ionic and water relation of red-beet at contrasting levels of sodium supply. Plant Physiol. 15: 767-776.
- Tardy F. and Havaux M. (1996) Photosynthesis, chlorophyll fluorescence, light harvesting system and photoinhibition resistance of a zeaxanthin -accumulation mutant of Arabidopsis thaliana. Journal of photochemistry and photobiology B: Biolgy. 34, 87-94
- Tester M., and Davenport R.J. (2003). Na+ tolerance and Na+ transport in higher plants. Ann. Bot., 9l: 503-527
- Tiburcio A.F., Besford R.T., Capell T., Borell A., Testillano P.S. and Risueno M.C. (1994). Mechanism of polyamine action during senescence responses induced by osmotic stress. J. Exp. Bot. 45: 1789-1800.
- Van Rensburg L. Kr ger ȕ G.H.J., Krȕger H. (1993). Proline accumulation as drought-tolerance selection criterion: its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L., J. Plant Physiol., 141, 188-194.
- Very A.A., and Sentenace H., (2003). Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol., 54: 575-603.
- Wang B., Luttge U. and Ratajczak R. (2004). Specific regulation of SOD isoforms by NaCl and osmotic stress in leaves of the C3 halophyte suoada salsa L. J. plant physiol. 161: 285-293.
- Weckx J, Vangronsveld J and Clijsters H (1993). Heavy metal induction of ethylene production and stress enzymes. I. Kinetics of the responses. In: PechJC, LatchйA, BalaguйC (eds) Cellular and Molecular Aspects of the Plant Hormone Ethylene. Kluwer Academic Publishers, Dordrecht, pp 238-239.
- Wei Y., Guangmin X., Daying Z. and Huimin, C., (2001). Transfer of salt tolerance from Aeluropus littoralis sinensis to wheat (Triticum aestivum L.) via asymmetric somatic hybridization. Plant Sci., 161: 259-266.
- Zhao D. L., Oosterhuis D. M., and Bednarz C. W. (2001): Influences of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosyntetica 39, 103-199.
- Yesck W.D. 1984. K-Na exchange at cellular membranes, inter cellular compartmentation of cations, and salt tolerance. In: Staples, R.C. and Tonniessen, G.H., (eds). Salinity tolerance in plants. NewYork, pp: 37-66.
- Yildirim E.; Turan M.; and Guvenc I., (2008). Effect of foliar salicylic acid applications on growth, chlorophyll and mineral content of cucumber (Cucumis sativus L.) grown under salt stress. Journal of Plant Nutrition, 31: 593-612.


