Changes in the methyl status of cytosine in symmetrical and asymmetrical sites of the csy1 gene promoter in corn leaves under irradiation with light of different wavelength
Автор: Fedorin D.N., Vlasenko A.A., Fedorina O.S., Eprintsev A.T.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Analysis of the nucleotide sequence of the CSY1 citrate synthase gene promoter allowed us to establish the presence of a CpG island, as well as the distribution of symmetric and asymmetric methylation sites by cytosine. Based on the analysis of the nucleotide sequence of the CSY1 gene promoter, primers for bisulfite sequencing were developed. The results of bisulfite sequencing showed that the main share of methylated cytosines falls on asymmetric CNN sites and is 56% of the total value of this indicator. The lowest value is characteristic of CNG sites, which apparently indicates their insignificant role in the formation of the methyl status of the mitochondrial citrate synthase gene promoter in maize leaves in the light. Changes in methylation of the promoter of the studied gene under different conditions of light irradiation are associated with the redistribution of the methyl status of the cytosine between symmetric (CG and CNG) and within the asymmetric CNN site.
Maize, citrate synthase, promoter, dna methylation, bisulfite sequencing
Короткий адрес: https://sciup.org/143184723
IDR: 143184723
Текст научной статьи Changes in the methyl status of cytosine in symmetrical and asymmetrical sites of the csy1 gene promoter in corn leaves under irradiation with light of different wavelength
Citrate synthase (CS, EC 2.3.3.1) is a tetrameric protein that carries out a condensation reaction to form citrate. In plant cells, there are two forms of this enzyme, mitochondrial and peroxisomal. In the mitochondrial matrix, participating in the Krebs cycle, citrate synthase is a component of the cell's energy metabolism. In the plant cell, there is a complex system for coordinating respiratory and photosynthetic processes, in which mitochondrial respiration plays an important role at the level of enzyme functioning. The activity of citrate synthase is regulated by the lighting regime, for example, when irradiated with red light, a decrease in the value of this indicator is observed (Eprintsev et al., 2018). Light is a key regulator of mitochondrial enzyme gene expression in plants, altering gene transcription through specific photoreceptors, in particular phytochromes (Fedorin et al., 2022, Eprintsev et al., 2020), which coordinate the expression of target genes with the participation of phytochrome-inducible transcription factors (Igamberdiev et al., 2014). This occurs through interaction with G-box sites, which are located in the promoter regions of genes. It has been shown that changes in the methyl status of individual CG dinucleotides of CpG island gene promoters regulate their activity in plant leaves under different illumination conditions (Fedorin et al., 2024, Eprintsev et al., 2023). DNA methyltransferases are capable of methylating cytosine residues in various contexts, including RNA-directed DNA methylation. This also allows them to methylate cytosine in asymmetric nucleotide sequences of CNN (Lin et al., 2017). There is significant competition between different methylation patterns, especially between the two types of non-CG methylation – CNG and CNN. The CMT2 enzyme functions to maintain CNG methylation, while CMT3 is involved in CNN methylation together with DRM (Lucibelli et al., 2022). Redistribution of the methyl status of cytosine between symmetric and asymmetric sites is due to the activation and/or inactivation of the corresponding types of DNA methyltransferases, regulated, among other things, by plant cell photoreceptors. The use of molecular methods for studying enzymes, in particular in the analysis of the transcriptional activity of genes, makes it possible to assess the role of the synthesis of protein components of enzymes in the organization of oxidative and synthetic cells. The epigenetic mechanism is an important point in the regulation of gene expression, which directly affects the level of their transcripts. Particular attention has recently been paid to the analysis of symmetric and asymmetric cytosine methylation sites in promoter regions of genes (Cao, Jacobsen, 2002, Kohli, Zhang, 2013).
MATERIALS AND METHODS
The objects of the study were maize leaves ( Zea mays L., cv Voronezhskaya 76), grown hydroponically at a constant temperature of 25°C and a 12-hour daylight period.
White light was obtained from fluorescent lamps in the Flora-1 setup. Red and far-red light was obtained using LEDs with an emission region of 640-680 nm (KIPD40M40-K-P6, Russia) and 710-750 nm (3L127A-5, Russia). The light intensity was 4 μmol quanta · m-2 · s-1. This light intensity is sufficient for the occurrence of signaling reactions associated with the participation of the phytochrome system, however, it does not lead to an intensification of photosynthesis (Eprintsev et al. , 2013).
The use of the phenol-chloroform method of DNA extraction made it possible to obtain them in the required quality and quantity (Chomczynski, Sacchi, 1987). The quality of nucleic acids was confirmed by electrophoresis in 1% agarose gel manufactured by Helicon (Russia).
The MethPrimer program was used to analyze the CSY1 gene promoter for the presence of CpG islands and select bisulfite sequencing primers. The nucleotide sequence of the promoter region of the maize mitochondrial CS gene was taken from the NCBI database (USA, . The primer sequence for bisulfite sequencing: forward – taatgggggttatgtgtatgtgtt, reverse – caaataaaaaatcccatcaaatcac. Bisulfite conversion of DNA (Patterson et al., 2011) followed by bisulfite sequencing was used to analyze the methyl status of cytosine.
Polymerase chain reaction with primers for bisulfite sequencing was performed using the Thermo Scientific DremTaq PCR MasterMix (2x) reagent kit (Thermo Scientific, Russia). The PCR reaction was carried out on a Tertsik device (DNA-Technology, Russia) with the following amplification parameters: preliminary denaturation at 95 °C for 10 min, then 35 cycles: 95 °C -20 sec, 56 °C - 20 sec, 72 °C - 30 sec, and finally 72 °C - 4 min.
The sequencing of the amplification products was performed at Evrogen. The calculation of the numerical values of the methyl status of the promoter of the studied gene was carried out based on a comparison of the nucleotide sequences of the promoter before and after bisulfite conversion. The values of the degree of methylation of the promoter were estimated by C-to-T substitutions (Li, Tollefsbol, 2011) and calculated from the total amount of symmetric and asymmetric cytosine methylation sites.
Each study was carried out in 3-4 replicates, analytical determinations for each sample were carried out in three replicates. Statistically significant differences are discussed at a significance level of p < 0.05 (Lakin, 1990).
RESULTS AND DISCUSSION
Analysis of changes in the expression of the gene encoding the mitochondrial isoenzyme of citrate synthase indicates its light dependence, since a decrease in the relative level of CSY1 gene transcripts was shown in the light and under the action of RC, which also indicates the participation of phytochrome in this regulation (Eprintsev et al. , 2018).
It was previously established that methylation plays a key role in the process of gene transcription, leading to chromatin condensation, which forms a structure that hinders access for transcription factors (Kass et al. , 1997). In such a situation, methylation of the promoter region of the gene, which ensures interaction with transcription factors, can lead to disruption of their binding to the nucleotide sequence and reduces its expression. In this case, not only CG dinucleotides, but also CNG and CNN sites play a special role in organizing the interaction of RNA polymerase and the gene promoter (Cao, Jacobsen, 2002).
To determine the methyl status of cytosines in symmetric and asymmetric methylation sites, bisulfite sequencing was used (Patterson et al. , 2011, Cokus et al. , 2008). Using the Methprimer software, primers were developed for the mitochondrial citrate synthase gene promoter for bisulfite sequencing. The amplicon obtained during PCR with the developed primers was sequenced and its sequence was analyzed for the nature of the distribution of methylated cytosine in symmetric and asymmetric sites. Analysis of the results of bisulfite sequencing of the CSY1 gene promoter in maize leaves irradiated with light of different wavelengths showed that a high level of methylation (85%) was observed in the “light” and “RL” variants. In the dark and under irradiation with FRL. RL+ FRL, a decrease in the methylation level of CG dinucleotides was shown, which was 80%, 75% and 80%, respectively (Table 1). A decrease in the methyl status of the promoter of this gene under these conditions is correlated with an increase in its transcriptional activity of mitochondrial citrate synthase.
At the same time, it was shown that CG dinucleotides at positions -531, -486, -462, -459, -405, -403, -396, -394 and -368 changed their methyl status. Most of them are located near the transcription initiation zone (position from -50 to -300), which ensures control of its interaction with transcription factors (Porto et al. , 2014, Peremarti et al. , 2010). Analysis of the position of methylated and unmethylated CNG sites of the CSY1 gene promoter in dark conditions showed significant changes in the redistribution of the methyl state of cytosine in them. Of the 12 sites, 5 were unmethylated in light conditions, which corresponds to a methylation status of 58.3% (Table 2). However, in dark conditions and under the influence of FRL, the methyl status increased and was more than 66.7%. The methylation status of CNG sites determines the high level of methylation of the entire promoter in the absence of an active form of phytochrome in the cell, which negatively affects the initiation of transcription of this gene during this period due to blocking the attachment of RNA polymerase.
It was shown that the methyl status of four CNG sites at positions -532, -528, -394 and -384 did not change and remained methylated in all variants of RL and FRL irradiation. All other CNG sites of the CSY1 gene promoter changed their methyl status depending on the maize illumination regime.
The obtained data on the study of the methylation status of CNN sites of the CSY1 gene promoter in maize leaves under different irradiation conditions indicate significant changes in the methyl status of cytosine. The methylation status of cytosine of CNN sites changes to the greater side when plants are irradiated with FRL and in the dark (Table 3). When plants are irradiated with FRL and under heterogeneous white light, the methyl status value is 79.1% and 66.7%, respectively. Consequently, the presence of the active form of phytochrome in maize cells under RL irradiation leads to a decrease in the methyl status of CNN sites.
It should be noted that in all irradiation modes of maize plants only two CNN sites remained unmethylated all the time, these are sites at positions -546 and -452. In addition, the presence of CNN sites in the CSY1 gene promoter, which are methylated in all experimental variants, is shown. Such sites have positions -494, -462, -459, -458, -456, -450, -442, -441, -435, -433, -429, -428, -400, -386 and -375.
The study of the distribution pattern of symmetric and asymmetric sites in the analyzed sequence of the mitochondrial citrate synthase gene promoter fragment showed the presence of 20 - CG, 12 - CNG and 48 -CNN methylation sites. Comparative analysis of genomic DNA and amplicon sequences revealed 3 converted thymines in CG dinucleotides, which corresponds to 85% methylation of these elements of the CSY1 gene promoter in maize leaves in the light (Fig. 1). For CNG sites, 3 converted thymines were also found, therefore, methylation for this type of symmetrical sites is 75%. The lowest methylation level was observed in CNN sites, where the analyzed indicator was 63.8%, which corresponds to 17 converted thymines in the amplicon sequence. Analysis of the distribution of the shares of symmetric and asymmetric sites in the context of the total value of cytosine methylation of the CSY1 gene promoter from maize leaves in the light showed that the greatest contribution to this indicator is made by asymmetric CNN sites, the share of which is 54%.
Symmetrical CG and CNG sites account for 46% of the methylation level of the studied gene promoter, which is less than asymmetrical sites. Among the symmetrical sites, CG is of the greatest importance, since it determines 30% of the methylation level of the mitochondrial citrate synthase gene promoter in maize leaves in the light.
Study of the obtained amplicon of the studied gene promoter from the leaves of the "dark" variant revealed 4 converted thymines in the CG dinucleotides, which corresponds to 80% of the methylation of these elements of the CSY1 gene promoter in maize leaves in the light (Fig. 2). For CNG sites, 3 converted thymines were found, therefore, methylation by this type of symmetrical sites is 75%. The lowest methylation level was observed in CNN sites, where the analyzed indicator was 48.1%, which corresponds to 14 converted thymines in the amplicon sequence.
Analysis of the distribution of the shares of symmetric and asymmetric sites in the context of the total methylation value of cytosine of the CSY1 gene promoter from maize leaves in the dark showed that the greatest contribution to this indicator is made by asymmetric CNN sites, the share of which is 66.7%.
Symmetrical CG and CNG sites account for 33.3% of the methylation level of the studied gene promoter, which is less than asymmetric sites. Among the symmetric sites, CG is the most important, since it determines 57.1% of the methylation level of the mitochondrial citrate synthase gene promoter in maize leaves in the dark.
The dynamics of changes in the methylation degree of symmetric and asymmetric sites of the CSY1 gene promoter when maize leaves are irradiated with red light indicates an important role of this epigenetic mechanism in regulating its transcriptional activity. Five cytosines were methylated at CG sites, 6 cytosines at CNG sites, and 12 at CNN sites. Under these irradiation conditions, significant changes in the redistribution of the methyl status of cytosine between the analyzed sites were observed. In the composition of CG – 75%, CNG – 50.0%, CNN – 54.5% were cytosines in the methylated state (Fig. 3).
Analysis of the distribution of the shares of symmetrical and asymmetrical sites in the context of the total value of cytosine methylation of the CSY1 gene promoter from maize leaves under RL illumination showed that the greatest contribution to this indicator is made by asymmetrical CNN sites, the share of which is 52.2%. An increase in the methyl status is characteristic of symmetrical CG and CNG sites, and to a greater extent for CNG sites, where the increase was 11.8%.
When plants were irradiated with light with a wavelength of 730 nm, an increase in the methyl status of cytosines was revealed in symmetrical CG and CNG sites, with a decrease in the analyzed indicator in asymmetrical sites (Fig. 4). The main methylation occurs at CNN sites, and the smallest contribution to the total cytosine methylation during this period of seed germination is made by cytosines in the CG.
With sequential RL + FRL irradiation, an almost identical distribution of the methyl status of cytosine in symmetrical and asymmetrical sites was shown compared to the "FRL" variant. However, an increase in this indicator was shown for CNN sites. The degree of methylation reached 70.4% (Fig. 5).
Probably, with this irradiation variant, the main contribution to the total methyl status of the CSY1 citrate synthase gene promoter is made by CNN sites, which is largely associated with the type of control of the methyl status of DNA by the mechanism of RNA-dependent methylation (Lucibelli et al. , 2022).
Table 1. Methyl status of CG dinucleotides of the CSY1 gene promoter in maize leaves
|
Light |
Dark |
RL |
FRL |
RL+FRL |
|
|
Number of CG |
20 |
20 |
20 |
20 |
20 |
|
Number of unmethylated CG |
3 |
4 |
3 |
5 |
4 |
|
Methylation percentage |
85 |
80 |
85 |
75 |
80 |
|
Percentage of bisulfite conversion |
86,4 |
71,4 |
83,4 |
83,3 |
87,5 |
Legend: Light – plants illuminated with white light; Dark – plants kept in darkness; RL – plants illuminated with light with a wavelength of 660 nm; FRL – plants illuminated with light with a wavelength of 730 nm; RL+FRL – plants successively illuminated with light with a wavelength of 660 nm and 730 nm.
Table 2. Methylation status of CNG sites of the CSY1 gene promoter in maize leaves
|
Light |
Dark |
RL |
FRL |
RL+FRL |
|
|
Number of CNG |
12 |
12 |
12 |
12 |
12 |
|
Number of unmethylated CG |
5 |
3 |
6 |
4 |
4 |
|
Methylation percentage |
58,3 |
75 |
50 |
66,7 |
66,7 |
|
Percentage of bisulfite conversion |
86,4 |
71,4 |
83,4 |
83,3 |
87,5 |
Legend: see Table 1
Table 3. Methylation status of CNN sites of the CSY1 gene promoter in maize leaves
|
Light |
Dark |
RL |
FRL |
RL+FRL |
|
|
Number of CNN |
48 |
48 |
48 |
48 |
48 |
|
Number of unmethylated CG |
16 |
11 |
20 |
10 |
10 |
|
Methylation percentage |
66,7 |
77 |
58,3 |
79,1 |
79,1 |
|
Percentage of bisulfite conversion |
86,4 |
71,4 |
83,4 |
83,3 |
87,5 |
Legend: see Table 1
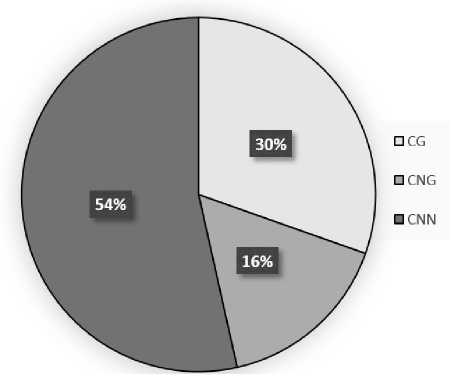
Figure 1: Distribution of methylated cytosines by symmetrical and asymmetrical sites of the CSY1 citrate synthase gene promoter in maize leaves in the light.
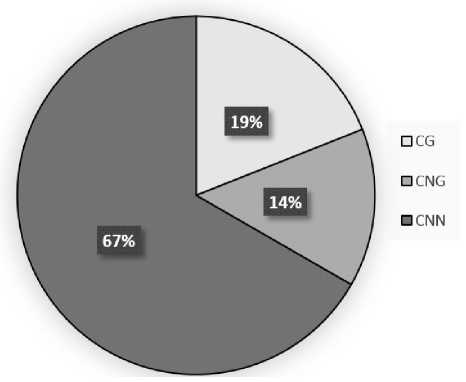
Figure 2: Distribution of methylated cytosines at symmetric and asymmetric sites of the CSY1 citrate synthase gene promoter in maize leaves in the dark.
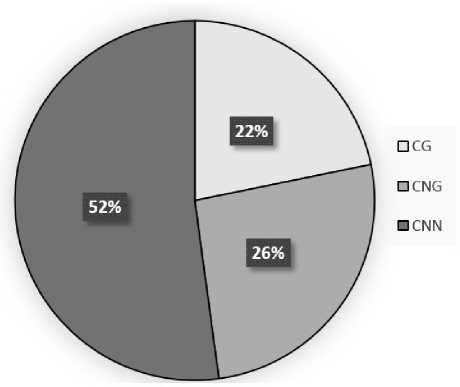
Figure 3: Distribution of methylated cytosines by symmetrical and asymmetrical sites of the CSY1 citrate synthase gene promoter in maize leaves under RL illumination.
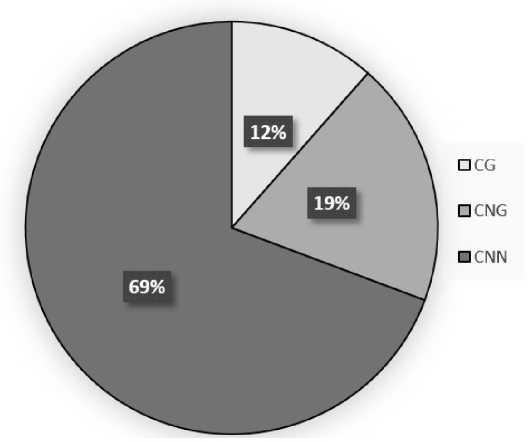
Figure 4: Distribution of methylated cytosines at symmetric and asymmetric sites of the CSY1 citrate synthase gene promoter in maize leaves under FRL illumination.
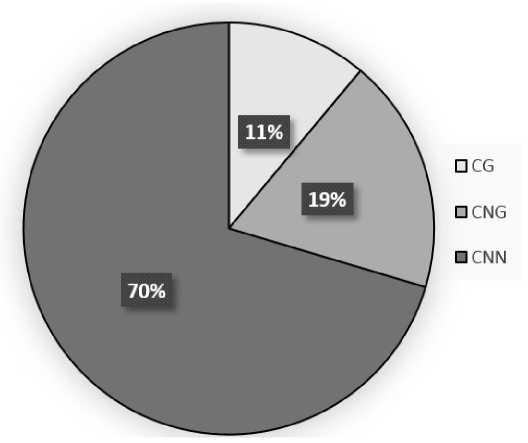
Figure 5: Distribution of methylated cytosines at symmetrical and asymmetrical sites of the CSY1 citrate synthase gene promoter in maize leaves under RL + FRL illumination.
CONCLUSION
The study of the nucleotide composition of the studied gene showed the nature of the distribution of CG dinucleotides in the promoter region of the studied objects. The CSY1 gene contains a CpG island in its promoters, which can cause regulation by changing the degree of their methylation. In addition, not only CG dinucleotides, but also CNG and CNN sites play a role in organizing the interaction of RNA polymerase and the gene promoter (Rountree, Selker, 1997, Erdmann,
Picard, 2020). Primers for bisulfite sequencing were developed to assess the change in the methyl status of cytosine in symmetric and asymmetric sites. The obtained results allow us to conclude that the change in methylation of the CS gene promoter in maize leaves when irradiated with light of different wavelengths is associated with the redistribution of the methyl status of the cytozone between symmetric sites (CG and CNG), and within the asymmetric CNN site. Moreover, in asymmetric sites, the change in the methyl status of cytosine in the presence of an active form of phytochrome in the cell was significant. Therefore, the main contribution to the epigenetic mechanism of regulation of the transcriptional activity of the mitochondrial citrate synthase gene in maize leaves is made by cytosine of CNN sites, the methylation of which is carried out by the enzymes DRM2 and CMT3 (Lucibelli et al., 2022). These types of DNA methyltransferases carry out their action with the participation of microRNAs via a plant-specific pathway known as RNA-directed DNA methylation (RdDM) (Grover et al., 2018). Since the change in the methyl status of the CSY1 gene promoter with a change in the light regime of maize is associated with CNN sites, RNA-directed DNA methylation is likely a key mechanism for controlling the transcriptional activity of this gene.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.


