Changes of respiration activities in cells of winter wheat and sugar cane suspension cultures during programmed cell death process
Автор: Lyubushkina I.V., Fedyaeva A.V., Stepanov A.V., Pobezhimova T.P.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.11, 2015 года.
Бесплатный доступ
Process of cell death in suspension cultures of winter wheat and sugar cane under high (50 °С) and negative (-8 °С) temperature treatment has been studied. It has been shown, that programmed cell death (PCD) process caused by the negative temperature in the culture of winter wheat was noted for slow rate of realization and it was carried out for 10 days. It has been state that rate of cell respiration was significantly higher than in the control culture. At the same time PCD processes induced by the high temperature in the culture of sugar cane and winter wheat and by the negative temperature in the culture of sugar cane realized for 24-48 h and was accompanied by graduate decrease of respiration activities. We can conclude that the main reason of PCD processes realization differences was a different level of respiration metabolism resistance to high and negative temperatures action.
Короткий адрес: https://sciup.org/14323929
IDR: 14323929
Текст научной статьи Changes of respiration activities in cells of winter wheat and sugar cane suspension cultures during programmed cell death process
Программируемая клеточная гибель (ПКГ) - это процесс саморазрушения клетки, активирующийся под действием внутренних или внешних факторов -на определенном этапе развития или как следствие неблагоприятных условий внешней среды (Kingston-Smith et al ., 2008). Он реализуется в течение некоторого периода времени - более короткого у животных (минуты и часы) и более длинного у растений (часы и даже дни) (Reape et al ., 2008; Zhou et al ., 2009). ПКГ у животных сопровождается характерными структурными и биохимическими изменениями в клетке -конденсацией протопласта, фрагментацией ДНК, усилением генерации активных форм кислорода (АФК) и рядом других (Williams and Dickman, 2008). Важную роль в этом процессе у животных играют митохондрии, поскольку в них локализованы многие гибель-стимулирующие факторы, например, белки семейства Bcl-2, цитохром с , APAF-1, апоптоз-индуцирующий фактор AIF, прокаспаза-3, ионы Ca2+ и АФК (Petit et al ., 1996; Jones, 2001; Bras et al ., 2005). У растений структурно-морфологические и биохимические изменения в клетках в процессе ПКГ могут значительно варьировать. (Krishnamurthy et al ., 2000). Так, при дифференциации трахеальных элементов наблюдается не характерное сокращение протопласта в объеме, а, напротив, его набухание (Rogers, 2005). Существуют работы, свидетельствующие о том, что повышение уровня АФК не является обязательным условием для активации ПКГ (Yu et al ., 1998; Dorey et al ., 1999;
Jabs, 1999). Функции митохондрий в процессе растительной ПКГ также до сих пор не ясны. Известно, что выход цитохрома с может не происходить во время гибели растительных клеток (Yao et al ., 2004; Vacca et al ., 2006). Не наблюдается у растений и формирование апоптосомы, характерное для реализации ПКГ посредством митохондрий у животных (Diamond and McCabe, 2007; Logan, 2008). Тем не менее, митохондрии являются поставщиками энергии как у животных, так и у растений, хотя их значение при наличии в клетке хлоропластов может быть не таким очевидным.
ПКГ - активный процесс, требующий для своей реализации постоянного притока энергии, поэтому поддержание метаболизма глюкозы и постоянный синтез АТФ могут быть не менее важными факторами для развития ПКГ, чем выход гибель-индуцирующих факторов из митохондрий в цитозоль (Kim et al ., 2006). В то же время, в растительных митохондриях содержится ряд белков, нехарактерных для животных, которые, вероятно, могут оказывать значительное влияние на ход ПКГ. К таковым относится, например, альтернативная оксидаза (АО), являющаяся второй терминальной оксидазой в электрон-транспортной цепи (ЭТЦ) растительных митохондрий (Siedow and Umbach, 2000).
В связи с этим, целью данной работы было изучение интенсивности дыхания в суспензионных культурах клеток озимой пшеницы и сахарного тростника в процессе ПКГ, вызванной действием повышенной и отрицательной температур, и выявление взаимосвязи между этими двумя процессами.
MATERIALS AND METHODS
В работе использовали суспензионные культуры клеток озимой пшеницы (Triticum aestivum L.), полученную из зрелых зародышей, и сахарного тростника Saccharum officinarum (L.), сорт POJ2878, линия, устойчивая к аноксии, полученную в ИФР РАН и любезно предоставленную к.б.н., с.н.с. лаборатории генетической инженерии СИФИБР СО РАН В.Н. Шмаковым. Культуры выращивались при 26 °С на МС-среде (Murashige and Scoog, 1962), содержащей сахарозу (3%), тиамин (1,0 мг/л), пиридоксин (0,5 мг/л), никотиновую кислоту (0,5 мг/л), 2,4-Д (2,5 мг/л), инозитол (0,01 %) и дитиокарбамат натрия (0,0005 %). Культуру пересевали каждые 14 дней с разведением свежей средой в 6 (культура озимой пшеницы) или 10 (культура сахарного тростника) раз. Культуры подвергали воздействию отрицательной температуры -8 °С в течение 6 и 2 ч или повышенной температуры 50 °С в течение 10 мин, затем переносили в контрольные условия (26 °С). Микроскопические методы исследования
Микроскопический анализ клеток проводили с помощью инвертированного флюоресцентного микроскопа AxioObserver Z1 («Carl Zeiss», Германия) с цифровой монохромной камерой AxioCam MRm3 и пакетом программного обеспечения для захвата и анализа изображений
«AxioVision Rel.4.6». В работе были использованы следующие фильтры: Filter set 15 (EX BP 546/12, BS FT 580, EM LP 590) и Filter set 10 (EX BP 450490, BS FT 510, EM BP 565).
Выживаемость клеток определяли с помощью двойного окрашивания флюоресцентными красителями: витальным красителем FDA (флюоресцеин диацетат, конечная концентрация 50 мкМ) и летальным красителем PI (пропидий йодид, конечная концентрация 7 мкМ). По 100 мкл культуры помещали в микроцентрифужные пробирки объёмом 2 мл, инкубировали в течение 2 мин при 26 °С на минитермошейкере TS-100 («BioSan», Латвия) и использовали для микроскопического анализа.
Определение скорости поглощения кислорода клетками проводили полярографически при 26 °С, используя платиновый электрод закрытого типа, в ячейке объемом 1,4 мл. Чтобы отделить митохондриальное дыхание от неспецифического поглощения кислорода, в полярографическую ячейку с культурой клеток последовательно добавляли 0,8 мМ цианид калия (KCN, ингибитор IV комплекса дыхательной цепи) и 2 мМ бензгидроксамовую кислоту (БГК - ингибитор альтернативной цианид-резистентной оксидазы). Поглощение кислорода, оставшееся после добавления ингибиторов, считали неспецифическим и не принимали в расчет дыхательной активности. Скорость дыхания культуры клеток выражали в нмоль поглощенного кислорода в мин на г сырой массы (нмоль О2/мин/г сырой массы).
Данные обработаны статистически. Приведены средние арифметические и стандартные отклонения (Glantz, 1998).
RESULTS
Отрицательная и повышенная температуры вызывают гибель клеток в суспензионных культурах озимой пшеницы и сахарного тростника
Активная гибель клеток реализуется в течение некоторого времени, у растений этот процесс может продолжаться несколько дней (Zhou et al., 2009). Мы наблюдали за процессом гибели клеток в суспензионных культурах озимой пшеницы и сахарного тростника после действия температур -8 и 50 °С. Оказалось, что самый продолжительный процесс гибели клеток вызывала отрицательная температура -8 °С в культуре озимой пшеницы - на протяжении 10 суток после обработки в данной культуре погибало около 60% клеток (Рис. 1а). Следует отметить, что период воздействия данной температуры, использовавшийся при обработке клеток озимой пшеницы, был весьма продолжительным - 6 ч. Меньшее время требовалось для активации процесса ПКГ отрицательной температурой в культуре клеток сахарного тростника. Нами показано, что воздействия температурой -8 °С на протяжении 2 ч было достаточно, чтобы индуцировать гибель 80% клеток сахарного тростника (Рис. 1в). Этот процесс гибели реализовывался значительно быстрее, чем процесс гибели в культуре озимой пшеницы - в течение 16-18 ч после воздействия. Вероятно, это обусловлено особенностями вида растения, из которого была получена данная культура. Так, озимая пшеница относится к морозостойким растениям, а сахарный тростник - к теплолюбивым.
Изучение действия повышенной температуры (50 °С) показало, что для активации процесса гибели клеток как в культуре озимой пшеницы, так и в культуре сахарного тростника, необходим очень короткий период воздействия - всего 10 мин. При этом процесс гибели в обеих культурах происходил в течение одинакового периода времени - на протяжение 48 ч (Рис. 1 б, г ). После такой обработки в культуре озимой пшеницы погибало около 40% клеток (Рис. 1 б ). В культуре сахарного тростника процесс шел немного интенсивнее и за 48 ч после обработки погибало около 50% (Рис. 1 г ).
Было выявлено, что действие повышенной температуры активирует сходный процесс гибели клеток в обеих изученных культурах, тогда как реализация процесса гибели клеток, вызванного действием отрицательной температурой, осуществляется аналогичным образом только в культуре сахарного тростника. В культуре озимой пшеницы процесс гибели клеток после обработки температурой -8 °С протекает очень медленно, в связи с чем можно ожидать отличий данного процесса на биохимическом уровне.
Изменение активности дыхания в процессе ПКГ, вызванном действием отрицательной и повышенной температур в суспензионных культурах озимой пшеницы и сахарного тростника
Нарушение транспорта электронов по дыхательной цепи под действием стрессовых факторов может действовать как пусковой механизм для запуска процесса ПКГ (Zhou et al., 2009). Изучение изменения активности дыхания в суспензионных культурах клеток озимой пшеницы и сахарного тростника после обработки отрицательной и повышенными температурами показало, что во всех случаях, кроме действия температуры -8 °С на культуру озимой пшеницы, обработка приводила к снижению интенсивности дыхания клеток в культуре (Рис. 2а-г). Так, действие повышенной температуры на культуру озимой пшеницы вызывало уменьшение скорости дыхания на 20% сразу после обработки, а через 48 ч, когда в культуре наблюдался наибольший процент мертвых клеток, скорость ее дыхания была ниже контрольной на 35% (Рис. 1б, 2б). Интенсивность дыхания клеток сахарного тростника сразу после действия повышенной температуры практически не изменялась, но через 48 ч снижалась на 75% (Рис. 2г).
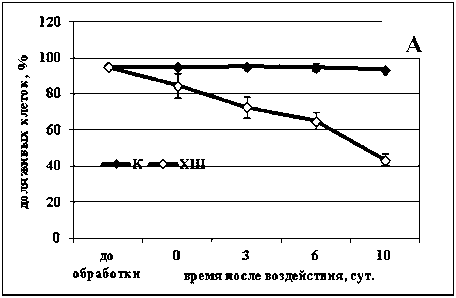
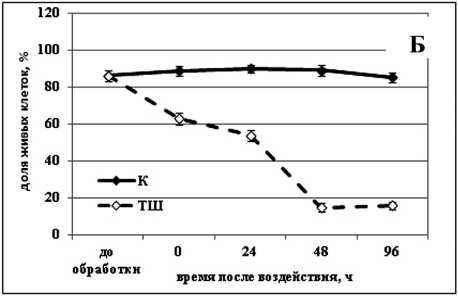
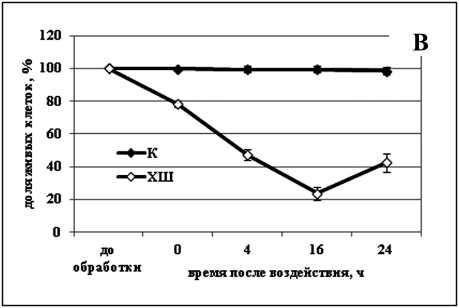
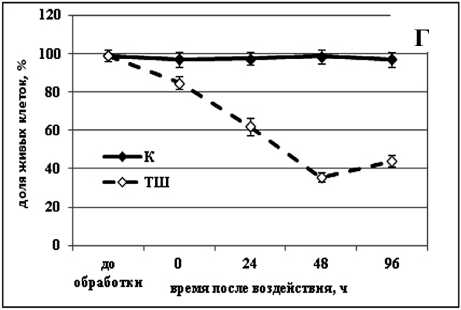
Figure 1. Гибель клеток в суспензионных культурах озимой пшеницы ( а, б ) и сахарного тростника ( в, г ) вызванная обработкой повышенной (50 °С) и отрицательной (-8 °С) температурами.
Обозначения: К - контрольная культура клеток, выращенная при температуре 26 °С; ХШ - культуры клеток, подвергнутые 6-ти часовой (культура озимой пшеницы) или 2-х часовой (культура сахарного тростника) обработке отрицательной (-8 °С) температурой; ТШ - культуры клеток, подвергнутые 10-ти минутной обработке повышенной (50 °С) температурой. M±S.D. n=3-5.
Как уже было сказано выше, действие отрицательной температуры на дыхание культуры озимой пшеницы было иным. Обработка данной культуры отрицательной температурой приводила к повышению интенсивности дыхания на 50% (Рис. 2а). Некоторое снижение дыхательной активности мы наблюдали лишь через 6 суток после воздействия - на 20% по сравнению с контролем (Рис. 2а). В то же время в культуре сахарного тростника данный тип воздействия вызывал постепенное снижение интенсивности дыхания -сразу после обработки мы наблюдали уменьшение скорости дыхания на 30%, а через 16 ч после обработки (в период, когда в культуре было наименьшая доля живых клеток) - на 90% (Рис. 2в). Интересно, что через 24 ч после обработки, когда гибель клеток прекращалась и начиналось постепенное восстановление культуры, скорость дыхания начинала постепенно увеличиваться (Рис. 2в).
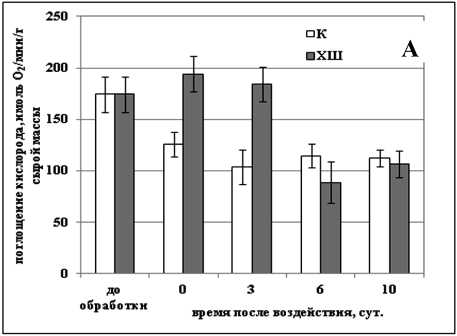
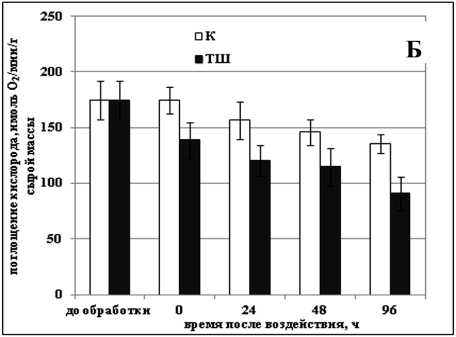
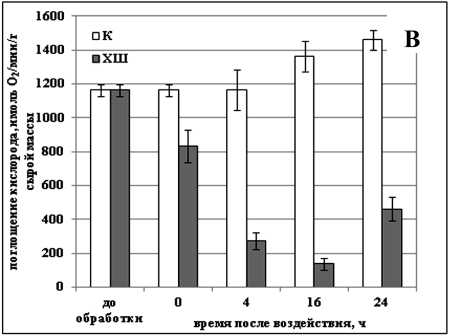
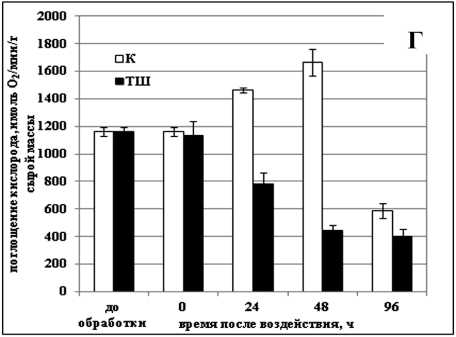
Figure 2. Изменение скорости дыхания клеток суспензионных культур озимой пшеницы ( а, б ) и сахарного тростника ( в, г ) в процессе гибели, вызванном действием отрицательной и повышенной температур.
Обозначения: К - контрольная культура клеток, выращенная при температуре 26 °С; ХШ - культуры клеток, подвергнутые 6-ти часовой (культура озимой пшеницы) или 2-х часовой (культура сахарного тростника) обработке отрицательной (-8 °С) температурой; ТШ - культуры клеток, подвергнутые 10-ти минутной обработке повышенной (50 °С) температурой. M±S.D. n=3-6.
Было показано, что в процессе ПКГ, вызванной действием повышенной и отрицательной температур в суспензионных культурах озимой пшеницы и сахарного тростника, усиление дыхания происходит только после обработки культуры озимой пшеницы температурой -8 °С. Именно при таком способе воздействия в культуре наблюдался самый длительный процесс гибели. В культуре сахарного тростника в процессе ПКГ, вызванной обработкой и повышенной и отрицательной температурами, наблюдалось, напротив, снижение скорости дыхания. Такие различия в реакции изученных культур могут быть обусловлены их изначально разной степенью устойчивости к действию стрессовых температур.
DISCUSSION
Одной из основных сложностей при характеристике процесса ПКГ у растений является невозможность предугадать, с какой скоростью будет протекать процесс гибели клеток при действии различных стрессовых факторов. Рядом авторов было показано, что при использовании в качестве индукторов гибели растительных клеток токсинов, гормонов или повышенных температур, а также в процессе нормального развития их гибель обычно наступает в пределах 24-96 часов (Reape et al., 2008; Zhou et al., 2009). Некоторые исследователи предлагали ограничиваться 6-ти часовым периодом после воздействия для изучения процесса ПКГ у растений (Reape et al., 2008). Однако в работах с пониженными температурами установлено, что гибель растительных клеток может продолжаться от нескольких суток до 3-4 недель (Ishikawa, 1996; Wu et al., 1997; Lyubushkina et al., 2014). Изучение процесса гибели клеток в суспензионной культуре озимой пшеницы под действием отрицательной температуры поставило перед нами важный вопрос: чем же обусловлена низкая скорость реализации ПКГ у растений в данных условиях? С одной стороны, это может быть обусловлено снижением скорости всех метаболических процессов, которые протекают в клетке, вследствие снижения температуры окружающей среды. С другой стороны, длительность процесса ПКГ в каждом случае могла быть результатом индивидуальной реакции каждого изученного растения на использовавшуюся в работе пониженную температуру, и полученные результаты не могут быть распространены на другие растительные виды.
В данной работе мы изучили процесс ПКГ в культуре сахарного тростника, вызванный действием отрицательной температуры -8 °С, и оказалось, что он осуществлялся в течение промежутка времени, сопоставимого по продолжительности с аналогичными процессами, активация которых происходила под действием иных факторов (Groover et al., 1997; Lan et al., 2004; Doyle and McCabe, 2010). Таким образом, причиной низкой скорости реализации ПКГ, наблюдавшейся в других работах, не могло быть только действие самой температуры. В нашей работе не могла она объясняться и низкой скоростью роста культуры озимой пшеницы (Lyubushkina et al., 2014), поскольку изучение процесса ПКГ, вызванного действием температуры 50 °С в данной культуре показало, что гибель клеток происходит в течение 48 ч после обработки, т.е. в рамках уже указанного временного промежутка (Reape et al., 2008). Мы можем предполагать, что медленное осуществление ПКГ в нашей работе было следствием особенностей метаболизма ее клеток, поскольку культура была получена из зародышей озимой пшеницы -растения, отличающегося высокой степенью холодо- и морозостойкости (Dorofeev et al., 2003).
Известно, что важными факторами для поддержания жизнедеятельности клетки являются наличие материалов, т.е. субстратов для пластического обмена, энергии, и сигналов, необходимых для координации различных процессов (Zhou et al., 2009). Те же самые факторы являются ключевыми и для процесса ПКГ (Bras et al., 2005). Кажется естественным тот факт, что главные отличия между этими двумя процессами будут заключаться именно в разнице воспринимаемых и передаваемых от компартмента к компартмету сигналов. Однако, на наш взгляд, не менее важным являются и различия, которые претерпевает энергообмен в процессе активации и реализации ПКГ. В нашей работе использовалась гетеротрофная культура клеток, у которой дыхание является единственным процессом, обеспечивающим клетку энергией. Поскольку ПКГ является активным процессом, требующим постоянного притока энергии для своего осуществления, то логично предположить, что активность дыхания будет снижаться постепенно на протяжении всего процесса гибели. Действительно, мы наблюдали снижение скорости дыхания в суспензионных культурах сахарного тростника и пшеницы в процессе ПКГ, вызванном действием температуры 50 °С (Рис. 2б, г). Однако изменения интенсивности дыхания в процессе ПКГ, вызванном действием температуры -8 °С, отличались. В культуре сахарного тростника мы наблюдали так же, как и после обработки ее повышенной температурой, постепенное снижение скорости дыхания (Рис. 2в). Следует отметить, что дыхательная активность клеток начала снижаться раньше, чем активировался процесс гибели клеток (Рис. 1в, 2в). Аналогичные изменения наблюдали и Vacca с соавт. (2004). Они установили, что снижение скорости поглощения кислорода клетками суспензионной культуры табака происходит уже через 2 ч после теплового шока, когда жизнеспособность культуры все еще составлет 100%.
Интересным является тот факт, что во время самого длительного процесса гибели, осуществлявшегося в культуре озимой пшеницы в течение 10 суток после действия отрицательной температуры (Рис. 1а), снижение скорости дыхания происходило только на 6 сутки после обработки (Рис. 2а). Известно, что энергетические затраты на поддержание жизнедеятельности организма при охлаждении возрастают, вследствие чего содержание АТФ в клетках уменьшается (Zauralon and Lukatkin, 1997; Grabelnych et al., 2014). Если в результате охлаждения интенсивность дыхания повышается, то это обычно рассматривается как адаптивное действие при условии, что увеличение не слишком значительно и не нарушается сопряжение дыхания и фосфорилирования. В случае сильного увеличения интенсивности дыхания в несколько раз, по всей видимости, наблюдается повреждение растения. Также повреждение дыхательных систем имеет место и при резком и значительном снижении дыхания (Klimov, 1998). Можно предположить, что в культуре озимой пшеницы вследствие действия отрицательной температуры запустилось два разнонаправленных процесса -адаптации и гибели. Однако, поскольку клеточные ресурсы ограничены, то постепенно, на протяжении 10 суток равновесие все-таки смещалось в сторону гибели клеток. Тем не менее, даже по окончании этого периода, в культуре озимой пшеницы все-таки оставалось около 40% живых клеток (Рис. 1а). Аналогичным образом Koukalová с соавт. (1997) отмечали, что при охлаждении клеток табака (линия BY-2) при температуре 5-6 °С в течение 5 недель небольшая доля живых клеток в культуре оставалась даже по истечении этого периода.
Таким образом, мы можем заключить, что устойчивость процесса дыхания к воздействию стрессовых факторов, действительно, является одним из важнейших факторов, определяющих скорость осуществления процесса активной гибели у растений.
ACKNOWLEDGEMENT
Работа выполнена при поддержке гранта РФФИ №14-04-32126 и №15-04-06533.
Список литературы Changes of respiration activities in cells of winter wheat and sugar cane suspension cultures during programmed cell death process
- Amor, Y., Chevion, M., Levine, A. (2000) Anoxia pretreatment protects soybean cells against H2O2-induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett., 477, 175-180
- Bras, M. (2005) Programmed cell death via mitochondria: different modes of dying. Biochem., 70, 231-239
- Diamond, M., McCabe, P.F. (2007) The mitochondrion and plant programmed cell death. Plant mitochondria, 308-334
- Dorey, S., Kopp, M., Geoffroy, P., Fritig, B., Kauffmann, S. (1999) Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol., 121, 163-172
- Dorofeev, N.V., Peshkova, A.A., Voinikov, V.K. (2003) Ozimaia pshenitsa v Irkutskoi oblasti. Irkutsk: Art-Press, 175 p
- Doyle, S.M., McCabe, P.F. (2010) Type and cellular location of reactive oxygen species determine activation or suppression of programmed cell death in Arabidopsis suspension cultures. Plant Sign.Behav., 5, 467-468
- Glantz, S.A. (1998) Primer of biostatistics. McGraw-Hill, New-York
- Grabelnych, O.I., Borovik, O.A., Tauson E.L., Pobezhimova, T. P., Katyshev, A.I., Pavlovskaya, N.S., Koroleva, N.A., Lyubushkina, I.V., Bashmakov, V.Yu., Popov, V.N., Borovskii, G.B., Voinikov, V.K. (2014) Mitochondrial energy-dissipating systems (alternative oxidase, uncoupling proteins, and external NADH dehydrogenase) are involved in development of frost-resistance of winter wheat seedlings. Biochemistry (Moscow), 79, 506-519
- Groover, A., DeWitt, N., Heidel, A., Jones, A. (1997) Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma, 196, 197-211
- Ishikawa, H.A. (1996) Ultrastructural features of chilling injury: injured cells and early events during chilling of suspension-cultured mung bean cells. Amer. J. Bot., 83, 825-835
- Jabs, T. (1999) Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol., 57, 231-245
- Jones, A.M. (2001) Programmed cell death in development and defense. Plant Physiol., 125, 94-97
- Kim, M., Lim, J.-H., Ahn, C.S., Park, K., Kim, G.T., Kim, W.T., Pai, H.-S. Mitochondria-associated hexokinases play a role in the control of programmed cell death in Nicotiana benthamiana. Plant Cell, 18, 2341-2355
- Kingston-Smith, A.H., Davies, T.E., Edwards, J.E., Theodorou, M.K. (2008) J.Exp.Bot., 59, 521-532
- Klimov, S.V. (1998) Povyshennoie otnoshenie fotosintez/dyhanie pri nizkih temperaturah -vazhnoie uslovie holodovogo zakalivaniia ozimoi pshenitsy Fiziologiia rastenii, 45, 419-424
- Koukalová, B., Kovařík, A., Fajkus, J., Široký, J. (1997) Chromatin fragmentation associated with apoptotic changes in tobacco cells exposed to cold stress. FEBS Lett., 414, 289-292
- Krishnamurthy, K.V., Krishnaraj, R., Chozhavendan, R., Christopher, F.S. (2000) The programme of cell death in plants and animals - a comparison. Curr. Sci., 79, 1169-1181
- Lan, S.-Y., Zhong, F.-X., Yang, Z.-M., Jin, D.-M., Xu, Z.-X. (2004) The starchy endosperm denucleation by a process of programmed cell death during rice grain development. J. Acta Biologiae Exp. Sin., 37, 34-44
- Logan, D.C. (2008) Having a swell time - mitochondrial morphology and plant cell death programmes. J. of Microscopy, 231, 215-224
- Lyubushkina, I.V., Grabelnych, O.I., Pobezhimova, T.P., Stepanov, A.V., Fedyaeva, A.V., Fedoseeva, I.V., Voinikov, V.K. (2014) Winter wheat cells subjected to freezing temperature undergo death process with features of programmed cell death. Protoplasma, 251, 615-623
- Murashige, T., Scoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant., 15, 473-497
- Petit, P.X., Susin, S.-A., Zamzami, N., Mignotte, B., Kroemer, G. (1996) Mitochondria and programmed cell death: back to the future. FEBS Lett, 396, 7-13
- Reape, T.J., Molony, E.M., McCabe, P.F. (2008) Programmed cell death in plants: distinguishing between different modes. J. Exp. Bot., 59, 435-444
- Rogers, H.J. (2005) Cell death and organ development in plants. Curr. Top. Dev. Biol., 71, 225-261
- Siedow, J.N., Umbach, A.L. (2000) The mitochondrial cyanide-resistant oxidase: structural conservation amid regulatory diversity. Biochem. Biophys. Acta., 1459, 432-439
- Vacca, R.A., Valenti, D., Bobba, A., Merafina, R.S., Passarella, S., Marra, E. (2006) Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiol., 141, 208-219
- Williams, B., Dickman, M. (2008) Plant programmed cell death: can't live with it; can't live without it. Mol. Plant Pathol., 9, 531-544
- Wu, J., Lightner, J., Warwick, N., Browse, J. Low-temperature damage and subsequent recovery of fab1 mutant Arabidopsis exposed to 2 °C. Plant Physiol., 113, 347-356
- Yao, N., Eisfelder, B.J., Marvin, J., Greenberg J.T. (2004) The mitochondrion - an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J., 40, 596-610
- Yu, I.C., Parker, J., Bent, A.F. (1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA, 95, 7819-7824
- Zauralov, O.A., Lukatkin, A.S. (1997) Posledeistvie ponizhennyh temperatur na dyhanie teploliubivyh rastenii. Physiologia rastenii, 44, 736-741
- Zhou, Z., Wang, L., Li, J., Song, X., Yang, C. (2009) Study on programmed cell death and dynamic changes of starch accumulation in pericarp cells of Triticum aestivum L. Protoplasma, 236, 49-58


