Characterization of the biochemical composition and antioxidant activity of Spinacia oleracea L. and Spinacia turkestanica Iljin.: a comparative study
Автор: Sokolova D.V., Solovieva A.E.
Журнал: Овощи России @vegetables
Рубрика: Селекция, семеноводство и биотехнология растений
Статья в выпуске: 4 (72), 2023 года.
Бесплатный доступ
Spinach is an economically important vegetable crop widely cultivated and consumed worldwide. This early ripening leafy vegetable is rich in bioactive components, fiber, micro and macro elements, vitamins, and has high antioxidant activity. Results of numerous studies on the effects of spinach on human health confirm its beneficial effect. The species S. oleracea L. is cultivated commercially. The ancestor of cultivated spinach is S. turkestanica Iljin, which has a breeding potential for different economically valuable traits. Its biochemical composition has been studied extremely little. The present article offers a comparative evaluation of the biochemical profile and antioxidant activity of cultivated and wild spinach species. The material for the study was a representative sample of 48 collection accessions of spinach from the N.I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR). The accessions were grown in 2019 and 2020 in the open ground of the Pushkin and Pavlovsk Laboratories of VIR. The antioxidant activity was studied spectrophotometrically by the DPPH assay of free radical scavenging at a wavelength of 515 nm. A significant similarity of the two species in most biochemical parameters was revealed, which confirms their phylogenetic relationship. Significant differences were found in the content of phenolic elements, which determine the elevated values of antioxidant and antiradical activity of S. turkestanica. The article presents correlation matrices of species biochemical composition, describes general trends, negative relationships and conjugated factors. The identified promising accessions of both cultivated and wild spinach are recommended for breeding for increased content of phenolic compounds, ascorbic acid and antioxidant activity. The result of the study helps to reveal the potential of the crop as a valuable source of bioactive components and high antioxidant activity.
Spinach, spinacia oleracea l, spinacia turkestanica iljin, antioxidant activity, phenolic compounds
Короткий адрес: https://sciup.org/140301891
IDR: 140301891 | УДК: 635.41:581.19-047.37 | DOI: 10.18619/2072-9146-2023-4-23-29
Текст научной статьи Characterization of the biochemical composition and antioxidant activity of Spinacia oleracea L. and Spinacia turkestanica Iljin.: a comparative study
Оригинальные статьи / Originalarticles УДК 635.41:581.19-047.37

Introduction he conditions of modern life put forward new requirements for human nutrition. In the market, there is a growing demand for natural products with high nutritiona value and revitalizing properties. Cultivated spinach (Spinacia oleracea L.) is an economically important vegetable crop widely cultivated and consumed worldwide. According to FAOSTAT, global production of spinach is growing every year, having reached 35 million tons in 2021 (FAOSTAT) [1]. Spinach is consumed fresh, used in the canning industry for the production of juices, purees, in baby and diet foods, as well as for the production of a green dye [2,3]. This leafy vegetable is rich in bioactive components and fiber [4]. It is valued for its high content of ascorbic acid, carotenoids, vitamins B1, B2, B3, B6, B9, H, K, E, P, and PP [5]. Also, it contains iron, sodium, potassium, calcium, magnesium, phosphorus, sugars, and protein. Due to the high content of various organic acids, the nutritional value of spinach does not change during canning and drying [6,7]. Spinach contains various active compounds such as flavonoids and other polyphenolic active ingredients that act synergistically as anti-inflammatory, antioxidant and anti-cancer agents. Epidemiological and preclinical data from studies on the health effects of spinach confirm its beneficial effects [8,9,10,11,12,13].
According to the APG II Classification System (2003) [14], Spinacia L. genus belongs to the Chenopodioideae subfamily of the Amaranthaceae family. In an earlier classification, spinach belonged to the Chenopodiaceae family. The genus is represented by three species: two 2003wild ones, S. turkestanica Iljin and S. tetrandra Stev., and cultivated S. oleracea L. The species S. tetrandra was first described by Christian von Steven, a Russian botanist of Swedish origin, while studying the flora of the Caucasus (1809) [15], and was long considered the only wild spinach species. The species S. turkestanica was isolated by M.M. Ilyin in 1934 as an independent species of wild spinach growing in Central Asia [16]. The distribution area of S. tetrandra is located mainly in Transcaucasia, while that of S. turkestanica is found in East and Central Asia, Uzbekistan, Turkmenistan, Afghanistan, and Iran [17,18,19].
The exact origin and the earliest date of S. oleracea cultivation are still unknown. It is believed that spinach was introduced into culture about two thousand years ago in Iran (former Persia), from where it spread to China, Europe, North Africa and America [20]. These assumptions were confirmed by recent transcriptome sequencing of 120 spinach accessions. It was shown that the most likely progenitor of cultivated spinach is S. turkestanica [21,22].
Wild S. turkestanica is of considerable interest for spinach breeding for such economically valuable traits as cold and drought tolerance, resistance to the most common diseases, abiotic stresses, as well as soil salinity and acidity. Its biochemical composition has been studied little. In light of the growing interest to the nutritional value and composition of spinach, the present study is relevant.
The present work was aimed at revealing features of the biochemical profile and antioxidant activity in the cultivated spinach species ( S. oleracea ) and its wild predecessor ( S. turkestanica ).
Materials and methods
The object of the study were two spinach species, S. oleracea and S. turkestanica (Fig. 1). The material for the experiment were 48 accessions from the spinach collection of the N.I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR). The plants were grown in the open field of the "Pushkin and Pavlovsk Laboratories of VIR" Research and Production Base (59°7111275´N, 30°43032647´E) in 2019, 2020. Seeds were sown manually on July 15 in a row with a 10 cm distance between plants and 70 cm between rows. Biomass was sampled for analyzing in the rosette phase on day 40 from the sprouts emergence. Soils in Pushkin are predominantly sod-podzols and sandy loams. The accessions were grown against a natural background without the use of fertilizers and pesticides.
The weather conditions of the second half of the summer of 2019 and 2020 were generally favorable for growing spinach and were characterized by moderate air temperatures at the level of long-term mean values (Fig.2). The growing season of 2020 was characterized by a large amount of precipitation: in June by 20 mm, in August - by 45 mm. Irrigation was carried out if necessary. The weather conditions of both years of testing made it possible to obtain plants identical in habit and weight of one plant with the closest possible biochemical parameters.

Fig.1.Experimentalspinach species:S.oleracea L. (left)and S. turkestanica Iljin. (on right).
Рис.1.Виды шпината в опыте:S.oleracea L. (слева)и S.turkestanica Iljin. (справа).
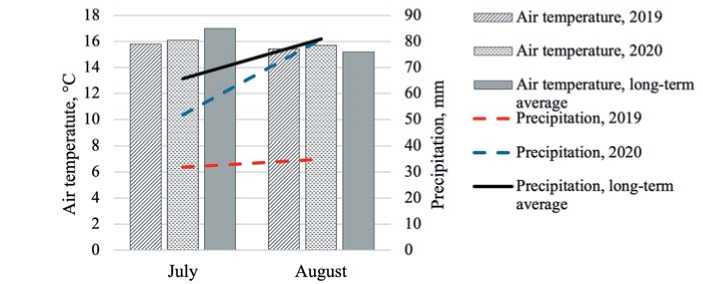
Source: PGR Automated Information Systems Department. Hydrometeorological station of VIR.
Fig.2. Climatic characteristics ofthe growing seasons 2019,2020 (Pushkin)
Рис.2.Климатическая характеристика вегетационных периодов 2019,2020 годов (г.Пушкин)
Biochemical analysis was carried out in the Department of Biochemistry and Molecular Biology of VIR. Accessions were processed and analyzed as described in Ermakov et al. [23]. Dry weight (in %) was measured gravimetrically. A 50 g portion of fresh substance was dried in a thermostat at 80 °C for 12 hours, and then at 105 °C for 1 hour to a constant weight. The content of ascorbic acid was determined by direct extraction from plants (10 g) with 1% HCl solution (according to I.K. Murri) followed by titration with 2,6-dichloroindophinol (Thielman's reagent) and expressed in mg/100 g. The total sugars content was determined by the Bertrand method. A 25 g sample was taken for the analysis. Oligosaccharides were preliminarily hydrolyzed with 10% HCl solution. The amount of cuprous oxide precipitate strictly corresponded to the amount of sugar in the solution. The settled precipitate of cuprous oxide was dissolved with iron sulfate (oxide) in the presence of sulfuric acid. In this case, copper oxide is oxidized completely, and ferrous oxide, in turn, is quantitatively oxidized with a titrated solution of potassium permanganate. The data are presented in percent. To measure the total acidity, a 25 g sample of fresh substance was homogenized in 250 ml of hot distilled water, then filtered, and 10 ml was titrated with 0.1 N alkali in the presence of an indicator. The results are expressed as a percentage, recalculated as oxalic acid. The protein content was measured by the Kjeldahl method [24]: a sample of dried and ground material was mineralized by heating with concentrated sulfuric acid at 420 °C for one and a half hours. Nitrogen was determined using a Kjeltec 2200 semi-automatic analyzer (FOSS, Sweden) followed by titration with 0.1 N sulfuric acid solution The total protein content was calculated from nitrogen with a factor of 6.25 (for vegetable crops). Pigments were isolated with 100% acetone, and their absorption was measured on an Ultrospec II spec- trophotometer (England) at different wavelengths (nm): 662 and 645 for chlorophylls a and b, 440 for carotenoids, and 454 for β-carotene. The total amount of soluble phenolic compounds was determined by Folin-Ciocalteu spectropho-tomety (phenolic compounds were extracted with 80% alcohol and kept for 12h in the dark at room temperature; absorption measured at 765 nm) modified by Singleton and Rossi [25]. The result was expressed as mg of gallic acid equivalent (GAE) per 100 g. For assessing antioxidant activity, free radical colorimetry was used as a method based on the reaction of the ethanol-dissolved DPPH (2,2-diphenyl-1-picrylhy-drazyl (C18H12N5O6, M = 394.33)) with an antioxidant sample [26]. The result was expressed as ascorbic acid equivalent (AAE). Alldata are given in terms of crude matter.
The data were statistically processed in the Statistica 10.0 program and in the R environment. The descriptive statistics (mean values, standard error of the mean, and coefficient of variation) were calculated for all parameters. Pearson correlation coefficient values of r<0.5 were considered as low, those in the range of 0.51>r ≥ 0.7 as medium, in the range of 0.71>r ≥ 0.9 as high, and those of r ≥ 0.9 as very strong.
Results and discussion
The VIR spinach collection is represented by three known species and features a wide variety of genotypes both in terms of origin and year of inclusion in the collection, and in terms of morphological characteristics. All the studied accessions are of European or Asian origin. The largest number of accessions belongs to the species S. oleracea ; 39 accessions are from European countries, Russia and Japan (Fig.3). S. turkestanica was represented by 9 accessions from Armenia, Kazakhstan, Uzbekistan, Tajikistan and China, which is consistent with historical data on the center of crop origin and ways of its spreading [19].
Netherlands Russia Romania Tajikistan Uzbekistan
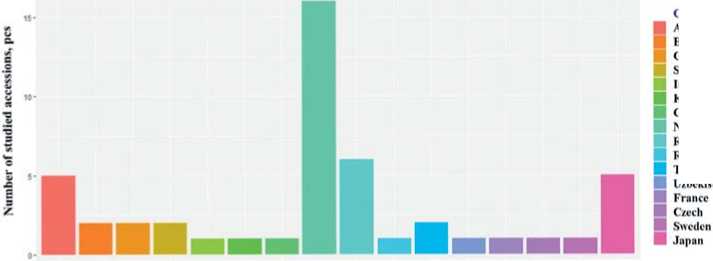
Fig. 3.Origin and number ofthe studied spinach accessions
Рис. 3.Происхождение и количество образцов шпината в опыте
Origin Armenia Bulgaria Ge плат Spain Itah Kazakhstan
Dry matter content. The content of dry matter is one of important indicators for judging the quality of vegetable raw matter. In the present study, the dry matter content did not differ significantly (p< 0.05) between accessions of the two species and averaged 10.5% for S. oleracea and 10.31% for S. turkestanica (Table 1). A weak variability of this trait is characteristic ( Сv =11-14%). The maximum content of 14.6% was noted in ‘Sp. Riccio D`America’ (k-942, Italy), an accession of S. oleracea . Dry matter content above the average was shown by 55.5% of the accessions of S. turkestanica .
Ascorbic acid content. A marked variation in the levels of ascorbic acid (vitamin C) among the tested genotypes was observed in the range from 24.8 to 62.0 mg/100 g. On the average, it was 40.9 mg/100 g. The maximum content of 62 mg/100 g was found in an accession of S. turkestanica from Tajikistan (k-942). No significant differences in the content of ascorbic acid were found between the two species. Similar data were obtained for S. oleracea by other authors when studying various genotypes grown in open ground conditions [27,28,29]. However, in protected ground conditions the differences between genotypes were much stronger. For instance, a study of a set of 34 S. oleracea genotypes in China has revealed a significant difference in the content of ascorbic acid [30]. This is explained by lighting conditions that affect the concentration of ascorbic acid in fruits and vegetables: a decreased light intensity usually leads to a decrease in its concentration and genotypic differences [31,32]. It may be assumed that the genotypes of S. turkestanica, which formed under conditions of increased photosynthetic activity in the center of origin of the crop, are able to synthesize ascorbic acid more actively. Several components, including ascorbic acid and phenolic compounds, have been reported to inhibit nitrite toxicity in spinach [33]. No doubt that spinach, which accumulates more ascorbic acid, will be more beneficial for human health.
Content of chlorophylls and total acidity. The content of organic acids and chlorophylls in the studied accessions of the two species was similar and had no significant differences ( p < 0.05).
Protein content. In terms of protein content, the group of S. oleracea accessions was superior to S. turkestanica . The maximum value of 29.11% was recorded for the ‘Ratnik’ variety (k-916, Russia). A general regularity was observed in the negative correlation between the content of protein and sugars (Fig. 4, 5). At the same time, this relationship was more significant ( r = ̶ 0.72 ( p <0.05)) in accessions of S. turkestanica vs. r = ̶ 0.57 ( p <0.001) in S. oler-acea .
Phenolic Content. Phenolic compounds are among the most common secondary plant metabolites [34]. Phenolic compounds found in spinach have a strong antioxidant effect due to the ability of their hydroxyl groups to scavenge free radicals. Extensive conjugation in the structure of flavonoids and numerous hydroxyl groups enhance their antioxidant properties [35]. Previous studies of spinach have shown that kaempferol (54%) predominates among
Table 1. Comparative characteristics of biochemical indicators of S. oleracea and S. turkestanica
Таблица 1. Сравнительная характеристика биохимических показателей образцов видов S. oleracea L. и S. turkestanica Iljin.
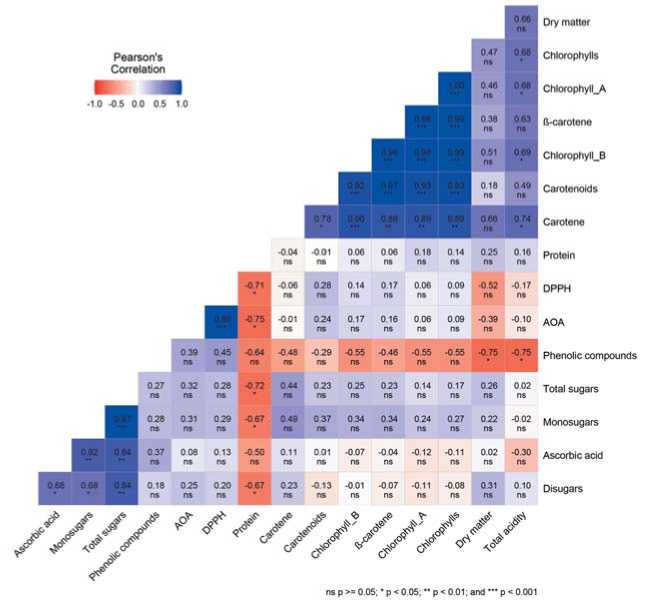
Fig.4. Correlation matrixofbiochemicalparameters in genotypes ofS.turkestanica
Рис.4.Корреляционная матрица биохимических показателей генотипов вида S. turkestanica Iijin
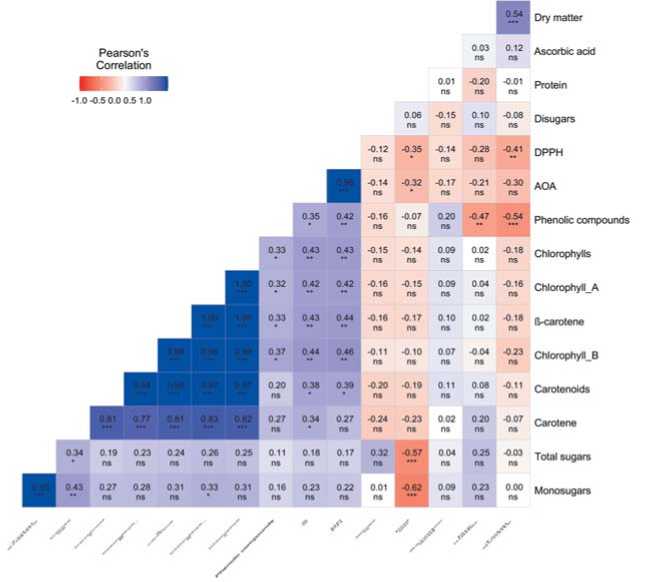
ns p »■ 0.05;e p < 0 05. ” p < 0.01: and ”* p < 0 001
Fig.5. Correlation matrixofbiochemicalparameters in genotypes ofS.oleracea
Рис.5.Корреляционная матрица биохимических показателей генотипов вида S.oleracea L.
the phenolic compounds, the content of gallic acid is 26% and that of galangin is 18% [36]. The content of phenolic elements in the studied accessions varied over a wide range. It should be noted that genotypes with a high content of phenolic elements were more common in S. turkestanica. The group with the above-average values included 23% of all S. oleracea and 34% of S. turkestanica accessions. Among accessions of the cultivated species, only those of Dutch and Russian origin turned out to be the leaders. The maximum value of 750.0 mg GAE/100 g was found in the accession ‘Gb. 25784ʼ (k-941, the Netherlands). Among S. turkestanica accessions, one from Armenia (vk-935) with 656.5 mg GAE/100 g and another one from Tajikistan (vk-942) with 604.3 mg GAE/100 g were singled out regarding this indicator. A general trend demonstrated by the correlation matrix for the crop is that the higher the content of dry matterand total acidity in plants, the less phenolic elements they contain. The negative correlation of these indicators in genotypes of the wild species was more significant: r= ̶ 0.75 (p<0.05) vs. r= ̶ 0.47-0.54 (p<0.01) in S. oleracea accessions. It was previously noted that the content of phenolic compounds in plants increases under conditions of high photosynthetically active radiation [37], as wel as with the advance of the crop to northern latitudes [38,39]. We assume that the origin of S. turkestanica genotypes, which formed in climatic regions with active solar radiation and thus got adapted to such conditions, is reflected in the ability to accumulate phenolic elements.
Antioxidant and antiradical activity. Spinach is one of the most valuable green crops with pronounced antioxidant properties [36,40]. Antioxidant activity (AOA) is the ability to inhibit the oxidation process, and antiradical activity (DPPH) reflects the ability of compounds to react with free radicals. There is strong evidence for the role of the vegetable antioxidant components in health maintenance and disease prevention [41,42,43]. Compared with lettuce and kale, the AOA and DPPH values in spinach are higher by 39.5% and 24.2%, respectively, and slightly lower than in broccoli [44]. Literature confirms that along with blueberries, spinach has a high ability to scavenge free radicals [45,46,47]. In our studies, high AOA levels in representatives of S. turkestanica were more common (observed in 67% of accessions), while it was true for only 46% of S. oleracea accessions. The maximum values were noted in S. turkestanica from Kazakhstan (k-775), Tajikistan (k-942) and Armenia (k-960) – 244.04, 243.81 and 256.10 µg AAE/100 g, respectively. These accessions were distinguished by a high content of sugars, a low content of protein, oxalic, citric and pyruvic acids, fatty acids and alcohols. In general, AOA of spinach negatively correlated with protein content and was high in S. turkestanica accessions. ( r = ̶ 0.75, p<0.05).
AOA of spinach is determined mainly by the pigment composition, i.e., chlorophylls and carotenoids, as well as by phenolic compounds. In the present study, the content of carotenoids in two spinach species did not have significant differences and averaged 32.0 mg/100 g. As shown in Figure 6 a close positive correlation between chlorophylls and carotenoids associated with AOA. Since the values of the pigment composition in the wild and cultivated species are close, it can be assumed that the increased AOA and DPPH values in spinach are associated with the accumulation of phenolic compounds.
It is known that the main carotenoid found in spinach leaves is lutein, which averages 39% of the total carotenoids [30]. This pigment is a natural protective filter for the eyes, maintaining visual acuity. The human body is not able to synthesize lutein, so its intake into the body is directly related to nutrition. Spinach is a promising crop, a source of lutein and high AOA. Its promotion and consumption will contribute to the revitalization of the population.
Conclusions
The conducted studies showed that the comparison of biochemical parameters of S. oleracea and S. turkestanica revealed a significant similarity of the two species in most biochemical parameters, which confirms their phylogenetic relationship. A negative correlation between the content of protein and sugars was noted to be characteristic of both species. Significant differences were found in the content of phenolic elements, which determine the increased values of the antioxidant and antiradical activity of S. turkestanica . The maximum content of phenolic elements (750.0 mg GAE/100 g) was recorded for an S. oleracea accession ‘Gb. 25784' (k-941, the Netherlands). In S. turkestanica , the highest values were demonstrated by accessions from Armenia (656.5 mg GAE/100g (vk-935) and Tajikistan (604.3 mg GAE/100 g) (k-942). These genotypes are of interest for breeding for an increased content of phenolic elements and AOA.
A negative relationship was revealed between dry matter content and total acidity with phenolic elements, which is more significant in S. turkestanica . Among the genotypes of the wild species, an accession from Tajikistan (k-942) is a source of high content of ascorbic acid (62 mg/100 g). For breeding for an increased AOA, S. turkestanica accessions from Kazakhstan (k-775) and Armenia (k-960) can be recommended.
In general, the biochemical composition of spinach is quite rich and has a beneficial effect on human health. The results of the study help to reveal the value of S. turkestani-ca and recommend its inclusion in breeding programs.
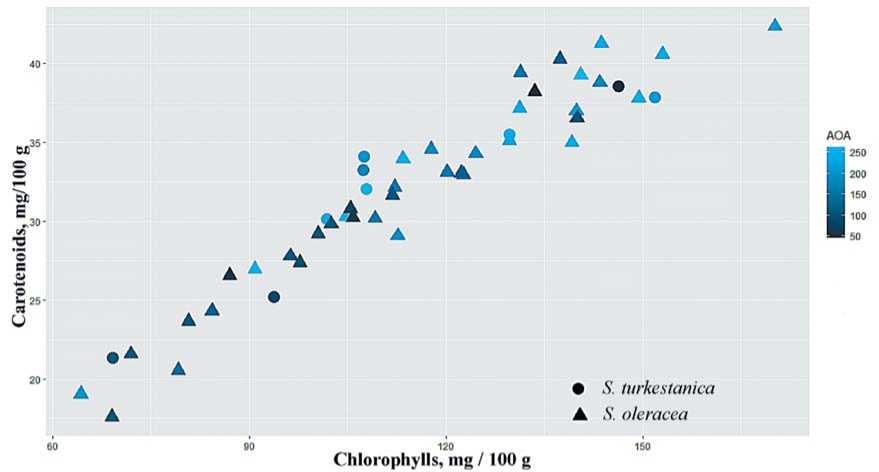
Fig.6.Scatterplotofspinach chlorophylls and carotenoids
Рис.6.Диаграмма рассеяния хлорофиллов и каротиноидов шпината. 1 – S. turkestanicaIijin.,2 – S.oleracea L.
Об авторах:
Диана Викторовна Соколова – старший научный сотрудник Отдела генетических ресурсов овощных и бахчевых культур, куратор коллекции свеклы, амаранта и шпината, , автор для переписки,
Aboutthe Authors:
Diana V. Sokolova – Senior Researcher,
Department of Genetic Resources of Vegetable and Cucurbit Crops,
Beet, spinach and amaranth collection curator, , Correspondence Author,
Alla E. Solovieva – Senior Researcher,
Department of Biochemistry and Molecular Biology,
Список литературы Characterization of the biochemical composition and antioxidant activity of Spinacia oleracea L. and Spinacia turkestanica Iljin.: a comparative study
- Food and Agriculture Organization of the United Nations (FAO), FAOSTAT, 2021. Available online: www.fao.org/faostat/en/#data/QCL (date of access 13 января 2023 года).
- Mukhanova Yu.I., Trebukhina K.A. Expand assortment. Potato and vegetables. 1987;(1):23-25. (in Russian)
- Pivovarov V.F. Vegetables of Russia. Moscow: Russian seeds. 1994. (in Russian)
- Hu J., Wu F., Wu S., Cao Z., Lin X., Wong M. H. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere. 2013;91(4):455-461. https://doi.org/10.1016/j.chemosphere.2012.11.066
- Morelock T.E., Correll J.C. Spinach. In: Prohens J., Nuez F. (eds.) Vegetables I. Handbook of plant breeding. Vol 1. Springer, New York. 2008.
- Tang L., Hamid Y., Sahito Z. A., Gurajala H. K., He Z., Feng Y., Yang X. Evaluation of variation in essential nutrients and hazardous materials in spinach (Spinacia oleracea L.) genotypes grown on contaminated soil for human consumption. Journal of Food Composition and Analysis. 2019;(79):95-106. https://doi.org/10.1016/j.jfca.2019.03.012
- Manzoor M. F., Ahmed Z., Ahmad N., Aadil R. M., Rahaman A., Roobab U., … Siddeeg A. Novel processing techniques and spinach juice: Quality and safety improvements. Journal of Food Science. 2020;85(4):1018-1026. https://doi.org/10.1111/1750-3841.15107
- Longnecker M.P., Newcomb P.A., Mittendorf R., Greenberg E.R., Willett W.C. Intake of carrots, spinach, and supplements containing vitamin A in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev.1997;6(11):887-892.
- Lomnitski L., Bergman M., Nyska A., Ben-Shaul V., Grossman S. Composition, Efficacy, and Safety of Spinach Extracts. Nutrition and Cancer. 2003;46(2):222-231. https://doi.org/10.1207/s15327914nc4602_16
- Edenharder R., Keller G., Platt K.L., Unger K.K. Isolation and Characterization of Structurally Novel Antimutagenic Flavonoids from Spinach (Spinacia oleracea). Journal of Agricultural and Food Chemistry. 2001;49(6):2767-2773. https://doi.org/10.1021/jf0013712
- Kotake-Nara E., Kushiro M., Zhang H., Sugawara T., Miyashita K., Nagao A. Carotenoids Affect Proliferation of Human Prostate Cancer Cells. The Journal of Nutrition. 2001; 131(12):3303-3306. https://doi.org/10.1093/jn/131.12.3303
- Nyska A., Suttie A., Bakshi S., Lomnitski L., Grossman S., Bergman M., … Maronpot R.R. Slowing Tumorigenic Progression in TRAMP Mice and Prostatic Carcinoma Cell Lines Using Natural Anti-Oxidant from Spinach, NAO-A Comparative Study of Three Anti-Oxidants. Toxicologic Pathology. 2003;31(1):39-51. https://doi.org/10.1080/01926230390173833
- Maeda N., Matsubara K., Yoshida H., Mizushina Y. Anti-cancer Effect of Spinach Glycoglycerolipids as Angiogenesis Inhibitors Based on the Selective Inhibition of DNA Polymerase Activity. Mini-Reviews in Medicinal Chemistry. 2011;11(1):32-38. https://doi.org/10.2174/138955711793564042
- An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141(4):399-436. https://doi.org/10.1046/j.1095-8339.2003.t01-1-00158.x
- Steven C.C. Decas plantarum nondum descriptarum Iberiae et Rossiae meridionalis (Mmoires de la socit Impriale des naturalistes de Moscou, 1809, V. II, p. 173-183) (in Latine)
- Ilyin M.M. Family III. Chenopodia - Chenopodiaceae Less. Flora URSS : in 30 volumes / editor-in-chief V. L. Komarov. - M.; L. Publishing House of the Academy of Sciences of the USSR. 1936. V.6. (ed. B. K. Shishkin) (in Russian)
- Uotila P. Spinacia. In: Reichinger K.H. (ed). Flora Iranica. Akademische Druck und Verlagsanstalt, Graz. 1997.
- Andersen S.B., Torp A.M. Spinacia. Chapter 13. In: Kole C. (ed.) Wild crop relatives: genomic and breeding resources vegetables. Springer, Berlin, 2011.
- Hallavant C., Ruas M.P. The first archaeobotanical evidence of Spinacia oleracea L. (spinach) in late 12th-mid 13th century A.D. France. Vegetation History and Archaeobotany. 2014;23:153-165. https://doi.org/10.1007/s00334-013-0400-8
- Dekandol' A. Place of origin of cultivated plants: Translation from the 2nd fr. ed. with add. according to later sources. Dr. Chr. Gobi, prof. St. Petersburg. university (ed.). St. Petersburg: K. Ricker, 1885. (in Russian)
- Xu C., Jiao C., Zheng Y., Sun H., Liu W., Cai X., … Wang Q. De novo and comparative transcriptome analysis of cultivated and wild spinach. Scientific Reports. 2015;(5):1-9. https://doi.org/10.1038/srep17706
- Xu C., Jiao C., Sun H., Cai X., Wang X., Ge C., Zheng Y., Liu W., Sun X., Xu Y., Deng J., Zhang Z., Huang S., Dai S., Mou B., Wang Q., Fei Z., Wang Q. Draft genome of spinach and transcriptome diversity of 120 Spinacia accessions. Nature Communications. 2017;(8):15275. https://doi.org/10.1038/ncomms15275
- Ermakov A.I. Biochemical research methods of plants. Leningrad, 1987. (In Russian)
- Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. [New Method for the Determination of Nitrogen in Organic Substances.] Zeitschrift für analytische Chemie. 1883;(22):366-383. https://doi.org/10.1007/BF01338151
- Ainsworth E.A., Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols. 2007;2(4):875-877. https://doi.org/10.1038/nprot.2007.102
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25-30. https://doi.org/10.1016/s0023-6438(95)80008-5
- Girenko M.M. Initial material for breeding of leafy green crops in the northwestern zone of the USSR (spinach, lettuce, dill) [dissertation]. Leningrad; 1964. (in Russian)
- Sychova I.V. Peculiarities of ecological methods for assessing the source material for the creation of heterotic spinach hybrids [dissertation]. Moscow; 2000. (in Russian)
- Koh E., Charoenprasert S., Mitchell A. E. Effect of Organic and Conventional Cropping Systems on Ascorbic Acid, Vitamin C, Flavonoids, Nitrate, and Oxalate in 27 Varieties of Spinach (Spinacia oleracea L.). Journal of Agricultural and Food Chemistry. 2012;60(12):3144-3150. https://doi.org/10.1021/jf300051f
- Bergquist S.Å.M., Gertsson U.E., Nordmark L.Y.G., Olsson M.E. Ascorbic Acid, Carotenoids, and Visual Quality of Baby Spinach as Affected by Shade Netting and Postharvest Storage. Journal of Agricultural and Food Chemistry. 2007;55(21):8444-8451. https://doi.org/10.1021/jf070396z
- Mozafar A. Plant Vitamins Agronomic, Physiological, and Nutritional Aspects. CRC Press: Boca Raton (1st edition). 1993. https://doi.org/10.1201/9781351075800
- Proietti S., Moscatello S., Colla G., Battistelli Y. The effect of growing spinach (Spinacia oleracea L.) at two light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. The Journal of Horticultural Science and Biotechnology. 2004;79(4):606-609. https://doi.org/10.1080/14620316.2004.11511814
- Walker R. Nitrates, nitrites and N-nitrosocompounds: A review of the occurrence in food and diet and the toxicological implications. Food Additives and Contaminants. 1990;7(6):717-768. https://doi.org/10.1080/02652039009373938
- Zaprometov M.N. Phenolic compounds. Distribution, metabolism and functions in plants. Moscow. 1993. (in Russian)
- Cao G., Sofic E., Prior R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radical Biology and Medicine. 1997;22(5):749-760. https://doi.org/10.1016/s0891-5849(96)00351-6
- Deng G.-F., Lin X., Xu X.-R., Gao L.-L., Xie J.-F., Li H.-B. Antioxidant capacities and total phenolic contents of 56 vegetables. Journal of Functional Foods. 2013;5(1):260-266. https://doi.org/10.1016/j.jff.2012.10.015
- Mikulic-Petkovsek M., Schmitzer V., Slatnar A., Stampar F., Veberic R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. Journal of the Science of Food and Agriculture. 2014;95(4):776-785. https://doi.org/10.1002/jsfa.6897
- Åkerstrom A., Jaakola L., Bång U., Ja ̈ derlund A. Effects of Latitude-Related ̈ Factors and Geographical Origin on Anthocyanidin Concentrations in Fruits of Vaccinium myrtillus L. (Bilberries). Journal of Agricultural and Food Chemistry. 2010;58(22):11939-11945. https://doi.org/10.1021/jf102407n
- Latti A K., Jaakola L., Riihinen K.R., Kainulainen P.S. Anthocyanin and ̈ Flavonol Variation in Bog Bilberries (Vaccinium uliginosum L.) in Finland. Journal of Agricultural and Food Chemistry. 2010;58(1):427-433. https://doi.org/10.1021/jf903033m
- Lomnitski L., Carbonatto M., Ben-Shaul V., Peano S., Conz A., Corradin L., … Nyska A. The Prophylactic Effects of Natural Water-Soluble Antioxidant from Spinach and Apocynin in a Rabbit Model of Lipopolysaccharide-Induced Endotoxemia. Toxicologic Pathology. 2000;28(4):588-600. https://doi.org/10.1177/019262330002800413
- Kanner J., Frankel E., Granit R., German B., Kinsella J. E. Natural antioxidants in grapes and wines. Journal of Agricultural and Food Chemistry. 1994;42(1):64-69. https://doi.org/10.1021/jf00037a010
- Salah N., Miller N.J., Paganga G., Tijburg L., Bolwell G.P., Riceevans C. Polyphenolic Flavanols as Scavengers of Aqueous Phase Radicals and as Chain-Breaking Antioxidants. Archives of Biochemistry and Biophysics. 1995;322(2):339-346. https://doi.org/10.1006/abbi.1995.1473
- Bergman M., Varshavsky L., Gottlieb H.E., Grossman S. The antioxidant activity of aqueous spinach extract: chemical identification of active fractions. Phytochemistry. 2001;58(1):143-152. https://doi.org/10.1016/s0031-9422(01)00137-6
- Chu Y.-F., Sun J., Wu X., Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. Journal of Agricultural and Food Chemistry. 2002;50(23):6910-6916. https://doi.org/10.1021/jf020665f
- Wang H., Cao G., Prior R.L. Total Antioxidant Capacity of Fruits. Journal of Agricultural and Food Chemistry. 1996;44(3):701-705. https://doi.org/10.1021/jf950579y
- Prior R.L., Cao G., Martin A., Sofic E., McEwen J., O’Brien C., … Mainland C.M. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. Journal of Agricultural and Food Chemistry. 1998;46(7):2686-2693. https://doi.org/10.1021/jf980145d
- Gil M.I., Ferreres F., Tomás-Barberán F.A. Effect of Postharvest Storage and Processing on the Antioxidant Constituents (Flavonoids and Vitamin C) of Fresh-Cut Spinach. Journal of Agricultural and Food Chemistry. 1999;47(6):2213-2217. https://doi.org/10.1021/jf981200l


