Chemical fingerprint based involvement of plant metabolites and osmoregulatory solutes in providing abiotic stress tolerance to invasive plant lantana camara
Автор: Nischal Priya, Sharma Arun Dev
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.15, 2019 года.
Бесплатный доступ
Background: Invasive alien species (IAS) are broadly distributed all over the world by disturbing or reducing the growth and development of native vegetation. Lantana camara is basically a noxious weed and have a potential to invade a region's indigenous vegetation. Even after knowing all its harmful effects, there has been a little research on various mechanisms followed by this plant to harm the plant species. Results: Random sampling was performed to take plant samples without any bias, to study various mechanisms carried out in plant species. Chemical fingerprinting of samples were then performed in various abiotic stress conditions (cold and hot) to study changes in L. camara under these stressful conditions in order to find the reason behind the invasiveness of this plant species. Stress indicator like malondialdehyde (MDA)/lipid peroxidation was also performed and increased lipid peroxidation during both extremities showed that plant is experiencing oxidative stress...
Chemical fingerprinting, chromatograph, invasiveness, lantana camara, lipid peroxidation, secondary metabolites
Короткий адрес: https://sciup.org/143168577
IDR: 143168577
Текст научной статьи Chemical fingerprint based involvement of plant metabolites and osmoregulatory solutes in providing abiotic stress tolerance to invasive plant lantana camara
Invasive alien species are the plants which have been accidentally escaped from their natural habitat and introduced into the ecosystems which are non-native to them. They have broad distribution all over the world in natural and agricultural areas, and are trying to create its own dominating world by displacing native vegetation (Henkel et al. 2009). Out of many plants being introduced to a new habitat, only a few become invasive which suggests that there is existence of some special characters or traits which enable invasiveness in particular plant. If we talk about various threats to global biodiversity, invasion is one of them majorly and is very widespread . There are certain definitions of invasive species known yet, which are given by different organizations which are engaged in invasion research. It is basically the potential of invasive species that make them so strong invaders and colonizers of the environment, whether introduced with will or by chance. It is still a matter of debate that why the invasive alien species are so invasive.
Various environmental factors are there which adversely affect plant’s growth and limits plant production, drought, nutrient imbalance, high temperature, etc. are the major factors among them. Changes in temperature occur rapidly as compared to other changes in stress such as salinity, drought, etc. With the help of nature, plants have developed various strategies to tackle certain stress conditions. It is already known that the acquisition of large number of natural products entirely depends on their growing conditions like temperature, light and nutrient supply (Falk et al. 2007; Ballhorn et al. 2011). Different stressful conditions will also influence the metabolic pathways responsible for the acquisition of various secondary plant products, that further leads to biochemical changes in plants (Scheel et al. 2016). Variety of experiments have shown that plants under stressful conditions accumulate high level of secondary metabolites like in complex phenols, numerous terpenes (Selmar et al. 2013). Therefore, there is no doubt that onset of various stresses enhances the level of secondary plant products.
The fingerprint techniques are one of the modern approaches for analyzing several metabolites (Grauwet et al. 2014; Esslinger et al. 2014). Chemical portrait given by fingerprinting is representative of ecosphere where plant grows, evidencing secondary metabolites pattern being driven in quantity and amount of compounds by the sensitivity of plants to ecological variables. The plant fingerprints might help in biomonitoring and biological research. Chemical fingerprinting of plant's various secondary metabolites could be performed with the help of different methods, that provide a graphical representation of the qualitative changes in secondary metabolites. Chromatograph approaches are obviously the most useful tool to reach the fingerprint target in terms of sensitivity, and even they are fast with high information content. The advantage of using this technique is to study the changes in chromatographic pattern without knowing the components of an unknown compound (Dunn and Ellis 2005). Chemical fingerprinting using chromatography is an important tool to study different patterns of plant metabolic variations in different conditions. Chemical fingerprinting coupled with chromatography separation is used for analyzing various components, that are existing in number of traditional medicines and various medicinal plants (Sermakkani et al. 2012).
Recently, biological insights have also been obtained from fingerprinting analysis of abiotic stress (Bailey 2003). These techniques are proved to be useful for analysis of the metabolic profile of plants under various environmental stress like conditions. Kuleff et al. (2003), also made a mention that by using chemical fingerprinting, plants can be demarcated on the basis of their species and geographical strain. It has various applications:(a) evaluation of different chemical components in different stress conditions; (b) the possibility to compare different groups of plants.
Lantana camara is a noxious and ornamental weed form family Verbenaceae and it is commonly known as red sage or wild sage is an evergreen strong smelling shrub (Thamotharan 2010) native to the American tropics. It has been expanding in various regions of the world and also includes India. There is very little knowledge about the various mechanisms involved in the tolerance of Lantana camara to stressful environmental conditions. Knowledge of these mechanisms of this invasive plant may give us idea about its growth and development in variety of areas under stressful conditions which can help us in controlling the growth of L. camara. Thus, the present study was carried out in order to know the possible cause of vast adaptability and invasiveness of the plant L. camara. It was hypothesized that there is a production of various cell metabolites in these plants in response to stress conditions which provide adaptation against stress. Therefore, we have studied the changes in chemical fingerprint profile using AKTA prime Plus chromatography system to obtain chemical fingerprints of Lantana under different temperature conditions in their natural environment.
MATERIALS AND METHODS
Plant material
The samples (leaves/flowers, in 10 batches) of Lantana camara : pink and violet that were at reproductive stage and growing in natural conditions, were collected from four different areas of Jalandhar. Varieties of the plant species can be differentiated by its flower color. Area of Jalandhar experiences three different type of seasons in year i.e. low temperature (February), moderate temperature (March), high temperature (April) (Table.1). This region experiences extremities of cold and hot. Jalandhar is located at a latitude of 71°31’ east and longitude of 30°33’ north. The experiments were conducted from February 2018 to April 2018. The temperature of the days was recorded (given in table). Random sampling was then conducted so that the study remain unbiased. Plant samples were pooled together for analysis. The sampling sites were marked previously so that no bias could occur in our study.
Extraction
The collected tissues of Lantana camara (leaves and flowers, 5g) were homogenized in 15ml of each of the solutions (1,2-Dichloroethane/Acetone/Ethanol/ Methanol/Water). The crude extracts were then centrifuged for 15min at 10,000rpm at room temperature. The supernatant was collected. An appropriate aliquot of each extract was filtered in 0.2µm (PALL Life Sciences) syringe filters, stored in 2 ml storage vials at -20°C prior to further analysis.
Chemical fingerprinting
Metabolic fingerprint analysis above filtered supernatants was carried out using AKTA prime PLUS system (GE healthcare, Sweden), equipped with gradient pump, auto sampler, and Prime view 5.0 software for data acquisition and processing. The analysis was carried out using HiTrapTM column (5 x 5 ml)with flow rate 0.5 mLmin-1, operating pressure, 1MPa (145 psi). The fingerprints were registered at wavelength λ = 254 nm and an aliquot of 1 ml was injected for acquiring chromatograms. A pre-equilibration period of 10 min with 20% ethanol was used between individual runs.
Identification and Quantization
The eight chemical standards — Rutin (in methanol), Phenylalanine (in distilled water), Glutathione Reduced (in distilled water), Glutathione Oxidized (in distilled water), Tannic acid (in ethanol), Glycine Betaine (in ethanol), Proline (in ethanol) and Vanillic Acid (in methanol) were prepared in appropriate solvents. The concentrations of standards used for calibration were 5, 10, 15, 20, and 25 (mg/ml). The standard curves were performed by analyzing standard reference solutions in triplicate and calibrated using linear regression equation derived from the peak areas. The limit of detection (LOD) and limit of quantification (LOQ) for each analyte at S/N of 3 and 10, respectively. All the solutions were filtered through 0.2µm filters prior to analysis. Identification of analytes namely Glycine Betaine, Rutin, Phenylalanine, Glutathione Reduced, Glutathione Oxidized, Tannic Acid, Proline and Vanillic Acid was conducted by comparing their retention times with those of the extracts. Quantization was established by preparing calibration curves with pure analytes.
Lipid Peroxidation (LP) and ROS staining
LP was estimated by measuring malondialdehyde (MDA) level. MDA content was calculated by given equation: MDA (µMgm-1 FW) = [(A532 - A600 ) / 156] x 103 x dilution factor. Histochemical detection of ROS (H2O2) was done by using 3,3’-Diaminobenzidine (DAB) as the chromogenic substrate. Plant material was collected at random (n=10). The leaves were immersed in DAB (1mg/ml) staining solution for detection of H2O2 The petri plates were wrapped in aluminium foil and kept overnight at room temperature. H2O2 was visualized as reddish brown stains formed on the leaves by the reaction of DAB with the endogenous H2O2.
RESULTS AND DISCUSSION
Invasive alien plants have encroached various ecosystem worldwide and affected biodiversity and crop productions at large. However, their biological mechanism to tolerate abiotic conditions under natural environment is still a matter of conjuncture. It is well known that major environmental stresses like heat, cold, drought dramatically affect metabolite synthesis through the synthesis of physiological and biochemical responses in plants. For plants, any external factor that exerts negative influence and produces changes and responses may be considered stressful. Being naturally present in plant (leaves and flowers), secondary metabolites and derivatives can be analyzed and used as stress indicators. In this study the effect of abiotic stress like cold and heat under natural conditions on changes in metabolic profiles by chemical fingerprint was studied in invasive alien plant Lantana camara .
Lipid peroxidation and ROS imaging
Chemical fingerprint analysis
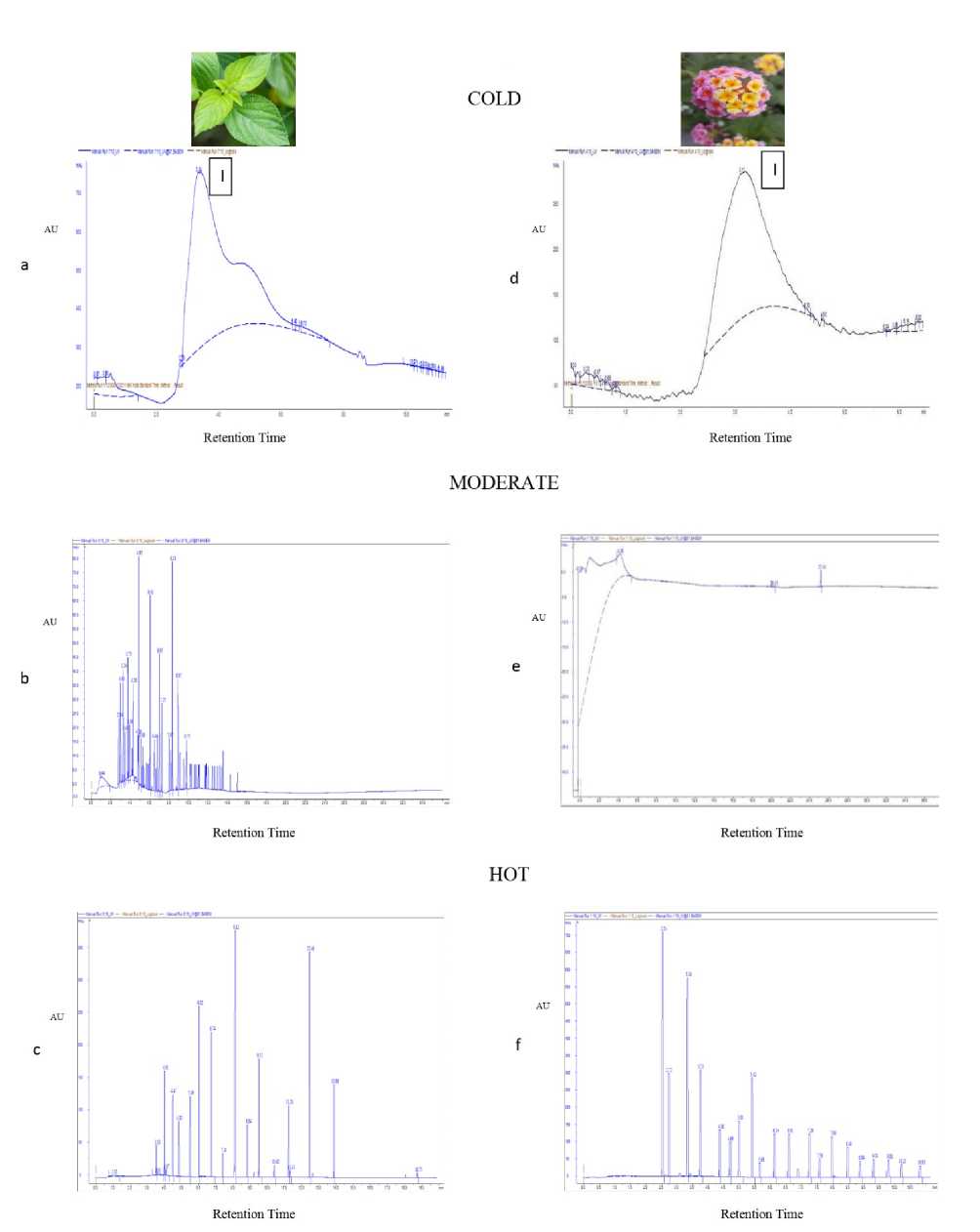
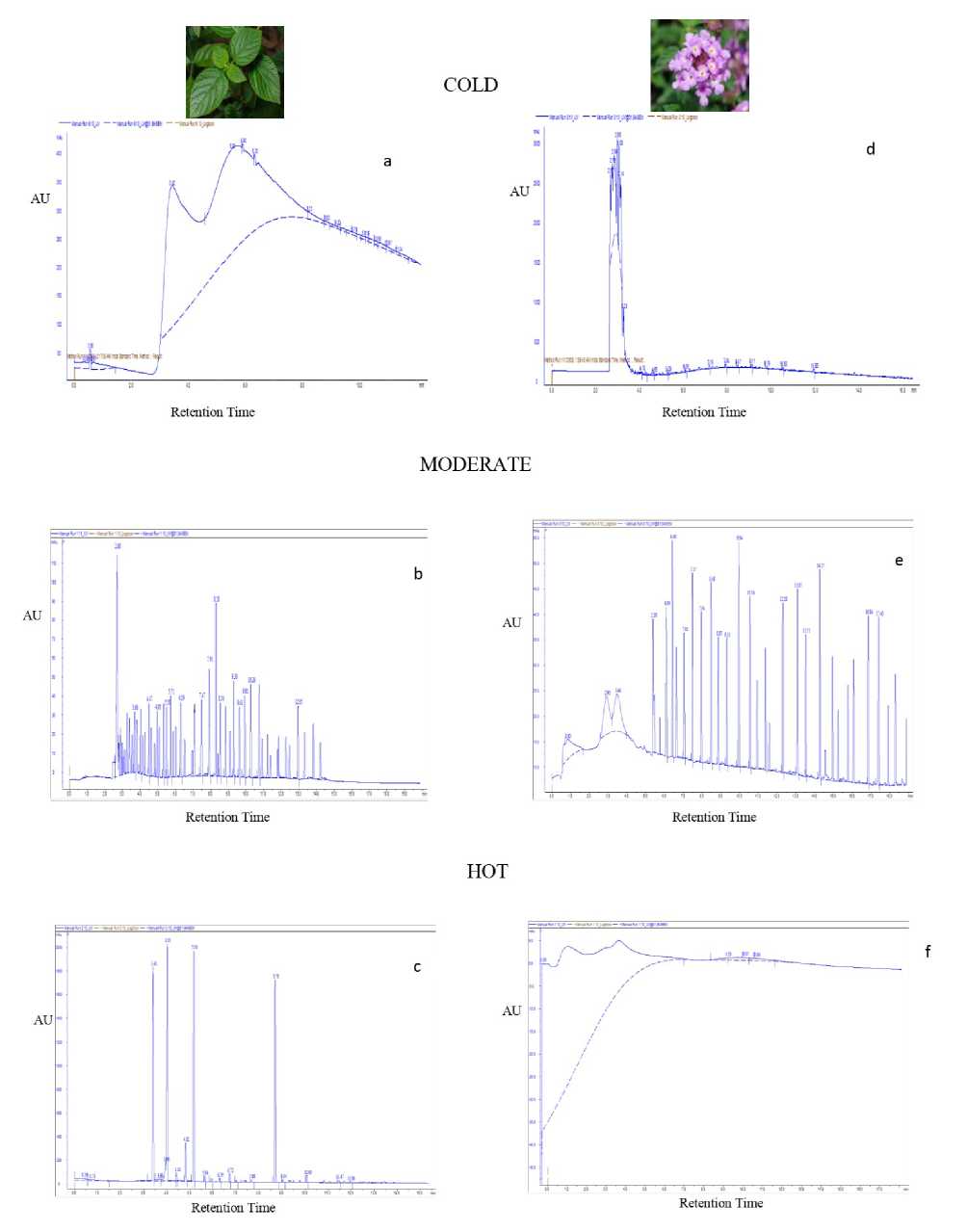
In the present study, Chemical Fingerprinting Profile of flowers and leaves of both the varieties of Lantana camara under different temperature regimes were studied in four different solvents: 1,2-Dichloroethane, Acetone, Ethanol, Methanol. As the three solvents, Acetone, Ethanol & Methanol did not give much information about the chemical fingerprint profile of this plant; we have not proceeded with these three solvents. Therefore, our whole study of Chemical Fingerprinting is based on the solvent - 1,2-Dichloroethane (Fig.3-4). Chemical fingerprint analysis of L. camara extracts (both leaves and flowers) revealed substantial amount of significant secondary metabolites as evident from chromatogram peaks (Fig. 3, Table 2) under adverse abiotic conditions. Results also indicated that the contents of secondary metabolites varied greatly among Lantana varieties samples collected in different environmental conditions. Different fingerprint profiles among both varieties were quite expected. It is plausible that plants collected at different times may considerably differ in chemical components, thereby, resulting in different profiles. It is generally believed that these differences are essentially caused by various environmental conditions where the plants are grown and harvested. In case of pink leaves, number of chromatographic peaks (major and minor) was detected in environmental condition dependent manner (Fig. 2). For instance, under both adverse conditions as compared to moderate temperature, although numbers of peaks were less, however, total area under peak was substantially higher by many folds (Table 1).
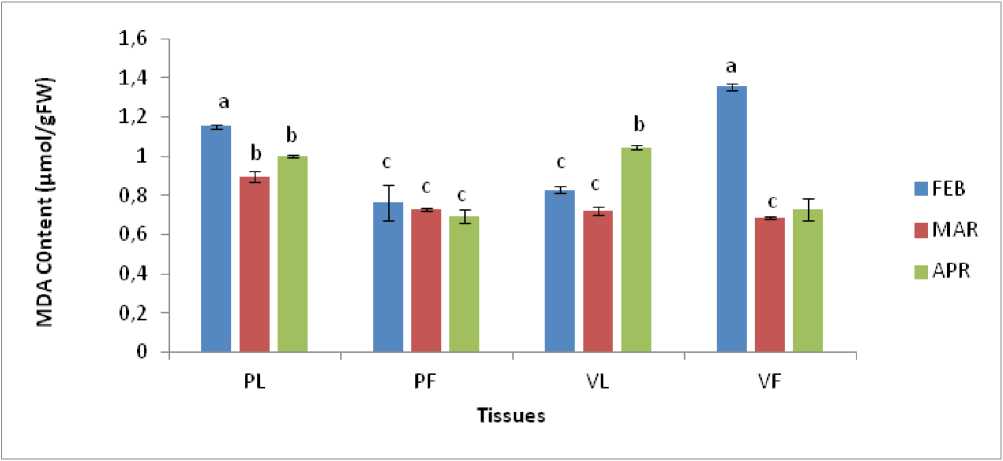
Figure 1: MDA Content in leaves and flowers of Lantana camara under conditions of different temperature regimes.
Symbols used PL=Pink leaves, PF=Pink flowers, VL=Violet leaves, VF=Violet flowers. Values are means of three replicates±SE. Means with different letters are significantly different at P<0.05 using Tukey’s multiple range test.

COLD MODERATE HOT
Figure 2 : Representative image of ROS histochemical staning of leaves of Lantana camara Pink (A) and Violoet varieties (B). [n=10]
Retention Time
Retention Time
COLD
Retention Time
Retention Time
Retention Time
HOT
Retention Time
MODERATE
Figure 3: Chemical Fingerprint Analysis of Pink leaves (a, b, c) and flowers (d, e, f) extracted in 1,2-Dichloroethane in the three different temperatures, where 'I' represents Glycine betain (having content 8mg/gFW in pink leaves and 9.7mg/gFW in pink flowers).
MODERATE
AU
Retention Time
AU
Retention Time
Retention Time
Retention Time
HOT
AU
AU
AU
AU
Retention Time
Retention Time
COLD
Figure 4: Chemical Fingerprint Analysis of Violet leaves (a, b, the three different temperatures.
c) and flowers (d, e, f) extracted in 1,2-Dichloroethane in
Table 1: Temperature and day length during different months in Jalandhar area.
|
Month |
Max. Temperature (C) |
Min. Temperature (C) |
Day Length |
|
February |
19° |
5° |
9h 47min |
|
March |
25° |
14° |
12h 13min |
|
April |
40° |
26° |
13h 18min |
Table. 2: Chemical fingerprinting data of two varieties (pink & violet) of Lantana camara extracted in 1,2-Dichloroethane
|
S.No. |
Variety |
Temperature |
Total no. of detected peaks |
Total Area (mAu*min) |
Ratio (Peak Area/Total Area) |
|
1. |
Pink Leaves |
Cold |
20 |
710.05 |
0.9994 |
|
Moderate |
241 |
48.90 |
0.7097 |
||
|
Hot |
158 |
113.61 |
0.9832 |
||
|
2. |
Pink Flowers |
Cold |
20 |
166.99 |
0.9998 |
|
Moderate |
225 |
68.61 |
0.9818 |
||
|
Hot |
89 |
167.96 |
0.9734 |
||
|
3. |
Violet Leaves |
Cold |
32 |
669.80 |
0.9941 |
|
Moderate |
167 |
84.72 |
0.5786 |
||
|
Hot |
95 |
435.89 |
0.9627 |
||
|
4. |
Violet Flowers |
Cold |
178 |
525.85 |
0.8987 |
|
Moderate |
148 |
46.47 |
0.7372 |
||
|
Hot |
131 |
655.53 |
0.9696 |
Notably, under cold conditions, one major peak was detected with very large height and area which was absent in moderate temperature, indicating enhanced accumulation of metabolites under stress conditions. In pink flowers, as compared to moderate temperature, many minor and a very few major peaks were detected in adverse conditions (Fig. 3). Notably, under cold conditions, major peak having high peak area was detected which was totally absent in sample being collected under moderate temperature conditions. Similarly, in hot conditions, many minor peaks with high peak area were detected. Fingerprint data of violet leaves and flowers also revealed similar data with many minor and major peaks. Under adverse conditions, although number of peaks decreased, however, total area increased dramatically along with emergence of few major peaks. Notably, under cold conditions, two large peaks with very high area and height were detected which was absent in moderate conditions (Fig. 4).
The chromatographic fingerprints showed abundant diversity of chemical constituents which were further analyzed by identification and quantization in tissues. So eight analytes viz. Glycine Betaine , Rutin, Phenylalanine, Glutathione Reduced, Glutathione Oxidized, Tannic Acid, Proline and Vanillic acid were unambiguously recognized by comparing their retention times with those of the extracts. Notably only one analyte i.e. Glycine Betaine was detected under only under cold conditions (Fig 3). Guerrini and Sacchetti (2014) have also made a mention that type of plant fingerprint, that allow to better understand the grown capacity of a plant species, is dependent on a particular environment to which plant is exposed. It was cited that genetic and environmental factors influence metabolic pathway and produce different pattern chemical composition thereby causing variation in fingerprint. Therefore, on the basis of above findings, it could be speculated that more number of secondary metabolites are producing in cold and hot conditions in L. camara which showed that these metabolites favored the growth of this plant in both adverse conditions. Similar results were obtained by Lin et al. (2014), when studied the chemical fingerprint analysis of Phyla nodiflora extract prepared in methanol, where they found that contents of active compounds varied greatly among the samples collected at different periods, especially, the contents being higher in samples collected in the summer.
CONCLUSION
It can be concluded from the results that malondialdehyde (MDA), coupled with ROS accumulation, is an oxidative stress indicator that plant is experiencing in unfavorable conditions.. Large number of major and minor peaks that were observed in chromatograph of L. camara showed the presence or production of certain metabolites in plant species during extreme conditions as compared to cold condition, therefore, suggesting the role of these metabolites in providing protection or adaptation in plant species against certain extremities.
ACKNOWLEDGEMENTS
As the corresponding author, AD Sharma thanks Governing Council, of College for this research grant.
DISCLOSURE CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Список литературы Chemical fingerprint based involvement of plant metabolites and osmoregulatory solutes in providing abiotic stress tolerance to invasive plant lantana camara
- Apel K, Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annual Review of Plant Biology 5: 373-399
- Bailey JC, Oven M, Holmes E, Nicholson J, Zenk H. (2003). Metablomic analysis of the consequences of cadmium exposure in Silene cucubalus cell cultures via 1H NMR spectroscopy and chemometrics. Phytochemistry 62: 851-858
- Ballhorn DJ, Kautz S, Jensen M, Schmitt S, Heil M, Hegeman AD. (2011). Genetic and environmental interactions determine plant defenses against herbivores. Journal of Ecology 99: 313-326
- Dunn WB, Ellis DI. (2005). Metabolic current analytical platforms and methodologies. Trends in Analytical Chemistry 24: 258-294
- Esslinger S, Riedl J, Fauhl-Hassek C. (2014). Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Research International 60: 189-204
- Falk KL, Tokuhisa JG, Gershenzon J. (2007). The effect of sulfur nutrition on plant glucosinate content: physiology and molecular mechanisms. Plant Biology 9: 573-581
- Goel A, Sheoran IS. (2003). Lipid peroxidation and peroxide scavenging enzymes in cotton seeds under natural aging. Biologia Plantarum 46: 429-434
- Grauwet T, Vervoort L, Colle I, Van Loey A, Hendrickx M. (2014). From fingerprinting to kinetics in evaluating food quality changes. Trends in Biotechnology 32: 125-131
- Guerrini A, Sacchetti G. (2014). Chemical Fingerprinting of Medicinal and Aromatic Plant Extracts: HP-TLC Bioautographic Assays as Preliminary Research Tool to Match Chemical and Biological Properties. Medicinal and Aromatic Plants 3: 1-3
- Henkel SK, Kawai H, Hofmann GE. (2009). Interspecific and interhabitat variation in hsp 70 gene expression in native and invasive kelp populations. Marine Ecology Progress Series 386: 1-13
- Kuleff I, Djingova R, Markert B. (2004). Chemical fingerprinting of plants. Ecological Research 19: 3-11
- Lin FJ, Yen FL, Chen PC, Wang MC, Lin CN, Lee CW, Ko HH. (2014). HPLC Fingerprints and antioxidant constituents of Phyla nodiflora. The Scientific World Journal 2014: 528653
- Lu P, Sang WG, MA KP. (2008). Differential responses of the activities of antioxidant enzymes to thermal stresses between two Eupatorium sp. in China. Journal of Integrative Plant Biology 50: 1744-7909
- Scheel GL, Pauli ED, Rakocevic M, Bruns RE, Scarminio IS. (2016). Environmental stress evaluation of L. leaves from spectrophotometric fingerprints by PCA and OSC-PLS-DA. Arabian Journal of Chemistry 2016: 1-7
- Selmar D, Kleinwachter M. (2013). Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant and Cell Physiology 54: 817-826
- Sermakkani M, Thangapandian V. (2012). GC-MS Analysis of Cassia italica leaf methanol extract. Asian Journal of Pharmaceutical and Clinical Research 5: 90-94
- Sharma A, Mamik S. (2017). Antioxidant response of the invasive alien species Parthenium hysterophorus L. under abiotic stress conditions with special emphasis on boiling stable antioxidant enzymes. Botanica Serbica 41: 25-36
- Thamotharan G, Sekar G, Ganesh T, Sen S, Chakrabarty R, Kumar S. (2010). Antiulcerogenic effect of Lantana camara leaves on in vivo test models in Rats. Asian Journal of Pharmaceutical and Clinical Research 3: 57-60
- Thankamani CK, Chempakam B, Ashokan PK. (2003). Water stress induced changes in enzymatic activities and lipid peroxidation in black pepper (Piper nigrum). Journal of Medicinal and Aromatic Plant Sciences 25: 646-650
- Vranova E, Inze D, Breusegem FV. (2002). Signal transduction during oxidative stress. Journal of Experimental Botany 53: 1227-1236


