Chromium Induces Genotoxicity in Root Tip Cells of Grass pea (Lathyrus sativus L., Variety Nirmal): A ROS-mediated Acute Toxicity Study
Автор: Dipan Adhikari
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.17, 2021 года.
Бесплатный доступ
Background: Heavy metal-induced pollution of water bodies has emerged out as a major environmental menace for the modern world in the twenty first century. Many industrial waste waters contain heavy metals including Chromium, which plays a major role in polluting our water and agricultural sustainability in the long run. Due to heavy anthropogenic manoeuvres chromium is released as a waste product from various industries such as electroplating, battery and smelters, leather tanning, textile printing etc. The compounds of Chromium have been known to be strong carcinogens and mutagens that can reach the target organs of human through drinking water and agricultural crops. Chromium is often admixed with industrial effluents that are used for irrigation. Purpose: The uptake of excess concentrations of heavy metals through this effluent irrigation adversely affects plant growth and development. The alternation in plant growth is correlated with the disruption of the physiological disturbances and genotoxicity in plant cell. Results: After the exposure to chromium at five concentrations (12.5, 10, 7.5, 5, and 2.5 mM) respectively the seed germination was adversely affected along with root length inhibition. At higher doses (5 mM onwards) chromium exhibited nucleolar disintegration (by AgNOR protein leaching). In germinating root tip cells above suboptimal concentration (2.5 mM) chromium stands out as potential Phyto-genotoxicant with other toxic effects i.e., lipid peroxidation, electrolyte leakage due to membrane disruption, ROS generation (histological staining of hydroxyl and superoxide radical generation) root cell apoptosis (by Evans blue staining) and disruption of root metabolic activity by inhibition of dehydrogenase activity (by 2,3,5-Triphenyl tetrazolium chloride (TTC) staining methods). Conclusion: These observations constitute a warning signal about the risks of the widespread and increasing presence of chromium into environment especially in agricultural point of view which demands a high throughput evaluation of chromium for its effects on other organisms, even on human health, due to large use of chromium compounds in different gadgets. Lathyrus sativus L. is an excellent model plant for the study of environmental ecotoxicology of different genotoxicants. Implication: Regulatory monitoring and assessment of plant health is necessary for the better understanding of mechanism of action of chromium and to reduce Cr contamination through seeds and the resultant vital genome loss is cash crops.
Grass pea, Chromium contamination, inhibition of seed germination, root length inhibition, lipid peroxidation, ROS outburst and metabolic inhibition, nucleoluar disruption and AgNOR
Короткий адрес: https://sciup.org/143173893
IDR: 143173893
Текст научной статьи Chromium Induces Genotoxicity in Root Tip Cells of Grass pea (Lathyrus sativus L., Variety Nirmal): A ROS-mediated Acute Toxicity Study
Chromium (Cr) is placed in the (VI-B) group in the modern periodic table 9 Avudainayagam et al., 2003)[1] of transition elements. Chromium has a range of oxidation numbers [Cr (II) to Cr(VI)] during different oxidation state. Chromium has a density of 7.19 g/cm3. It is being defined as a heavy metal because of 51.10 g/M molecular weight, and an atomic number 24. This metal has also been assigned the 21st position among the most abundantly available metals on the earth’s crust (Babula et al., 2008). The trivalent [chromite; Cr (III)] and the hexavalent [chromate; Cr (VI)] is known to be the most chemically stable Cr species (Zayed and Terry, 2003). In the oxygenated environment Cr (III) into Cr (VI) conversion takes place naturally and this hexavalent form of Cr is considered to be potentially most strongly oxidizing ion in the oxidation-reduction reactions. This hexavalent form has higher water solubility, mobility, and bioavailability which pass on the most toxic attributes in this form of Cr in comparison to other active Cr species ( Becquer et al., 2003). In the last century use of chromium has been excessively increased owing to its high demand and increasing use in various small, middle and heavy industries all over the globe (Shahid et al., 2017). India tops the position in terms of Cr usage in the world. (Pandey et al., 2016; Mukherjee et al., 2017). Several heavy, middle and small-scale industries contribute heavily to the hyper-accumulation of Cr putting forth eventually an alarming threat for the biological world.
It has been established that Cr (VI) and Cr (III) present different chemical, toxicological, and epidemiological manifestations in different organisms (Kimbrough et al., (1999). Cr (VI) is a powerful carcinogen for human beings (IARC Monographs, 1990). Cr (VI) has been reported to be toxic to different members of angiospemic plants (Shanker et al., 2005) aquatic animals (Velma et al., 2009), and soil microflora (Petrilli and De Flora, 1997). Plants are the sessile organisms which receive the first thrust because of increasing load of heavy metals’ in contaminated rhizosphere and unable to control their entry in their systems through roots from the very early stages of germination. In plants, particularly in different cash crops, Cr at low concentrations (0.05– 1mg L-1) was found to promote growth and increase yield. Ironically this heavy metal is not an macronutrient and not to be considered as essential to plants (Peralta-Videa et al., 2009: Paiva et al., 2009). In this context, accumulation of chromium in edible plants may represent a potential hazard to animals and ultimately for humans. Increased Cr accumulation in the agricultural land ultimately brings forth a disruption in plant growth and development at the cellular, organ, and tissue toxicity and ultimately at the genetic level bringing genomic losses (Wakeel et al., 2018).
In order to detect the effect of environmental toxicity in terms of heavy metal stress some quick, cost effective and precisely reproducible screening methods of bioassays are seriously warranted for environmental monitoring too. Due to the highly conserved nature of the genetic material, a broad variety of plant genera are being employed in such test as the first line of evaluation of xenobiotics including different heavy metals time to time. Response of plants to mutagenic treatments can be evaluated at different levels of organizations i.e., DNA, chromosome and genome in addition to the whole organism exhibited at the biochemical and morphological changes. Higher plants like Lathyrus sativus L.; though an “orphan crop”, represents an excellent model to access the effects of heavy metal-induced physiological, biochemical and cytogenetic toxicity (Adhikari, 2019) over Vicia faba (Cavusoglu et al., 2010), Hordeum vulgare (Gecheff, 1996) Crepis capillaris (Grant and Owens, 1998) and Allium cepa L. (Adhikari, 2019, etc., because of its chromosome complement (2n=14, bimodal karyotype) which gives a fine blend of physiological and genotoxic responses to different stress inducers including heavy metal stress, (Adhikari et al., 2020). Since the potential of in-situ monitoring of heavy metal toxicity especially of chromium has long been recognized in other crop plants, the present investigation was aimed to study the cytotoxic effects of graded concentrations of chromium (VI) on Lathyrus sativus L. to investigate the extent and degree of appearance of cellular responses found in different assays like membrane leakage, membrane lipid peroxidation, root cell oxidizability, cellular death in addition to seed germination and root length inhibition and interphase silver-stained nulceolar organizer regions (Ag-NOR) and associated changes in nuclear cytology (Leaching of nuclear proteins) as biomarkers of environmental bio monitoring to elucidate the mechanism of action of Chromium toxicity for other germinating cash crops in vitro.
MATERIALS AND METHODS
Plant material procurement and treatment of the test compound.
Lathyrus sativus L., (Variety Nirmal) seeds were used in the experiments. Nirmal is a commercial cultivar of winter common (Locally known as Khesari) obtained Pulse and Oilseed Research Station, Baharampur, Murshidabad, Ranibagan, Khagra, Berhampore, West Bengal 742101. The seeds were surface disinfected with 1% sodium hypochlorite for 5 min and vigorously rinsed with distilled water. Then they were 24 hours treated with K 2 Cr 2 O 7 (Potassium dichromate, SIGMA ALDRICH), olecular weight = 294.185 g mol-1. Fiver serially diulted concentrations (12.5 mM, 10 mM, 5mM, and 2.5mM respectively, 24 hrs imbibitions) have been prepared for each Potassium dichromate and were used for seed treatment. Control was set up by seed immersion in distilled water. The treated seeds were placed on moist filter paper in covered Petri dishes and then maintained in dark, at 23°C, in order to germinate. The seeds were then spread over moist cotton kept in Petri-dishes (15 cm diameter) at 24 ±2 ° C temperatures for further observation (Adhikari et al., 2020).
Determination of Germination percentage and measurement of radicle length in Lathyrus sativus L:
The germination potential of seeds and radicle (embryonic root) length (measured using a millimeter ruler) were analyzed at every 24 h interval up to 72 hrs. The experiment was repeated three times under similar conditions. For Lathyrus sativus L ., the seed germination percentage was measured (after a span of 48 hrs). The germination percentage was measured as Germination %= germinated seed/total seeds x 100. (Adhikari et al., 2020).
Determination of Membrane Permeability/ Electrolyte Leakage after Cr treatment on etiolated roots of Lathyrus sativus L.
Electrolyte leakage (EL) was assayed by measuring the ions leaching from tissue into deionised water. Fresh root samples (treated and control; 100 mg) were cut into small pieces (about 5 mm segments) and placed in test tubes containing 10 ml deionised water. Tubes were kept in a water bath at 32 ° C for 2 h. After incubation, electrical conductivity (EC1) of the bathing solution was recorded with an electrical conductivity meter (Systronics M-308, Kolkata, India). The samples were then autoclaved at 121 °C for 20 min to completely kill the tissues and release all electrolytes. Samples were then cooled to 25 ° C and final electrical conductivity (EC2) was determined. The EL was expressed as a percentage by the formula, EL%= EC1/EC2X100. (Adhikari et al., 2020).
Determination of Tissue Lipid Peroxidation on etiolated roots of Lathyrus sativus L.
i Histochemical analysis : The Schiff’s reagent was used for histochemical detection of lipid peroxidation level in roots of Lathyrus sativus L. (Pompella et al., 1987). The germinating roots (after 72 hrs) were incubated in Schiff's reagent for 60 minutes. The roots were then rinsed with 0.5% K 2 S 2 0 5 (w/v) (prepared in 0.05 M HCl) until the root colour became light red. All stained roots were then examined and pictures were captured using bright-field single-shot mode at 10× magnification (Olympus CH20i microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software.
ii Spectrophotometric determination : Lipid peroxidation, an indicator of tissue injury induced by reactive oxygen species was measured as thiobarbituric acid reactive substance roots of Lathyrus sativus L. (TBARS) [22]. The amount of tissue TBARS was be measured by the thiobarbituric acid assay (TBA) as previously described in the method (Adhikari et al., 2007) . The results were expressed as µmol TBARS/g wet tissue weight.
Evaluation of metabolic/mitochondrial activity
TTC (2,3,5-Triphenyl tetrazolium chloride) staining is a novel approach to check the viability of cells. The seeds of Lathyrus sativus L., were treated with different concentrations of K2Cr2O7 solution for 24 h. The same set-up was followed for positive and negative control taken as 0.1% hydrogen peroxide and distilled water, respectively. All the roots were immersed in 0.5% (w/v) TTC stain for 5 h in dark. Subsequently, the roots were washed in distilled water. Absorbance was measured at 490 nm using a spectrophotometer against 95% ethanol as blank. We considered Positive Control (Hydrogen Peroxide O.D.) as 100% metabolic/Respiratory activity and respective test O.D.s were converted into subsequent activity (in terms of metabolic activity percentage) (Adhikari et al., 2020).
Cell death measurement and visualization with Evans Blue staining.
Ten germinating roots of Lathyrus sativus L., after incubations at different treatments in K 2 Cr 2 O 7 solution plus controls were incubated in a 0.25% aqueous Evans blue solution for 15 min at room temperature, according to the protocols of with little modification (Vijayaraghavareddy et al., 2017). The roots were then washed twice with double-distilled water and left in distilled water overnight. All stained roots were then examined and pictures were captured using bright-field single-shot mode at 10× magnification (Olympus CH20 microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software.
Histochemical staining for hydrogen peroxide and superoxide
-
i. NBT staining for histochemical detection of Superoxide:
To detect the presence of superoxide in root apex grown after incubation (with different concentrations of K 2 Cr 2 O 7 solution for 24 h) of the seeds of Lathyrus sativus L. after 72 hrs of germination, were incubated for 5 min in the staining solution of 300 μM nitroblue tetrazolium salt (NBT; Fluka,Germany) dissolved in 0.1M Tris-HCl,0.1M NaCl,0.05M MgCl 2 (pH= 9.5) with minor modifications (Daudi et al., 2012). NBT stained root tips were observed for the presence of superoxide using a light microscope. All stained roots were then examined and pictures were captured using bright-field single-shot mode at 10× magnification (Olympus CH20i microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software.
-
ii. DAB staining for histochemcial detection of H 2 O 2
To detect the presence of superoxide in root apex grown after incubation (with different concentrations of
K 2 Cr 2 O 7 solution for 24 h) of the seeds of Lathyrus sativus L., after 72 hrs of germination were then stained with 1% (W/V) 3-diaminobenzinidine (DAB; pH 3.8) for 1 hr and subsequently rinsed with deionized water to detect the presence of H 2 O 2 ( Thordal-Christensen et al., 1997). All stained roots were then examined and pictures were captured using bright-field single-shot mode at 10× magnification (Olympus CH20i microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software.
AgNOR staining of Lathyrus sativus L., root tip cell resting nucleolus
Twenty root tips of Lathyrus sativus L. (to observe nucleoli changes in germinating root tip cells after Cr pre- treatment for 24 hrs), were cut, fixed in 3 parts of 95% ethanol: 2 parts of acetic acid for overnight and hydrolysed in 1M HCL, 95% ethanol and acetic acid (5:3:2, v/v) for 10 minutes at 60°c, followed by squashing in 45% acetic acid , dried in an oven at 40°c, and after 2 days stained with 50% of Silver nitrate (w/v) (Shi et al., 2017). Slides were then observed under microscopes. All stages were examined at 10 x, 40 x and 100 x oil immersion objective using a 100 × eyepiece of a compound microscope (Olympus CH20 microscope) fitted with CMOS Camera (IS 500, 5.0 MP) and its attachment with a computer with the aid of VIEW 7 image analysis software.
Statistical Analyses
All the values are presented as Mean ± SD (standard deviation, n=6). Statistical analyses were performed with analysis of variance (ANOVA) followed by post-hoc Dunnet’s Test. The p values less than 0.05 were considered significant. In the statistical analysis, differences between the groups were tested by analysis of variance (ANOVA) in GRAPH PAD PRIZM-version 6 computer program.
RESULTS
Determination of Germination percentage after Cr treatment
At 72 h after germination following Cr treatment, in comparison to control group, 2.5 mM promoted rapid seed germination and could augment insignificant change in seed germination inhibition. However, from 5 mM onwards i.e., 7.5, 10 and 12.5 mM cromium induced significantly inhibition of seed germination was accounted when compared to control (p< 0.001). At 12.5 mM chromium exposure (the highest concentration tested significantly inhibited seed germination (p< 0.001) compared to control which was found to be less than 20%. (Figure:1a)
Effect of Cr treatment on root length inhibition
In untreated seeds of Lathyrus sativus L., the root length increased with the increase in time after 72 h (Figure 2). In comparison to control group, 2.5 mM could not hamper root length inhibition. A significant decrease in root length were accounted when compared to control (p< 0.001) in seeds exposed to 5, 7.5, 10 and 12.5 mM of Cr treatment after 72 h in comparison to control. (Figure: 2)
Determination of disruption of membrane permeability/electrolyte leakage after Cr treatment on etiolated roots of L. sativus L.
In untreated seeds of Lathyrus sativus L., the root electrolyte leakage was minimal which was below 15% (Figure 3). After incubation at different Cr treatments when the seeds were germinated after 72 hrs the etiolated roots could show a differential response in terms of electrolyte leakage/membrane permeability. There was a concentration dependent disruption of membrane leakage in a significant manner in etiolated roots of Lathyrus sativus L., in comparison to control (Figure 3).
Determination of Tissue Lipid Peroxidation on etiolated roots of L. sativus L.
a Histochemical analysis: Qualitative detection by colour formation (LPO) in growing root tips.
Enhanced lipid peroxidation upon Cr (VI) treatment was confirmed by the in situ histochemical localization with Schiff’s reagent, (Fig. 4a). In the control roots there was insignificant colour development after reactions with Schiff’s base in contrast to the treatment sets where with increasing concentrations of Cr pre-incubations significantly developed colour formation up on reactions with Schiff’s base. The increasing colour development confirmed the localization and intensity of LPO formation as a result of Cr induced membrane damage as a result of ROS formation (Figure 4a).
-
B. Quantitative estimation of MDA (TBARS) by spectrophotometric assay.
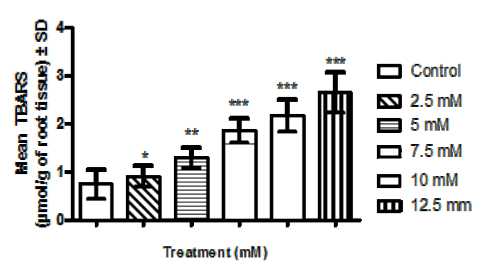
In this spectrophotometric quantification it was found that pre-incubation of seeds of Lathyrus sativus L. seeds upon germination produced significant increase in lipid peroxidation in a dose dependent fashion in comparison to control measured as TBARS formed (in terms of coloured MDA formed) as an end result (Fig 4b).
Cell death measurement and visualization with Evans Blue staining in germinating roots of L. sativus L.
Uptake of Evan’s blue by the root meristems of Lathyrus sativus L., as a parameter of cell death served as a biomarker of cytotoxicity. In this experiment it was found that Cr pre-incubation produced significant death in the germinating root meristem tissues in L. satiuvs L. The colour development (qualitative) was evident from the lower doses in the developing regions of the roots in 2.5 and 5 mM treatments. However the intensity of colour formation was much lesser that the higher concentration treated seeds. From 7.5 mM treated germinating roots both apical and developing regions are intensely coloured extending to permanent regions; after 12.5 mM of Cr treatment, the whole root becomes blue showing the death region extending from root cap to permanent region. denotes the extension and range of cellular death in the growing roots.
Evaluation of root metabolic activity (Root oxidizability test)
The effect different concentrations of chromium on root metabolic activity (dehydrogenase) activity on germinating roots of Lathyrus sativus L. TTC staining was employed in the present study as an indicator to evaluate the effect of plant extract on mitochondrial metabolism. In TTC staining 2, 3, 5-triphenyl tetrazolium chloride is reduced to red formazan by mitochondrial enzymes. The result showed a dose dependent decrease in mitochondrial activity as visualised by decrease in staining and absorbance in comparison to positive control (p< 0.001) (Fig. 6). Positive control remained unstained with minimum absorbance indicating least mitochondrial activity and negative control showed maximum activity. In the case of treatments, it was found that at 25, 5 and 7.5 mM there was an increase in root metabolic activity (dehydrogenase activation). But at 10 mM treatment there was significant lowering of root metabolic activity in comparison to positive control. But at the highest concentration i.e. at 12.5 mM treatment there was a total loss of root metabolic activity probably due to cellular poisoning and disruption of dehydrogenase activity (72 hrs).
Staining of Peroxyl and Superoxide Radical by Histochemical reaction in germinating root tips of L sativus L.
i NBT Staining for histochemical detection of Superoxide:
Here we tried to detect the distribution of superoxide in roots of Lathyrus sativus L using a histochemical staining method, NBT staining. When NBT compound reacts with superoxide, formazan visualized as blue precipitation in cells is immediately formed. Superoxide (O2•-) is known as one of ROS which controls cell proliferation. As shown in 7a , No NBT staining pattern in root apex region was observed in control set. However, we could observe that Cr pretreatment in germinating roots of Lathyrus sativus L strongly promoted the burst of superoxide generation after 72 hours of germination. Only at 2.5 mM treatment we could observe a lesser extent of colour development owing to lower concentrations of fromazan formation in comparison to other treatments.
ii Histochemical DAB Staining for hydroxyl radical Detection
Histochemical detection of ROS was performed for studying the specific localization of different free radicals and their pattern of accumulation in the tissue. As a result, it was observed that no accumulation of H2O2 took place in the control roots (Fig. 7b); but significant H2O2 localization was observed both at apical and growing region of the germinating roots of Lathyrus after imbibing the seeds with increasing doses of Cr, after 72 hrs. Also a significant accumulation of the H2O2 molecule at the growing region of the roots was detected. The levels of both H2O2 and O2-·, in the root elongation zone were significantly increased in a dose dependent manner, while in the maturation zone only at higher treatments (7.5, 10 and 12.5 mM) significant alteration in H2O2 and O2-· production was observed. At the higher concentration i.e. at 12.5 mM and 10 mM Cr pretreatment the root cap region was deeply stained, indicating that Cr toxicity affects the root cap also to exert cytotoxicity. The histochemical staining also showed increased staining density because of H2O2 and O2- production in roots with an increasing concentration of Cr treatment-induced acute ROS generation.
Silver stained nuclei and nucleoloar AgNOR detection in resting nucleus after sliver nitrate staining in growing root tips of L. sativus L.
Generally, there are 1 to 2 dark-brown nucleoli in the nucleus of L sativus L root tip cells (Figure 8A). The toxic effects of Cr (VI) induced acute toxicity on nucleol varied with the concentrations used. Three phenomena were observed under Cr induced stress. Firstly, at low concentration (2.5 mM Cr, 24 h incubation), in the growing root tip cells nucleoli were found to be irregular in morphology (Figure 8B showing irregular nucleoli with heteromorphic-paired micro-nuclei (PNhet) in comparison to control. Secondly, some tiny silver-stained particles together with the nucleolus were observed in the nucleus of some root tip cells exposed to 2.5, 5 and 7.5 mM Cr after 72 hrs in the germinating root tip cells (Figure 8D, F, G, H). Thirdly, the particles were accumulated and leached out from the nucleus to the cytoplasm after 10 and 12.5 mM of Cr exposure at growing root tips after 72 hrs of germination. The amount of this particulate material increased progressively in cytoplasm (Figure 8 G, H, and I) and nearly occupied the whole cytoplasm. At 10 and 12.5 mM exposure there was almost complete degradation of the nucleus and the whole nuclear proteins (AgNOR) leached out and the nuclear structure had been collapsed showing apoptosis and nucleolar degreadation (Fig 8 H and I). We could observe some tiny particulates containing the argyrophilic proteins in the nucleus of the root tips exposed to 5 mM Cr treatment (Figure 8D). More particulates were accumulated in it with increasing Cr concentration mainly on the nucleoli. The phenomenon was noted that some particulates containing the argyrophilic proteins were extruded from the nucleus into the cytoplasm in the group treated with 7.5, 10 and 12.5 mM Cr treatments. Figure 8C showed that the leaching materials were located near the nucleus. Finally, the material enclosed the nucleus i.e., the nucleolar proteins were extruded from the nucleus into the cytoplasm, leading to celluar apoptosis leading to cellular death (Fig 8I).
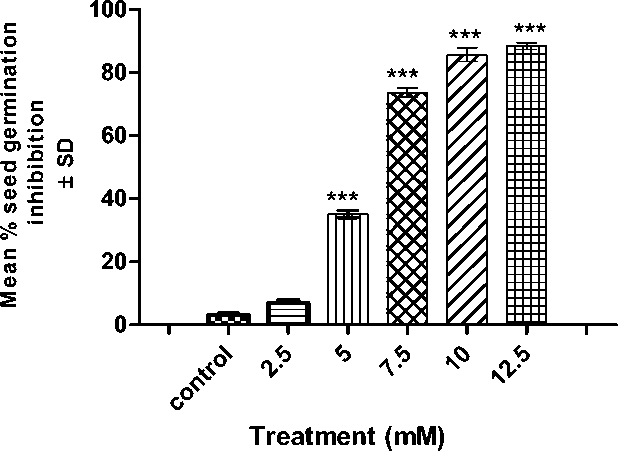
Figure 1a: Histogram showing seed germination inhibition after 48 hours of recovery from treatment. P verses control (P<0.0001) following ANOVA and Dunnet’s multiple comparison test with each treatment with control. Seed germination percentage of P. ovata with 48 h after Cr (VI) stress. The data are represented as mean ± standard error of mean (SEM) (n = 5). Asterisks denote the level of significance; *p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
lb. Effect of Cr treatment on seed germination (figure plates)

Picture lb: Pictorial representation of Seed germination inhibition after incubation at different concentrations of Cr salt (direct plating, after 48 hrs). A= Control, B= 2.5 mM
Figure 1b: Pictorial representation of Seed germination inhibition after incubation at different concentrations of Cr salt (direct plating, afier 48 hrs). A= Control, B= 2.5 mM treated germinating seeds, C: 5 mM treated germinating seeds, D: 7.5 mM treated germinating seeds, E= 10 mM treated germinating seeds and F= 12.5 mM treated germinating seeds. It was seen that at the from 7.5 ppm onwards there was inhibition of seed germination frequency and at 12.5 mM treatment it was found that there was almost cessation of seed germination in comparison of control.
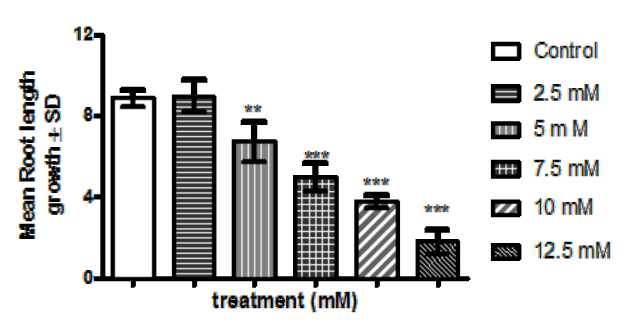
Figure 2. Histogram showing differences in root growth inhibition in L. sativus L. after 72 hours recovery (after treatment) in comparison to control. P versus control (a= <0.0001) after Dunnet’s multiple comparison test with control and all the treatments. The data are represented as mean ± standard error of mean (SEM) (n = 5). Asterisks denote the level of significance; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
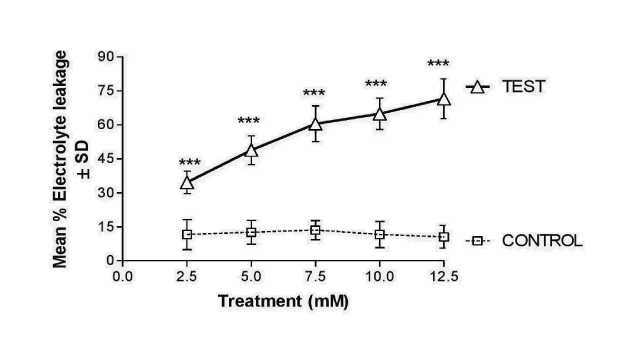
Figure 3. Line diagram showing the values are expressed as mean % electrolyte leakage ±SD (n=6), ANOVA is followed by post-hoc Dunnet’s test (vs control). Asterisks denote the level of significance; ***p ≤ 0.001.
a. Histocnemicai analysis: qualitative detection oy colour tormation (Lipid peroxidation) in growing root tips.
Control (Root tips showing LPO formation Treatment with different mM concentrations of after reaction with Schiff’s base after 72 chromium (Root tips showing LPO fonnation after hrs of germination). reaction with Schiff’s base after 72 hrs of germination).
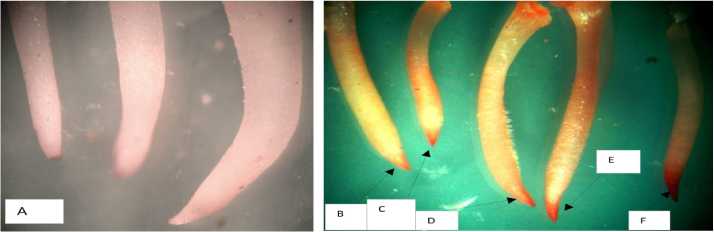
Figure 4a: Visualization and detection LPO in germinating Lathyrus sativus L. root tips after Cr (VI) treatment by histochemcial reaction with Schiff’s reagent. A= Control, distilled water treated germinating roots, B= 2.5 mM treated germinating roots, C= 5 mM treated germinating roots, L) 7.5 mM treated germinating roots, E= 10 mM treated germinating
Figure 4a: Visualization and detection LPO in germinating Lathyrus sativus L, root tips after Cr (VI) treatment by histochemcial reaction with Schiff‘s reagent. A: Control, distilled water treated germinating roots, B: 2.5 mM treated germinating roots, C: 5 mM treated germinating roots, D: 7.5 mM treated germinating roots, E: 10 mM treated germinating roots and F: 12.5 mM treated germinating roots. Arrow heads denote the region showing the occurrence of lipid peroxidation after reaction with Schiff‘s base.
Figure
4b. Histogram showing effect of different concentrations of Cr salt preincubation on germinating roots of Lathyrus sativus L. roots. Values are expressed as mean TBARS content (umol/g of fresh root tissue) ±SD (n=10),*P<0.05 by post-hoc Dunnet’s test (vs control), **P<0.001 by post-hoc Dunnet’s test (vs control) and ***P<0.0001 by post-hoc Dunnet’s test (vs control).
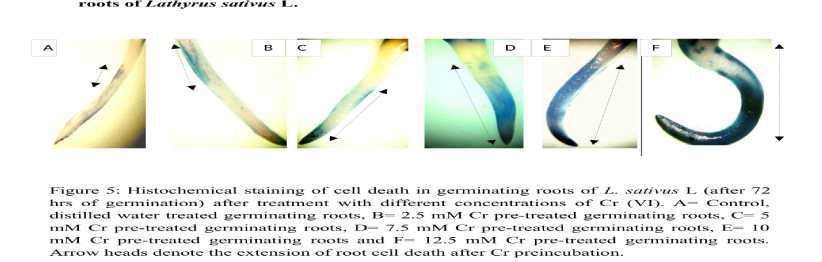
Figure 5. Histochemical staining of cell death (Evans Blue staining) in germinating roots of L. sativus L (after 72 hrs of germination) after pretreatment with different concentrations of Cr salt. A: Control, distilled water treated germinating roots, B= 2.5 mM Cr pretreated germinating roots, C= 5 mM Cr pretreated germinating roots, D: 7.5 mM Cr pretreated germinating roots, E: 10 rnM Cr pretreated germinating roots and F: 12.5 mM Cr pretreated germinating roots. Arrow heads denote the degree and extension of root cell death in germinating root cells.
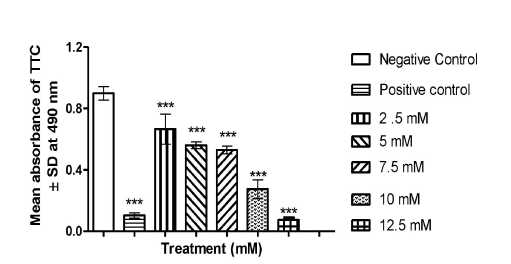
Figure 6. Determination of root metabolic activity (dehydrogenase activity by TTC staining) after chromium treatment in germinating roots of Lathyrus sativus L. TTC staining - Graph showing the effect of different concentrations of copper on mitochondrial activity. (NC) Negative control - Distilled water; (PC) Positive control - 0.1% hydrogen peroxide showing the mean O.D. of formazan produced in root tissues of L. sativus L.after 48 hours of growth due to root dehydrogenase activity. P verses negative control (**=<0.001, ***=<0.0001) following ANOVA and Dunnet’s multiple comparison test with each treatment with negative control.
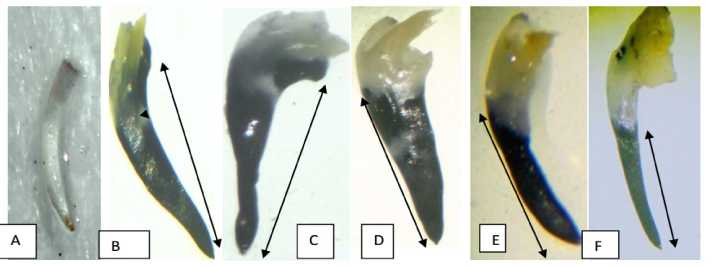
Figure 7a: Microphotographs of detection of superoxide formation in germinating roots of L. sativus L., (after 72 hrs of germination) after pretreatment with different concentrations of Cr (VI). A= Control, distilled water treated germinating roots, B= 12.5 mM treated germinating roots, C= 10 mM treated germinating roots, D= 7.5 mM treated germinating roots, E= 5 mM treated germinating roots and F= 2.5 mM treated germinating roots. Arrow heads denote the regions of superoxide radical formation and degree of expansion in the germinating roots.
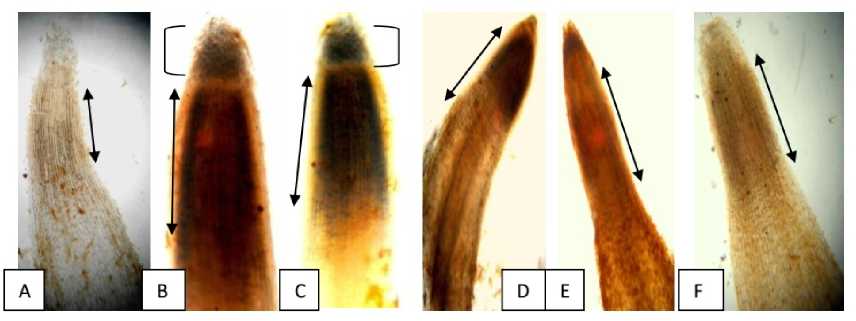
Figure 7 b: Visualization of (a) H 2 0 2 by DAB staining in roots of Lathyrus sativus L, after 72 hours of germination after preincubation of the seeds with different concentrations of Cr salt. A: Control, distilled water treated germinating roots, B: 12.5 mM pretreated germinating roots, C: 10 mM pretreated germinating roots, D: 7.5 mM pretreated germinating roots, E: 5 mM pretreated germinating roots and F: 2.5 mM pretreated germinating roots, Arrow head denotes the extension of production of H 2 0 2 the growing roots. Arrow heads denote the regions of hydroxyl radical formation.
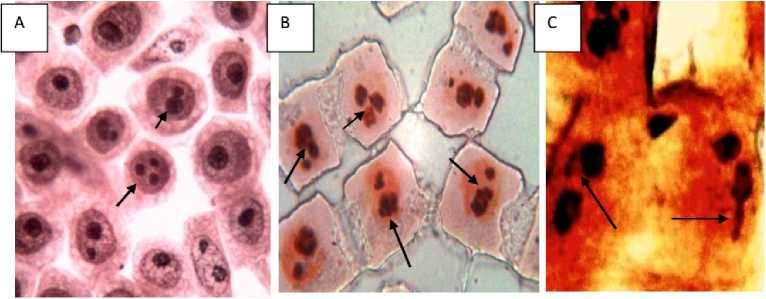
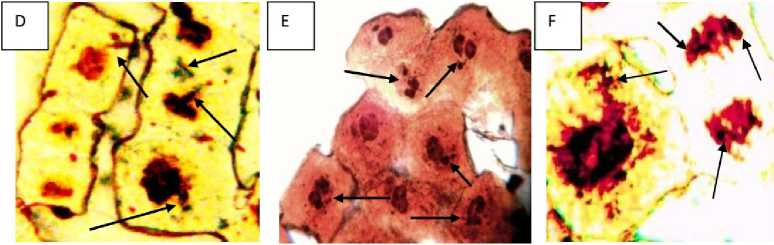
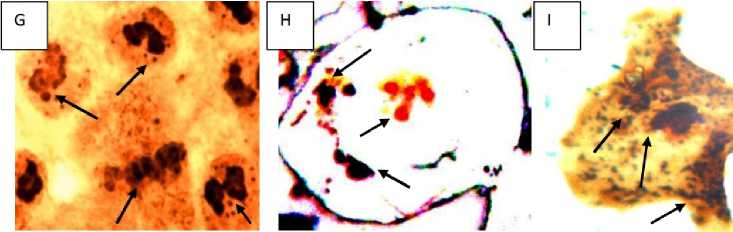
Figure 8: Effects of Cr on nucleoli in the root tip cells of Lathyrus sativus L. A= Control cells, B= Cells showing irregular nucleoli with heteromorphic-paired nuclei (PNhet) indicated by the arrow heads after 2.5 mM Cr pre-incubation in germinating root tip cells, C-D= cells showing silver-stained nucleolar proteinaceous materials extruded from the nucleus in to the cytoplasm, indicated by the arrow heads after 5 mM Cr pre-incubation in germinating root tip cells (under 100x oil immersion microphotograph). E= cells showing silver-stained nucleolar proteins and materials from the nucleus are scattered in the cytoplasm, indicated by the arrow heads after 7.5 mM Cr preincubation in germinating root tip cells, F= cells showing extrusion of nucleolar proteins stained with silver in the cytoplasm and diminishing AgNOR in the adjacent cells at the anaphase cell indicated by arrow head after 7.5 mM Cr pre-incubation in germinating root tip cells (100x oil immersion microphotograph), G= showing silver-stained nucleolar proteins and materials from the nucleus are scattered in the cytoplasm showing swollen nucleolus in the nucleus which occupied large areas of the nucleus, indicated by the arrow heads after 10 mM Cr pre-incubation in germinating root tip cells (100x oil immersion microphotograph). H= cell showing silver-stained nucleolar proteinaceous materials from the nucleolus are scattered in the cytoplasm, indicated by the arrow heads after 12.5 mM Cr pre-incubation in germinating root tip cells (100x oil immersion microphotograph), I= showing silver-stained nucleolar proteins and materials from the nucleus are scattered in the cytoplasm showing and degraded and multiple fragmented nucleus which occupied large areas of the cytoplasm , indicated by the arrow heads after 12.5 mM Cr pre-incubation in germinating root tip cells (100x oil immersion microphotograph) indicating apoptotic cell leading to cellular death.
DISCUSSION
Grass pea or Chickling vetch ( Lathyrus sativus L.) is under a worldwide cultivation practice for its high protein content as dietary supplement along with its great nutritive values. This pulse is especially consumed by human beings along with as animal fodder in third world countries (Bhagat et al ., 2015). Recently, the ecotoxicological effects of Cr2+ on germination and early seedling growth of six pulses (soybean ( Glycine max (Linn.); mung bean ( Vigna radiata (Linn.) Wilczek.),; hyacinth ( Lablab purpureus (Linn.) Sweet); sweet pea ( Lathyrus odoratus Linn.); adzuki bean ( Vigna angularis (Willd.) and black bean ( Dumasia villosa DC.) were investigated (Jun et al , 2009). In our present study we could find some inhibitory effects of Chromium (VI) supplied as on germinating seeds of Lathyrus sativus L. In Experiment I, Cr (VI) treatments suppressed seed germination and root growth significantly from 5 mm onwards. At 2.5 mM concentration there was a partial increase in seed germination (insignificant). This particular concentration did not augment any significant reduction in root length. (Fig 2). Toxicity has been defined as a function of exposure (cumulative dose), and exposure is directly proportional to concentration and treatment time (Rozman et al , 2010). In plants Cr toxicity depends upon their capacity to keep up germination in Cr contaminated environment by its inner ability for tolerance/withstanding mechansims (Peralata et al ., 2001)30]. In fact, it’s an established fact that Cr toxicity differs greatly in different plant species and the source (Lopez-Luna et al. 2009). Scientists (Peralata et al ., 2001) had compared the germination frequency of
Avena sativa, T. aestivum, and Sorghum bicolor soil amended with tannery sludge having proportionate amounts of Cr(III) in addition to with soils contaminated with Cr(VI) on germination of Avena sativa, T. aestivum, and Sorghum bicolor x Sorghum sudanense. Out of these seeds they could report that all the seeds exhibited lesser germination frequency in comparison to A. sativa (Lopez-Luna et al., 2009). Time to time different scientists had reported that Cr(VI) can alter the germination percentage in different families of plants such as Phaseolus vulgaris (Parr and Taylor, 1982), Cucumis sativus, Lactuca sativa, and Panicum miliaceum (Gorsuch et al., 1995), Medicago sativa (Peralta et al., 2001), Echinchloa colona (Rout et al., 2000), Saccharum sp hybrid cv. CoLk8102 (Jain et al., 2000), Casuarina equisetifolia (Zhou and Li, 2004), Triticum aestivum (Datta et al., 2011), Glycine max, Vigna radiata and V. angularis (Jun et al., 2009), Apium graveolens (Scoccianti et al., 2006), Beta vulgaris, Raphanus sativus, Daucus carota, Solanum melongena,and Lycopersicon esculentum (Lakshmi and Sundaramoorthy, 2010) and Brassica oleracea var. acephala (Ozdener et al., 2011). Phytotoxic effects of different chromium concentration on seed germination and seedling growth in various vegetable crops, Daucus carrota (L.), Raphanus sativus (L.), Beta vulgaris (L.), Lycopersium esculentum (L.) and Solanum melongena (L.), Vigna radiata (L.), Vigna an- gularis (L.), Lablab purpureus (L.), Lathyrus ordoratus (L.), Triticum aestivum (L.) were reported time to time (Lakshmi and Sundaramoorthy, 2010; Jun et al., 2009; Isak et al., 2013). Vajpayee et al., (2011) had reported that Cr (III) nanoparticles (25–100 μg ml-1) found to inhibit germination and growth in T. aestivum. Researcherare of opinion that this reduction in germination upon Cr (III, VI) exposure has been supposedly the decreased activity of α-amylase and β-amylase (hydrolytic enzymes). This reduced enzymatic activity could augment a rate limiting factor for the supply of sugars to developing embryos (Zeid, 2001) and starch to amylase is the most essential factor for sugar supply to developing embryos. Decrease in amylase activity under Cr treatment decreases sugar availability to developing embryo which may contribute to inhibition of seed germination (Zeid 2001). Eventually in our work we could see the same response of Lathyrus sativus L seeds on germination upon Cr exposure which could also be corroborated with a decrease in α and β amylase activities under Cr stress. Cr (VI) caused a decrease in the activities of ribonuclease, acid phosphatase, phytase, invertase, and β-amylases and enhanced maltase activity in P. sativum seedlings (Dua and Sawhaney, 1991), thereby suggesting that Cr(VI) disturbs carbohydrate, RNA, and phosphate metabolism. Disturbed ribonuclease activity restricts the supply of ribonucleotide and also affects the turnover of RNA species. The reduction in activity of hydrolytic enzymes such as α-amylase, β-amylase, maltase, and invertase implied hampering of the supply of glucose required for respiration of germinating seedlings, thereby affecting plant growth (Dua and Sawhaney, 1991). In another study Scoccianti et al., (2008) found that at 10mMa total inhibition kiwifruit pollen germination was detected. In our study we could see that at 12.5 mM there was almost total seed germination inhibition.
Thus it can be said that Cr (VI), depending on the magnitude of the dose, either might inhibit or stimulate root growth indicating toxicity. Chromium toxicity interferes with several metabolic processes in plant, causing reduced seed germination or early seedling growth. In another study, Cr (VI) concentrations up to 2 mM supplied as K2Cr2O7 did not affect germination of pea seeds, Vigna angularis and Lathyrus ordoratus reported by Jun et al., (2009). In our experiment 2.5 mM concentration did not affect seed germination at all. Increasing concentration of heavy metal significantly reduced the strength of germination as compare to the lowest concentration of heavy metal which has the least harmful influence on the germination (Peralta et al., 2001). So we may conclude that 2.5 mM Cr (VI) is the suboptimal tolerable concentration for Lathyrus sativus L. (variety Nirmal).
A significant decrease in root length were accounted when compared to control (p< 0.001) in seeds exposed to 5, 7.5, 10 and 12.5 mM of Cr treatment after 72 h in comparison to control. (Figure: 2). At 2.5 mM concentration there was an insignificant but partial promotion of root length which was totally reversed during treatment with higher doses as can be seen from the figure 2. Recently Marzban et al., (2017) had demonstrated that Lathyrus sativus L. (rangeland species) after being treated with 0, 2, 4, and 6 mM cadmium nitrate concentrations root length and root dry weights were reduced significantly with increased cadmium concentrations. This finding is in agreement with our finding where from 5 mM onwards we could see significant reduction in root length growth (Fig 2). Stimulation of root growth under low concentrations of chromium was also described in Amaranthus sp (Zou et al., 2006). For example, Peralta et al., (2001) found out that roots of alfalfa plants exposed to 5mg L-1 of Cr(VI) grew 166% more than the controls as root growth is frequently affected by heavy metals while at higher doses (20 and 40 mg L-1) there was marked dose vs root length inhibition effect. Reportedly, Cr (VI) in concentrations up to 200 mg L-1 decreased growth of paddy (Oriza sativa L.) (Sundarmurthy et al., 2010). Sensitive mungbean cultivars also showed decreased root growth when exposed to Cr (VI) (Routh et al., 1997). It was found that there was no root elongation in mung bean exposed to Cr(VI) concentrations between 96 and 1928 μM, in addition to the halted development of lateral root (both in number and length); but in lower concentrations, sensitive cultivars showed root elongation by lower concentrations of Cr exposure similar to the control (Samantary et al., 2002). Moreover, roots of Zea mays L. treated with Cr(VI) were shorter in length and brownish in colour and presented less number of roots hairs (Mallick et al., 2010). L´opez-Luna et al., (2009) found that root growth of oat and sorghum was decreased by Cr concentrations in the soil contaminated with of 100 mg Cr(VI) kg-1. Munzuroglu and Geckil, (2002) demonstrated that inhibition of root elongation is considered to be the first evident manifestation chromium toxicity in plants (population biomarker), but after the seed germination, chromium [Cr (VI)] is considered strongly toxic. Decrease in root growth in presence of Cr(VI) can be explained by a multi-cascade reaction with a disruption of membrane architecture owing to membrane leakage (Fig 3), root tissue lipid peroxidation (Fig 4), due formation of ROS (Fig 6) and cellular death (Fig 5), decreased root oxidizability (Fig 8) and decreased root cell division and/or elongation due to nuclear degradation (Fig 9) which might have resulted in tissue collapse. As a compound effect of these consequent incapacities, the roots fail to absorb water and nutrients from the medium (Barcel´o et al., 1985) combined with disruption in chromosome division and of cell cycle (Sundarmurty et al., 2010).
In previous experiments it was found that Pb and Cu with increasing concentrations propounded membrane leakage (Adhikari, 2019; Adhikari et al., 2020) as a result of membrane lipid peroxidation, ultimately resulting leakage of cell electrolytes. Electrolyte leakage is a hallmark of stress response in intact plant cells. This phenomenon is widely used as a test for the stress-induced injury of plant tissues and ‘a biophysical marker’ of plant stress tolerance (Lee and Zhu, 2010). The electrolyte leakage is ubiquitous among different species, tissues, and cell types, and can be triggered by all major stress factors, heavy metals ( Demidchik et al., 2003), oxidative stress (Demidchik et al , 2003, 2010). he electrolyte leakage is detected almost instantaneously after the application of a stress factor and lasts from a few minutes to several hours. Despite the high physiological importance and direct correlation with stress tolerance, the mechanisms of electrolyte leakage are far from understood. Pharmacological analyses have demonstrated that this reaction is sensitive to reactive oxygen species (ROS) generation. We propose that in this ROS-activated plant cells trigger programmed cell death (PCD) (Demidchik, 2012). Electrolyte leakage may be induced by a range of factors, such as oxidative degradation of the lipid bilayer. Potassium efflux from roots treated by heavy metals was first established and this reaction was initially thought to be caused by pores in the membrane that are induced by lipid peroxidation (De Vos et al., 1993). Electrolyte leakage is a constituent part of the plant's response to stress. The stress-induced electrolyte leakage is always accompanied by ROS generation and often leads to PCD. These phenomena are not independent of each other. Recent data demonstrate that ROS (hydroxyl radicals and H2O2) are capable of activating annexins catalysing K+ efflux from plant cells. Moreover, K+ efflux has been shown to cause PCD under oxidative stress. Potassium ions seem to block intracellular proteases and endonucleases; therefore, their efflux stimulates these hydrolytic enzymes, leading to PCD. (Demidchik et al , 2014). Cr (VI) also interferes with the activity of plasma membrane H+-ATPase (Shanker et al., 2005) and Na+/K+-dependent ATPase (Pauls et al., 1980). The oxidative damage induced by Cr (VI) exposure was also evident from the enhanced electrolyte leakage indicating membrane disintegration due to Cr toxicity (Panda 2007). Influence of membrane permeability of chromium stress on Lathyrus sativus L root cell membrane is selectively permeable membrane, which can control and adjust the transport and exchange of intracellular substances. At the cellular level, it is the first site of stress injury. The permeability values reflect the amount of soluble substance leakage within the cell membrane, so is the evaluation index of the reaction of plants to environmental damage. Chromium stress most direct harm to the plant cell is the structure and function of cell membrane, with the membrane permeability increases, membrane stability decreases, passive leakage of ion cells and macromolecules. Therefore, the increased membrane penetrability is the direct evidence of cell membrane damage a biophysical marker of cytotoxicity.
In the present study, exposure to Cr (VI) incited a ROS-mediated oxidative stress in the germinating L. sativus L. roots. Upon exposure, the levels of ROS like MDA, enhanced as compared to the control in a timedependent manner. To quantify the Cr (VI) induced damage to the membranes, lipid peroxidation analysis was done. Exposure increasing concentrations of Cr (VI) enhanced MDA content, a measure of lipid peroxidation, as compared to the control (Fig 4b). MDA is a free radical generated as a by product of lipid peroxidation and enhanced levels of MDA upon exposure advocates that Cr (VI) increased lipid peroxidation, caused damage to the membrane and induced oxidative stress in the roots. It was confirmed by staining of roots with Evans blue that showed the damage to the membrane in a dose-dependent manner (Fig. 5). Enhanced lipid peroxidation upon Cr (VI) treatment was confirmed by the in situ histochemical localization with Schiff’s reagent, (Fig. 4a). Parallel to MDA accumulation, O2•-levels were increased upon expo-sure to Cr (VI) (Fig 7a). It was confirmed by in situ detection with NBT (Fig. 7b). In situ detection of H2O2 with DAB also supported the greater accumulation of H2O2, exhibiting greater colour intensity with increase in the dose duration (Fig. 7b). The observations made in the present study are corroborated by earlier reports that Cr (VI) act as an abiotic stress for plants and induce ROS (H2O2, O2•-) generation, resulting in enhanced lipid peroxidation and damage to membranes.
In previous experiment (Adhikari, 2019) it was found that Pb with increasing concentrations propounded membrane lipid peroxidation in L. sativus L. root tip cells upon pre-incubation with seeds. The metabolic alterations in plants due to Cr(III, VI) stress have been attributed to direct effect on enzymes and/or metabolites or to ROS generation resulting in lipid peroxidation and oxidative damage (Montes-Holguin et al., 2006). Earlier, it has been reported that Cr (VI) reduction to Cr (III) results in ROS generation (Kotas and Stasicka 2000). Several studies have reported that Cr(III, VI) exposure induces oxidative damage in the plants resulting in growth inhibition and enhanced malondialdehyde (MDA, an indicator of lipid peroxidation) content in roots and shoots of S. bicolor and the increase was greater in roots (Shanker and Pathmanabhan 2004). Pandey et al., (2005) also reported that under Cr (VI) stress, MDA accumulation is greater in roots than in shoots of B. juncea. Scoccianti et al., (2008) found an increase in lipid peroxidation in germinating pollen of kiwi (A. deliciosa var. deliciosa) upon Cr (III, VI) stress. The toxic effect upon ROS generation and lipid peroxidation is more pronounced with Cr(VI) than with Cr(III) (Shanker and Pathmanabhan 2004; Shanker et al., 2004a; Scoccianti et al., 2008). In roots of 15-day-old green gram (V. radiata) exposed to 50 μM Cr, increase in lipid peroxidation was seen 5 h after Cr(VI)-treatment compared to after 12 h in Cr(III)-treated roots (Shanker et al., 2004a). In the leaves of Saccharum sp, treatment with 30, 60, and 90 ppm of Cr(VI) enhanced lipid peroxidation as indicated by elevation in MDA level by 76.7, 85.5, and 89.3 %, respectively (Rai et al., 2006). Exposure to 1 μM Cr(VI) declined MDA content after 20 days in leaves of A. viridis; however, at 10 and 100 μM Cr(VI), MDA level increased by 10.9 and 25.4 %, respectively (Liu et al., 2008). Panda (2007) reported an increase in MDA content in O. Sativa after 18 h of Cr(VI) treatment. Subrahmanyam (2008) found an increase in MDA content in T. aestivum treated with ≥0.10 mM Cr(VI).
As a result of all biotic and abiotic stress responses eukaryotic membrane damage happens. The membrane architecture determines how a cell can sustain altered environmental conditions and cell death can be regarded as a cellular biomarker to assess stress-induced cell damage or death. Evan’s blue is an azo dye, has been widely used to assay the levels cell viability. Physiologically Evan’s blue dye happens to penetrate through ruptured or destabilized membranes to stain cells. Thus, when plant cells which are subjected to stress develop compromised membrane integrity/architecture, will allow the dye to permeate the stain Evan’s blue dye which is directly proportional to the amount of cell/tissue faced death. The intensity of colour development will be increased gradually with the levels of cellular stress compared to control cells that are not stressed. In contrast, live, healthy cells in control sets that are capable of maintaining membrane integrity do not take up Evan’s blue dye. Cells that have taken up Evan’s blue dye would have an accumulation of a blue protoplasmic stain and this degree of staining intensity can be qualitatively documented under bright field microscopy with or without the use of a camera. Using this analysis, the accumulation of dye in positively-stained cells corroborates the degree of cell membrane damage. Experimentally the amount of cells that are stained with Evan’s blue dye under various conditions is used as a “bioassay based” biomarker of cellular stress. Cell mortality of Cr (VI)-treated roots (2.5 to 12.5 mM) was determined with Evans blue staining under low power microscopy (Fig: 5). In untreated seedlings no Evans blue staining was observed ((Fig: 5). With a gradual increasing concentration treated germinating root tips after 72 h, there was gradual increase in staining in the roots. In the lower concentrations (i.e. at 2.5 and 5 mM treatment) there was development of colouration in the developing zone of the roots while from 7.5 ppm onwards the roots developed colouration in extending from apical to growing regions extending upto the permanent regions with qualitative escalation in intensity. (Fig 5d, e and f). This qualitative demarcation strongly proves the increasing concentrations of Cr (VI) brought about cellular death in the germinated roots even after 72 hours.
Plant roots are sessile organs that are exposed to a diverse array of stress factors. The plant membrane architecture is composed of lipids and glycoproteins playing the role of a protective barrier to control cell membrane fluidity. Once the cells are exposed to oxidative stress, cell membranes are greatly compromised to death. The reactive oxygen species (ROS) associated with oxidative stress can act on membrane lipids to decrease membrane stability. We adapted a reliable Evan’s blue staining technique that has been used extensively to assess cell death or membrane damage (Smith et al ., 1982; Oprisko et al ., 1990; Vemanna et al ., 2017) for instantly monitoring cellular stress. Evan’s blue is an acidic, non-permitting exclusion dye which stains dead or damaged cells only. The dye does not enter live cells with stable membranes (Gaff and Okong’O-Ogala, 1971). One advantage of this method is that it is highly reproducible and easily subjected to microscopic visualization or even under naked eyes (Baker and Mock, 1994).
Recently, Mukherjee and Arya (2014) observed that CdCl2 exposure induced cell death in root cells of both A. cepa and V. faba where a ~ 2 - 3.5 fold increase in Evan’s blue uptake was observed in A. cepa and V. faba, indicating cytotoxic effect of Cd on plants. Cell death or the senescence as evaluated by Evan’s blue test could be attributed to the induction of high MDA content by lipid peroxidation contributed by high production of ROS leading to cell death in V. faba making it more sensitive towards Cd. Moreover, the loss of membrane integrity, a primary marker of loss of cell viability is in accordance with the observed peroxidation of lipids and is consistent with the increase of cell senescence and death, because of higher exposure to Cd. In our study we may corroborate that up on exposure to all the concentrations (from 2.5 to 12.5 mM) Cr (VI) imparted cellular death through ROS production thus disrupting the membrane architecture by lipid peroxidation in germinated roots of L. sativus L.
Increased root oxidizability (RO) indicates a greater diffusion of oxygen from the roots, primarily to counter the toxic materials around the site of action. TTC salt used to measure RO actually absorbs electrons from the mitochondrial transport In other words, enhanced RO is also an indicator of higher ROS generation. Subrahmanyam (2008) found an increase in MDA content in T. aestivum treated with ≥0.10 mM Cr(VI). Dixit et al ., 2002) reported that Cr (VI) affects redox reactions in root mitochondria in P. sativum and induces lipid peroxidation in mitochondrial membranes. The present study showed that root oxidizability assessed through TTC-reduction was lesser in the roots at higher concentrations (Fig 6). A conspicuous decrease in root oxidizability indicated a reduction in the rate of root respiration and root oxidizability points toward probable cellular death (apoptosis) via mitochondrial poisoning (Fig 6). A decrease in root oxidizability also indicated that the higher concentrations of Cr (VI) amended cellular stress in germinating seeds via ROS generation. After root cell membrane being hurt/disrupted by ROS (after the treatment with heavy metals, e.g., copper), intracellular ion and organic substances leak greatly (evident from Fig 3) resulting in physiological metabolism disorder (Adhikari et al ., 2020). It can be clearly said that Cr stress has obvious effects on physiological and biochemical characteristics of Lathyrus roots. When the seeds are subjected Cr concentration at 7.5 mM, Lathyrus root activity decreased significantly, showing a clear downward trend in 10 and 12.5 mM treatments too. In addition, we can also infer that with increasing concentrations of Cr exposure brought about deeper damage to germinated roots, which would have lead to nutrient deficiency (owing to electrolyte leakage) and further accelerate the physiological metabolism disorder leading to root cell death (Fig 5).
Reports are being published to state that Cr (VI) stress induced generation of reactive superoxide radicals (O2•-), and hydrogen peroxide (H2O2) in plants (Shanker et al., 2004a; Panda 2007; Gangwar and Singh 2011). Reporters claimed that Cr (VI) could bring forth the inactivation of mitochondrial electron transport chain ultimately resulting in enhanced O2•-generation (Dixit et al., 2002) [78]. Shanker et al., (2004a) reported a significant enhancement of O2•- from 5 h treatment up to 120 h under Cr(VI) stress in addition to H2O2 content increased (in the same protocol) after Cr(VI) treatment, respectively, in V. radiata roots. In Saccharum roots in comparison to shoots there was enhancement of H2O2 content after treatment with 30, 60, and 90 ppm of Cr(VI) (Rai et al., 2006). Panda (2007) had reported that 100 mM Cr(VI) treatment could double H2O2 content after 48 h in addition to O2•-content increased by 33 % in developing O. sativa seedlings. Recently, Cr (VI) at ≥500 μM has been reported to enhance MDA content and H2O2 accumulation in M. sinensis (Sharmin et al., 2012). In our experiment with Lathyrus the observed increase in lipid peroxidation, an indicator of increased oxidative damage severely affected the functioning of plasma membrane and finally lead to the death of the cells which had been established by other scholars in different plants (Kappus 1985; Panda and Choudhury 2005). Interestingly at the higher concentration i.e. at 12.5 mM and 10 mM Cr pre-treatment the root cap region was deeply stained by DAB, indicating that Cr generated a burst of H2O2 which directly affected the root cap also to exert cytotoxicity in germinating roots of Lathyrus sativus L. (fig 7b, B and C)
According to Boulon et al ., (2010) nuclei have been regarded as the site of response of cellular toxicity due to abiotic stress. Because of abiotic stress both the structure and function of the nucleolus are directly affected. Such altered changes might include the modification and alteration in the processes of RNA production and proteins in dividing cells. It is therefore of worth mentioning that alterations in the structure of nuclear structure and changes in the proteins thereof might serve as a powerful cytological marker which have been employed widely in plants and animals during the effect of different stressors in environmental studies (Liu et al ., 1994). Several nucleolar parameters such as as number of nucleoli per cells, nuclear volume and the number of cells with heteromorphic-paired nucle
(PNhet) have been considered as nuclear biomarker in plants (Arkhipehuk and Garanko, 2002) because of simplicity, low costing and high rate of reproducibility. According to Arkhipehuk (1995), cells which have small number of NOR with fixed nucleoli characteristics are more desired for cytological evaluations in functional activities of the rRNA genetic changes over the other species having many nucleoli. Fitting into this parameter, Lathyrus sativus L., represent a small number of NOR, which can be considered as a model plant system to bioassay the action of environmental pollutants for the study of nuclear parameters as a biological marker. In the current study the AgNOR method was employed to study the nucleolar activity of aberrant cells as a biomarker for genotoxicity induced by Cr and to test the impact of it in identifying the presence of nucleolus in resting micronucleus. Here the variation in number of nucleoli in L. sativus meristamatic cells has been considered as “the most sensitive biomarker” parameter with regard to cytotoxicity in comparison to chromosomal aberrations, mitotic index and micronucle formation. This particular parameter i.e. the “changes in nucleolar activity” becomes the most prominent assay to establish and comprehend the complete information on the toxic, genotoxic and cytotoxic effects of different pollutants and cytotoxic substances including our test substance i.e., the heavy metal chromium (lima et al ., 2019).
In our result it could be seen that the prominent changes in number of nuclear activity as a function of nucleolar characteristics observed mostly in this particular bioassay for the determination of cytogenotoxicity of Cr. The number of nucleoli per cell and the number of cells with heteromorphic-paired nuclei with altered AgNOR were observed with increasing concentration of Cr with pre-treatment. This in turn altered the number of active NORs and the intensity of the transcriptional activity of the NORs in resting nucleus exerting its cytotoxic stress via ROS generation. This reduced amount of NORs being actively transcribed for rRNA synthesis could have been a direct outcome of ROS burst and consequently the reduction in the cell division cycle could result in cellular death in the actively growing region of root tip cells. Nucleoli are the ribosome factory of the cells and play several crucial functions in the nucleus. Nucleolus is well known as the site of transcription of ribosomal genes (Shaw and Jordan, 1995), which contains NORs being defined as nucleolar components containing a set of argyrophilic proteins, which are selectively stained by the silver method (Trerè, 2000). It is proved that proteinic carboxyls might initially combine and deoxidize certain silver cations (Ag–), which then attract silver cations being continued to deposit at the reaction locus. So after silver-staining, the nucleoli are selectively stained in dark brown and the NORs can be easily identified as black dots in the coloured background (Trerè, 2000). Nucleolin is one of the main proteins in nucleolus and oxidative stress could induce the cleavage of it (Jiang et al, 2014). Van der Aa et al., (2006) indicated that the nuclear pore complex (NPC) was the most important channel for nuclear material. The phenomenon that the nucleolar material was extruded from the nucleus into the cytoplasm could be explained by the fact that the nuclear membrane proteins (mainly Lamin proteins) would have been affected after Cr induced ROS generation, causing the NPC to lose selectivity.
It was reported Al could induce nucleolar material extruded from the nuclei into the cytoplasm in root cells of Allium cepa exposed to Al (Fiskesjö, 1983). After that, reports were published that besides Al, some heavy metals such as Cd, Pb, Cu, Ni, Co, Mg and Hg could also affect nucleoli and induce nucleolar disruption and leaching of AgNORs in the cytoplasm in Allium cepa , A. sativum , Vicia faba and Zea mays (Liu et al., 1995; Liu et al., 2003, Liu et al., 2009, Qin et al., 2013, Jiang et al., 2014). Our results with Cr are in compliance with the previous reports showing the same trend.
The Cr-induced phenomena observed in this investigation were that some tiny particles containing argyrophilic proteins were scattered in the nucleus of root tip cells and leached out from the nucleus to the cytoplasm (Figure 2). Once the nucleolus was affected, the root growth of Lathyrus sativus L was clearly inhibited. These toxic effects of Cr on nucleoli are in agreement with the previous observations where toxic effects of Cd on nucleoli in root tips of A. sativum (Liu et al. 2003, 2009) and V.faba (Zhang et al. 2009, Qin et al. 2013) were investigated. Nucleolus has been known to play important roles in the regulation of many fundamental cellular processes, including cell cycle regulation, apoptosis, telomerase production, RNA processing, monitoring and response to cellular stress (Boisvert et al. 2007). This altered expression of nucleolus in Lathyrus sativus L may mirror an enhancement and dysfunctional nucleolar activity which is an important aspect of the cellular/nucleolar response to Cr stress as a function of ROS burst leading to genotoxicity, nucleolar dysfunction and cellular toxicity leading to cell death.
CONCLUSION
Grass pea is a diploid (2n = 14 with its big complex 8.2-Gb genome) could be employed as a fantastic model plant to evaluate cytogenotoxic assays in addition to other standardized models like Allium cepa L. The findings of the present investigation confirm that (1) Cr is a strong genotoxicant which can affect nuclear structure and functioning of nucleolus in the root tip cells of Lathyrus sativus L inducing extrusion of silver-stained materials containing argyrophilic proteins from the nucleolus into the cytoplasm augmenting severe genotoxicity during the early stages of germination, which are an ultimate resultant of ROS outburst. Chromium contamination hampers seed germination, root length growth inhibition, membrane leakage, membrane lipid peroxidation and cellular death in Lathyrus sativus L. This plant system can be well employed as a useful testing model for the early detection of different heavy metal pollution including chromium in different cash crops and pulses. The data obtained here can provide valuable information for monitoring and forecast early effects of exposure to Cr in polluted environments. 2. Industrial demands are spewing alarming amounts of toxic substances in our environment that are having so many unforeseen consequences on human health including the quality of life in plant world. All commercial cultivars and cash crops are no exception but prone to this alarming effects of heavy metal pollution which indirectly brings forth genomic loss. Chromium is a very toxic heavy metal which when comes in contact with the seeds via water gets readily absorbed by the seeds and upon germination exerts its toxic effect in a multi-cascade fashion. Chromium causes ROS outburst in living root tip cells upon germination which is highly cytotoxic and impairs the genome of cash crops. So health hazards and loss of crop productivity resulting from Cr contaminations need to be identified and policies need to be formulated to regulate the use of Chromium notwithstanding economic consequences.
ACKNOWLEDGMENTS
The author extends his indebtedness to the Post Graduate Dept of Botany, Hooghly Mohsin College, for providing the physical space and necessary experimental set up to complete this work.
FUNDING
The Author sincerely and gratefully acknowledges the financial support accorded by University Grants Commission (UGC Minor Research Project No- F. PSW - 088/10 - 11(ERO)), Govt of India.
CONFLICTS OF INTEREST
Список литературы Chromium Induces Genotoxicity in Root Tip Cells of Grass pea (Lathyrus sativus L., Variety Nirmal): A ROS-mediated Acute Toxicity Study
- Adhikari, D. (2019) Augmentation of Mitodepressive and cytogenetic effects of Lead upon Acute exposure on Grass Pea (Lathyrus sativus L.) root tip cells. American Journal of Biological Sciences., 1(1), 14-22.
- Adhikari, D, Samanta, S, Roy, A., Dutta, A., Vedasiromoni, J. and Sen, T. (2007) In vitro hemolysis and lipid peroxidation inducing activity of the tentacle extract of the sea anemone (Paracondylactis indicus Dave.) on rat erythrocytes. Indian Journal of Pharmacology., 39(3), 155-159.
- Arkhipchuk, V.V. and Garanko, N.N. (2002) A novel nucleolar biomarker in plant and animal cells for assessment of substance cytotoxicity. Environ Toxicol., 17, 187–194.
- Arkhipchuk, V.V. (1995) The use of nucleolar characteristics in biotesting. Tsitol Genet., 29, 6–12.
- Arya, S.K. and Mukherjee, A. (2014) Sensitivity of Allium cepa and Vicia faba towards cadmium toxicity. Journal of Soil Science and Plant Nutrition., 14 (2), 447-458.
- Avudainayagam, S., Megharaj, M., Owens, G., Kookana, R.S., Chittleborough, D. and Naidu, R. (2003) Chemistry of chromium in soils with emphasis on tannery waste sites. Reviews of Environmental Contamination and Toxicology., 178, 53–91.
- Babula, P., Adam, V., Opatrilova, R., Zehnalek, Havel, L. and Kizek, R. (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environmental Chemistry Letters., 6(4), 189–213.
- Baker, C.J. and Mock, N.M. (1994) An improved method for monitoring cell death in cell suspension and leaf disc assay using Evan’s blue. Plant Cell Tissue Organ Cult., 39(1), 7-12.
- Barcel´o, J., Poschenrieder, C. and Guns´e, J. (1985) Effect of chromium (VI) on mineral element composition of bush beans. Journal of Plant Nutrition., 8, 211–217.
- Becquer, T., Quantin, C., Sicot, M. and Boudot, J.P. (2003) Chromium availability in ultramafic soils from New Caledonia. Science of the Total Environment., 301(1–3), 251–261.
- Bhagat, G.J., Kamdi, S.R., Neharkar, P.S., Ghate, S.R., and Kadu, P.R. (2015). Influence of integrated nutrient management on paddy-lathyrus cropping system in eastern Vidarbha region. International Journal of Tropical Agriculture., 4(2), 16-20.
- Boisvert, F.M., Koningsbruggen, S. van., Navascués, J. and Lamond, A.I. (2007) The multifunctional nucleolus. Nature Reviews Molecular Cell Biology., 8, 574–585. DOI: 10.1038/nrm2184.
- Boulon, S., Westman, B.J., Hutten, S., Boisvert, F.M. and Lamond, A.I. (2010) The nucleolus under stress. Mol Cell., 40, 216–227.
- Cavusoglu, K., Yalcin, E. and Ergene, A. 92010) The investigation of cytotoxic effects of refinery wastewater on root tip cells of Vicia faba L. J. Environ. Biol., 31, 465-470.
- Datta, J.K., Bandhyopadhyay, A., Banerjee, A. and Mondal, N.K. (2011) Phytotoxic effect of chromium on the germination, seedling growth of some wheat (Triticum aestivum L.) cultivars under laboratory condition. J Agric Technol,; 7, 395–402.
- Daudi, A., Cheng, Z., O'Brien, J.A., Mammarella, N., Khan, S. and Ausubel, F.M. (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern triggered immunity. Plant Cell., 24 (1), 275–287.
- Demidchik, V., Shabala, S.N., Coutts, K.B., Tester, M.A. and Davies, J.M. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. Journal of Cell Science., 116, 81–88.
- Demidchik. V. (2010) Reactive oxygen species, oxidative stress and plant ion channels. In: Demdichik V, Maathuis FJM, eds. Ion channels and plant stress responses. Heidelberg: Springer-Verlag., pp 207–232.
- Demidchik, V. (2012) Reactive oxygen species and oxidative stress in plants. In: Shabala S, ed. Plant stress physiology. Wallingford, UK: CAB International., pp 24–58.
- De Vos, C., Bookum, W T., Vooijs, R., Schat, H. and DeKok, L. (1993) Effect of copper on fatty acid composition and peroxidation of lipids in the roots of copper tolerant and sensitive Silene cucubalus. Plant Physiology and Biochemistry., 31, 151–158.
- Demidchik, V., Straltsova, D., Medvedev, S.S., Pozhvanov, G.A., Sokolik, A. and Yurin, V. (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment, Journal of Experimental Botan., 1-12.
- Dixit, V., Pandey, V. and Shyam, R. (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant Cell Environ., 25, 687–693.
- Dua, A. and Sawhaney, S.K. (1991) Effect of chromium on activities of hydrolytic enzymes in germinating pea seeds. Environ Exp Bot. 31, 133–139.
- Fiskesjö, G. (1983). Nucleolar dissolution induced by aluminium in root cells of Allium. Physiologia Plantarum., 59, 508–511.
- Gaff, D.F. and Okong'O-Ogola, O. (1971) The use of non-permeating pigments for testing the survival of cells. J Exp Bot., 22, 756-758.
- Gangwar, S. and Singh, V.P. (2011) Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: implication of oxidative stress. Sci Hortic., 129, 321–328.
- Grant, W.F. and Owens, E.T. 91998) Chromosome aberration assays in Crepis for the study of environmental mutagens. Mutat. Res., 410, 291-307.
- Gecheff, K.I. (1996) Production and identification of new structural chromosome mutations in barley (Hordeum vulgare L.). Theor. Appl. Genet., 92, 777-81.
- Ghosh, T. Mukherjee, S. and Adhikari, D. (2020) Evaluation of Acute Toxicity Studies on Copper–Induced Oxidative stress in Lathyrus sativus L., (variety Ratan) Germinating seeds: A Biomarker based risk assessment. Journal of Advance Scientific Research., 11(4), 243-254.
- Ghosh, I., Ghosh, M. and Mukherjee, A. (2017) Remediation of Mine Tailings and Fly Ash Dumpsites: Role of Poaceae Family Members and Aromatic Grasses. In Enhancing Cleanup of Environmental Pollutants; Springer: Cham, Germany., 117–167.
- Gorsuch, J.W., Rittler, M. and Anderson, E.R. (1995) Comparative toxicities of six heavy metals using root elongation and shoot growth in three plant species. In: The symposium on environmental toxicology and risk assessment. Atlanta, GA, USA. pp 26–29.
- Isak, R.S., Parveen, R.S., Rafique A.S. and Alamgir, A.S. (2013) Phytotoxic Effects of Heavy Metals (Cr, Cd, Mn and Zn) on Wheat (Triticum aestivum L.) Seed Germination and Seedlings Growth in Black Cotton Soil of Nanded, India,” Research Journal of Chemical Sciences., 3(6), 14-23.
- International Agency for Research on Cancer. Chromium, nickel and welding: in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 1990; 49, The International Agency for Research on Cancer, Scientific Publications, Lyon , France.


