Chronic Stress Enhances Hepatotoxic Effects of Sorbic and Benzoic Acids in a Rat Model
Автор: D.O. Karimov, Yu.V. Ryabova, E.F. Repina, Ya.V. Valova, A.A. Gizatullina, T.G. Yakupova, D.A. Smolyankin, O.A. Khmel, M.V. Kurilov
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.21, 2025 года.
Бесплатный доступ
Background: The individual effects of psychological stress and food preservatives on the liver—the body’s primary detoxification organ—are well-documented; however, little is known about their combined impact. The aim of this study was to evaluate the hepatic effects of sorbic and benzoic acid exposure in male rats subjected to chronic stress. Results: Over a 28-day period, four groups of male rats were studied: Control, Chronic Stress, Preservatives (sorbic acid at 500 mg/kg and benzoic acid at 100 mg/kg), and a Combined Exposure group. Gene expression analysis revealed increased Sod1 expression under stress conditions, while Nqo1 and Hmox1 were significantly downregulated following preservative exposure and remained suppressed in the combined group. Biochemical analysis demonstrated reduced ALT, AST, and ALP activities across all experimental groups, with the most pronounced decreases observed under combined exposure. LDH activity was elevated under stress but declined when stress was coupled with preservative intake. Lipid metabolism was disrupted, as evidenced by decreased triglyceride levels and altered cholesterol concentrations. Total protein and albumin levels were significantly reduced only in the combined group. Despite preserved hepatic architecture, these molecular and biochemical changes suggest early signs of functional decompensation. Conclusions: Co-exposure to chronic psychological stress and high doses of food preservatives resulted in a non-linear and potentially synergistic disruption of hepatic redox homeostasis, protein synthesis, and lipid metabolism. Further studies incorporating mitochondrial assessment, long-term exposure models, and quantitative interaction analysis are warranted to clarify the mechanisms underlying this toxicological synergy.
Chronic stress, food preservatives, liver, gene expression, Sod1, Hmox1, Nqo1, rats
Короткий адрес: https://sciup.org/143185121
IDR: 143185121
Текст научной статьи Chronic Stress Enhances Hepatotoxic Effects of Sorbic and Benzoic Acids in a Rat Model
Chronic sychological stress re resents an increasingly ervasive issue in modern society, with far-reaching consequences on both the individual and o ulation levels. Its im act s ans sychological wellbeing, social functioning, and quality of life (Ceccato et al., 2018), as well as rofessional erformance, healthcare outcomes, and ublic health metrics (Shchaslyvyiс et al., 2024; Lukan et al., 2022; Bird et al., 2024; Crielaard et al., 2021).
Sustained activation of stress-res onse systems im oses a continuous burden on the body's ada tive ca acity, leading to ersistent biochemical, hysiological, and frequently structural alterations. One of the rimary targets of chronic stress is the liver, the central organ of metabolism and detoxification. Activation of the hy othalamic– ituitary–adrenal axis under stress results in elevated circulating glucocorticoids, which are redominantly metabolized in the liver via oxidation, reduction, and conjugation. While this detoxification rocess yields inactive metabolites, glucocorticoids have been shown to rovoke inflammatory res onses and romote he atocyte a o tosis, notably through u regulation of the Fas antigen. Interestingly, studies have demonstrated that glucocorticoid ex osure does not directly induce Fas ex ression in cultured he atocytes (Chida et al., 2004), suggesting that their he atic effects in vivo are mediated indirectly through systemic mechanisms (Chida et al ., 2004; de Sousa Rodrigues et al., 2017). Chronic stress has also been shown to im air he atic microcirculation, leading to decreased tissue erfusion and diminished vascular regulatory ca acity (Potekhina et al., 2024). These hemodynamic disturbances contribute to hy oxia, Ku ffer cell activation, and increased leukocyte infiltration, am lifying local inflammation and disru ting he atic homeostasis (Joung et al., 2019). Moreover, chronic stress is now recognized as a contributing factor in the rogression of he atitis, liver fibrosis, cirrhosis, and even he atocellular carcinoma (Reichel et al., 2018).
Food reservatives, while essential for extending shelf life and reventing microbial s oilage, may also ose toxicological risks. Potassium sorbate, a widely used reservative, has been associated with oxidative damage through its ro-oxidant otential (Taghavi et al., 2016), and sorbic acid may im air he atic li id metabolism (Chen et al., 2020). Benzoic acid, although not conclusively shown to induce oxidative stress, has been linked to metabolic cascades that accom any oxidative imbalance; elevated tissue levels of benzoic acid are associated with oxidative stress-related outcomes, albeit without direct evidence of reactive oxygen s ecies generation (Ló ez-González et al., 2023).
We hy othesized that combined ex osure to sorbic and benzoic acids under chronic stress would induce more ronounced structural and functional alterations in the liver than either factor alone. Given that both chronic stress and dietary reservatives can disru t redox homeostasis and metabolic athways, we ex ected additive or even synergistic interactions, articularly reflected in altered ex ression of antioxidant defense genes, serum biochemical markers, and he atic histology. At the same time, we considered the ossibility of antagonistic effects in selected arameters — articularly those reflecting rotein synthesis, li id metabolism, or mor hological integrity—where co-ex osure might attenuate or mask the individual effects of stress or reservative ex osure, resulting in a nonlinear or com ensatory res onse attern.
Accordingly, the aim of this study was to evaluate the he atic effects of sorbic and benzoic acid ex osure in male rats subjected to chronic stress, focusing on antioxidant gene ex ression, serum biochemical markers, and liver mor hology.
MATERIALS AND METHODS
Ethical Approval. Ex erimental modeling of chemical ex osure under chronic stress conditions was a roved by the Institutional Bioethics Committee (Protocol No. 01-02, dated February 8, 2024). All rocedures for animal housing, feeding, care, and euthanasia were carried out in accordance with the legislation of the Russian Federation on the treatment of laboratory animals, as well as the Euro ean Convention for the Protection of Vertebrate Animals used for Ex erimental and other Scientific Pur oses (ETS No.
123) and Directive 2010/63/EC of the Euro ean Parliament and of the Council on the rotection of animals used for scientific ur oses.
Animal Model and Group Allocation. The study design followed the ARRIVE guidelines to ensure high-quality re orting of animal ex eriments. Outbred male white rats (body weight 190–210 g, age 10–12 weeks at the start) were randomly assigned to four grou s (n=6 er grou ). One grou was ex osed to stressors according to the rotocol throughout the entire duration of the ex eriment (“Chronic Stress” or “Stress”). Another grou received daily intragastric administrations of aqueous solutions of food reservatives – sorbic acid (500 mg/kg body weight, 100 mg/mL) and benzoic acid (100 mg/kg body weight, 20 mg/mL) – at the same time each day (“Sorbic and Benzoic Acids” or “SBA”). A third grou was subjected to combined ex osure to chronic stress and food reservatives (“Chronic Stress + Sorbic and Benzoic Acids” or “Comb”). The final grou served as the control (“Control” or “Ctrl”), and animals received a daily intragastric administration of an equivalent volume of the reservative vehicle, which was distilled water.
The doses of sorbic and benzoic acids were set to ten times the average daily intake for an adult human, based on the list of food roducts a roved by R2.1.10.3968-23 (Moscow, 2023) and TR TS 029/2012 (ado ted by the Eurasian Economic Commission Council, No. 58 dated July 20, 2012). It is im ortant to note that this study did not aim to re licate real-world consum tion atterns of sorbic or benzoic acids for any s ecific o ulation grou ; rather, it sought to identify otential toxic effects on the liver under chronic stress conditions at high-dose ex osures to these widely used sorbic and benzoic acids as food reservatives. In summary, the study design evaluated (1) the im act of chronic stress, (2) the im act of food reservatives – sorbic and benzoic acids, and (3) their combined effects on the liver enzymes, mor hology, gene ex ression in liver tissue. An overview of the ex erimental design is resented in Figure 1.
Chronic Stress Protocol. Chronic stress was induced following the method described by Matisz et al. (2021). Animals were subjected to mild, un redictable stressors on a daily basis, com rising 2–3 randomly selected factors such as social isolation, immobilization, ex osure to noise, continuous lighting during the dark hase, and restricted access to food and water for a limited eriod. Each combination of stressors was a lied at random times over the course of a day, ensuring that the same stress factor was not re eated twice within a single day.
Evidence of chronic stress in the animals was confirmed by several behavioral and hysiological signs, including reduced weight gain com ared to the Control grou , alterations in locomotor activity, and a tendency toward diminished ex loratory behavior Gizatullina et al., 2024 a; Gizatullina et al., 2024 b), consistent with stress-induced changes described in related studies (Alexa et al., 2023).
Measurements of biochemical stress markers (e.g., corticosterone or cortisol) were deliberately omitted due to the substantial influence of circadian rhythms and rocedural interventions (blood sam ling) on hormone levels, which reduces the s ecificity of such assessments (Kim et al., 2018; Perhonen et al., 1995). Moreover, while cortisol is the rinci al stress hormone in humans, corticosterone serves this role in rats, com licating direct com arisons of these results with clinical data focused on human hysiology (Joëls et al., 2019).
Euthanasia, Blood and Tissue Collection. After 28 days of ex osure, the animals were euthanized by deca itation in accordance with the recommendations of the American Veterinary Medical Association (AVMA Guidelines for the Euthanasia of Animals: 2020 Edition). Blood sam les were collected immediately at the time of deca itation, and necro sy was erformed to harvest liver tissue sam les.
Serum Biochemistry. Biochemical arameters reflecting metabolic status and liver function – including alanine aminotransferase (ALT), as artate aminotransferase (AST), alkaline hos hatase (ALP), and lactate dehydrogenase (LDH) activity – were measured in serum using the Stat Fax 3300 hotometer (Awareness Technology, USA). Diagnostic kits from Vector-Best (Vector-Best, Russia) were em loyed according to the manufacturer’s instructions.
Histology . Liver sam les for histomor hological evaluation were fixed immediately after necro sy in 10% neutral buffered formalin. Tissues were rocessed through graded iso ro anol and embedded in araffin using standard histological rocedures. Sections 5–7 µm thick were re ared using an MS-2 microtome and stained with hematoxylin and eosin. Slides were subsequently imaged using a Celena X digital imaging system (Logos Biosystems, South Korea), and qualitative descri tive analysis was erformed on the resulting histological re arations. Mor hometric analysis was conducted using the o en-source software QuPath-0.5.1 (The Queen’s University of Belfast, United Kingdom). The nuclear and cyto lasmic areas of he atocytes were measured, and the nuclear-to-cyto lasmic (N/C) ratio was calculated for 50 cells selected from five random fields of view on each histological slide obtained from a single animal. Additionally, the number of binucleated and anucleated he atocytes was counted in 10 randomly selected fields of view er slide, with one slide analyzed er animal.
Gene Expression Analysis. To assess the ex ression of antioxidant defense genes, liver tissue sam les were collected immediately after deca itation and necro sy. Small fragments of liver were ra idly frozen in liquid nitrogen and stored in ExtractRNA solution (Evrogen, Russia). Total RNA was isolated using a TRIzol-based extraction method, followed by reverse transcri tion and real-time PCR am lification using the Rotor-Gene Q system (QIAGEN, Germany).
Com lementary DNA (cDNA) synthesis was erformed from urified total RNA using the MMLV RT kit and oligo(dT)₁₅ rimers (Evrogen, Russia). S ecific oligonucleotide rimers for real-time PCR were designed using PrimerQuest (Integrated DNA Technologies, USA) and synthesized by Evrogen (Russia). Am lification reactions were carried out in a total volume of 25 µL containing 2 µL of cDNA tem late and SYBR Green dye. The PCR conditions included an initial denaturation at 95°C for 3 minutes, followed by 45 cycles of 95°C for 15 seconds, 59°C for 25 seconds, and 72°C for 15 seconds.
Gene ex ression levels were normalized to Ga dh as a reference gene. Relative quantification was erformed using the ΔΔCT method as described by Livak and Schmittgen (2001). The ΔΔCT value was calculated as the difference between the mean ΔCT of the ex erimental grou and that of the control grou . Fold change (FC) values were derived by ex onential transformation of the ΔΔCT, reflecting the relative change in target gene ex ression com ared to the control.
To evaluate molecular alterations, we selected genes involved in oxidative stress regulation, detoxification, and antioxidant defense mechanisms: Superoxide dismutase 1 (Sod1), NAD(P)H quinone dehydrogenase 1 (Nqo1), Heme oxygenase 1 (Hmox1).
The Sod1 gene encodes the antioxidant enzyme su eroxide dismutase 1 (SOD1), which lays a critical intracellular role in maintaining basal oxidative balance by catalyzing the dismutation of su eroxide radicals generated in both the cytosol and mitochondria (Trist et al., 2021). The Nqo1 gene encodes NAD(P)H quinone dehydrogenase 1 (NQO1), a multifunctional cyto rotective enzyme with antioxidant ro erties that artici ates in redox cycling and cellular rotection against electro hilic stress (Preethi et al., 2022). The Hmox1 gene encodes heme oxygenase-1 (HO-1), a stress-res onsive heat shock rotein (HSP32) that contributes to cellular resilience through its antioxidant, anti-inflammatory, and homeostatic functions
(Kaszubowska et al., 2024). The functional characteristics of the studied genes are summarized in Table 1.
The selection of these genes is based on their critical roles in the athogenesis of oxidative liver injury induced by chronic stress and, as we hy othesize, by food reservatives–s ecifically, the widely used sorbic and benzoic acids. It is well established that chronic stress activates the hy othalamic- ituitary-adrenal axis, leading to elevated glucocorticoid roduction and, consequently, increased generation of reactive oxygen s ecies (ROS). This romotes oxidative damage to li ids, roteins, and DNA, contributing to the develo ment of various athologies –including, as demonstrated in a 12-week mouse study, increased levels of triglycerides and cholesterol, as well as the onset of non-alcoholic fatty liver disease (NAFLD) (Liu et al., 2014). NAFLD is currently one of the most revalent causes of liver disease globally, though regional differences exist. The estimated global revalence of NAFLD in adults is a roximately 32%, with higher rates observed in males (40%) com ared to females (26%) (Teng et al., 2022). The over roduction of ROS requires a com ensatory u regulation of endogenous antioxidant systems, articularly those involving SOD1 and HO-1 (Trist et al., 2021; Kaszubowska et al., 2024). Secondly, the liver – as the rimary organ for xenobiotic metabolism – is es ecially vulnerable to toxic ex osures. Benzoates and sorbates have been shown not only to inhibit cytochrome P450 activity but also to generate secondary free radicals (Pi er et al., 2017), reinforcing the im ortance of evaluating NQO1, a key hase II detoxification enzyme regulated by the Nrf2 athway (Preethi et al., 2022). Thirdly, the combined im act of these stressors may be additive or even synergistic, otentially exhausting the antioxidant reserves of he atocytes. Therefore, monitoring the ex ression of Sod1, Nqo1, and Hmox1 is critical for assessing the ada tive ca acity of liver cells under combined oxidative and xenobiotic stress.
Statistical Analysis. Statistical analyses were conducted using SPSS Statistics 21.0 (IBM, USA). Normality was assessed via the Kolmogorov–Smirnov test. For normally distributed data, one-way analysis of variance (ANOVA) followed by Tukey’s and Tamhane’s ost-hoc tests were a lied. Data are ex ressed as mean and standard error (SE). For non-normal distributions, the Kruskal–Wallis test (for com arisons of three or more grou s) and the Mann–Whitney test (for com arisons between two grou s) were used. Differences were considered statistically significant at < 0.05.
RESULTS
Expression of Antioxidant Defense Genes. The results are resented in Figure 2. The ex ression dynamics of genes involved in he atic antioxidant defense dis layed divergent trends across ex erimental grou s.
Sod1 ex ression (Figure 2A) was significantly elevated in the Chronic Stress grou (1.78 ± 0.30 arbitrary units vs. –0.12 ± 0.39 in the Control,
= 0.0005). In contrast, ex ression remained at control levels in the Sorbic and Benzoic Acids grou and in the Combined Ex osure grou (–0.00 ± 0.36 and 0.39 ± 0.09, res ectively; = 0.8194 and = 0.5871 vs. Control). However, airwise com arisons revealed significant differences between the Stress grou and the Sorbic and Benzoic Acids grou ( = 0.0004), and between the Stress and Combined grou s ( = 0.0001).
Nqo1 ex ression (Figure 2B) did not differ significantly from control in any ex erimental grou : – 0.79 ± 1.06 in Control, –1.66 ± 0.18 in Stress, – 2.62 ± 0.19 in SBA, and –2.94 ± 0.35 in the Combined grou ( = 0.8118, = 0.2565, and = 0.2048 vs.
Control, res ectively). However, intergrou com arisons revealed significant differences between the Stress and SBA grou s ( = 0.0012), and between the Stress and Combined grou s ( = 0.0065).
A similar attern was observed for Hmox1 ex ression (Figure 2C): while no significant differences were detected relative to Control (–0.18 ± 0.68 in Control, 0.34 ± 0.22 in Stress, –1.73 ± 0.22 in SBA, and –1.50 ± 0.26 in Combined; = 0.9358, = 0.1176, and = 0.2025 vs. Control), significant differences emerged between the Stress and SBA grou s, as well as between the Stress and Combined grou s ( = 0.0001 for both).
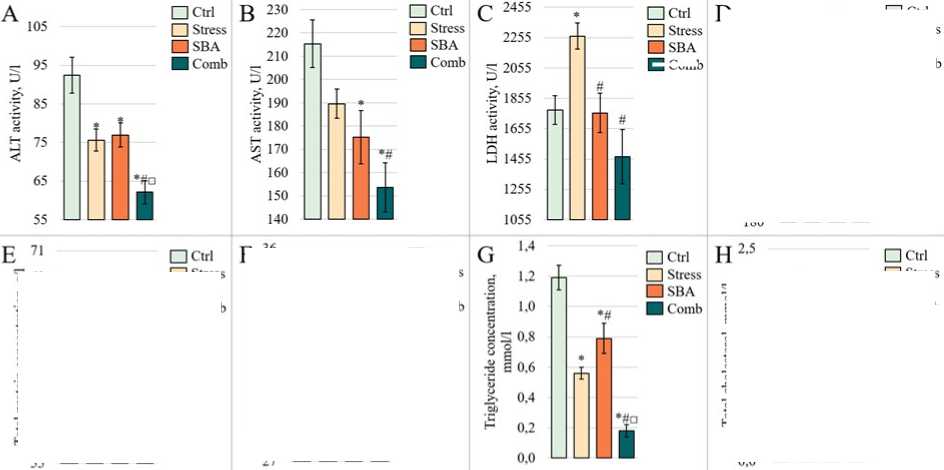
Liver Enzyme Activity. The results of the biochemical analysis are resented in Figure 3. ALT activity (Fig. 3A) was significantly reduced in the Stress grou (75.60 ± 2.87 U/L, = 0.0060) and in the Sorbic and Benzoic Acids grou (76.95 ± 3.12 U/L, = 0.0112) com ared to the Control grou (92.42 ± 4.63 U/L). A further decrease was observed in the combined ex osure grou (62.12 ± 3.02 U/L, = 0.0001). The combined ex osure grou also differed significantly from both single ex osure grou s ( = 0.0044 vs. Stress and = 0.0006 vs. SBA). AST activity showed a similar trend (Fig. 3B), with a decrease in the Stress grou (189.56 ± 6.30 U/L) that did not reach statistical significance vs. Control (215.32 ± 10.27 U/L, = 0.1032). However, AST activity was significantly reduced in the SBA grou (175.17 ± 11.41 U/L, = 0.0372), with a further significant reduction in the combined grou (153.68 ± 10.50 U/L, = 0.0001). A significant difference was also observed between the Stress and combined ex osure grou s ( = 0.0170). In contrast, LDH activity increased only in the Stress grou (2265.69 ± 86.57 U/L vs. 1777.02 ± 93.94 U/L in Control, = 0.0006) and remained at control levels in the SBA (1757.74 ± 129.78 U/L) and combined ex osure (1470.71 ± 180.06 U/L) grou s (Fig. 3C). ALP activity (Fig. 3D) was significantly reduced in the Stress grou (240.08 ± 29.77 U/L vs. 477.35 ± 22.31 U/L in Control, = 0.0001), remained unchanged in the SBA grou (469.50 ± 51.76 U/L, = 0.8884), and was again significantly decreased in the combined grou (268.20 ± 30.64 U/L, = 0.0001).
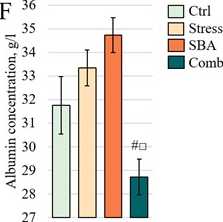
Assessment of Protein Metabolism Parameters. Total serum rotein concentration exhibited a decreasing trend in all ex erimental grou s (Fig. 3E); however, no statistically significant differences from the Control grou were detected ( > 0.05). A nearsignificant difference was observed between the Stress grou (63.38 ± 1.58 g/L) and the combined grou (57.56 ± 1.57 g/L, = 0.0582). Albumin levels (Fig. 3F) showed a slight, non-significant increase in the Stress (33.33 ± 0.76 g/L, = 0.2566) and SBA (34.73 ± 0.73 g/L, = 0.1292) grou s com ared to Control (31.75 ± 1.21 g/L). However, a marked decrease was recorded in the combined ex osure grou (28.72 ± 0.76 g/L), which differed significantly from both isolated ex osure grou s ( = 0.0001 for both com arisons) but not from the Control grou ( = 0.105).
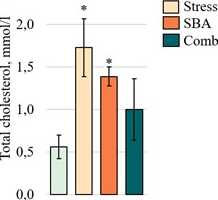
Assessment of Lipid Metabolism Parameters. Triglyceride levels in blood serum (Fig. 3G) were significantly decreased in all ex erimental grou s com ared to Control (1.19 ± 0.08 mmol/L), with the most ronounced reduction observed in the combined grou (0.56 ± 0.04 mmol/L in Stress, = 0.0001; 0.79 ± 0.10 mmol/L in Comb, = 0.0001 vs. Control and vs. each isolated grou , = 0.0001). Conversely, total cholesterol concentrations (Fig. 3H) were significantly elevated in the single ex osure grou s (0.56 ± 0.14 mmol/L in Stress, = 0.0070; 1.73 ± 0.34 mmol/L in SBA, = 0.0001) com ared to Control (1.39 ± 0.11 mmol/L). However, in the combined ex osure grou , cholesterol levels (1.00 ± 0.36 mmol/L) did not significantly differ from Control ( = 0.5908).
Histomorphology. Re resentative liver tissue microgra hs are shown in Figure 4. In rats ex osed to chronic stress, numerous small, immature he atocytes with double nuclei were observed, along with visually detectable anucleated he atocytes. In the Sorbic and Benzoic Acids grou , the liver reserved its ty ical radial arrangement of he atocyte cords relative to the central vein, which a eared mildly congested. He atocyte nuclei were clearly defined and rounded, and the cyto lasm was uniform with no signs of degeneration. In the ortal tracts, mild vascularization and occasional foci of leukocytic infiltration were observed, while hemorrhages and necrotic areas were absent. In the grou subjected to combined ex osure to reservatives and chronic stress, the he atic histoarchitecture remained intact. The radial trabecular attern was reserved; he atocytes across all zones exhibited round nuclei with rominent nucleoli and homogeneous cyto lasm. Central veins and sinusoids were moderately congested, and bile ducts were unobstructed. In the fields of view, clusters of small immature he atocytes were noted alongside areas containing anucleated he atocytes. No signs of inflammatory infiltration, necrosis, or aty ical cell roliferation were detected.
Quantitative mor hometric analysis revealed a series of statistically significant differences. The mean nuclear area of he atocytes in the control grou was 33.42 ± 0.52 μm². In the chronic stress grou , this arameter was significantly reduced (26.53 ± 0.54 μm², < 0.0001), whereas in the grou treated with sorbic and benzoic acids, a significant increase was observed (37.12 ± 0.80 μm², = 0.0002). Notably, in the combined ex osure grou , the nuclear area remained com arable to control values, although a trend toward an increase was detected (35.25 ± 0.78 μm², = 0.0537). No significant differences were found between the combined grou and the reservative-only grou ( = 0.0971), while a statistically significant difference was observed between the combined grou and the stress grou ( < 0.0001).
The cyto lasmic area of he atocytes in the control grou was 200.57 ± 4.67 μm². This value was significantly decreased in the stress grou (168.83 ± 3.29 μm², < 0.0001). In the reservative grou , cyto lasmic area did not differ from the control (207.96 ± 3.93 μm², = 0.2289), whereas in the combined grou , it was significantly reduced (180.84 ± 5.14 μm², = 0.0054). A significant difference was found between the combined and reservative-only grou s ( = 0.0001), but not between the combined and stress grou s ( = 0.0520).
Given that nuclear and cyto lasmic areas alone do not fully ca ture the cellular res onse, a nuclear-to-cyto lasmic (N/C) ratio was calculated. In the control grou , the N/C ratio was 0.17 ± 0.01. In the stress grou , this ratio was 0.16 ± 0.01, which did not differ from control ( = 0.0741). In the reservative grou , it was slightly higher at 0.18 ± 0.01, but not statistically significant ( = 0.1562). However, in the combined ex osure grou , the N/C ratio was significantly increased (0.20 ± 0.01, = 0.0005 vs. control), likely due to a decrease in cyto lasmic area along with a moderate increase in nuclear size. This ratio also differed significantly from the reservative grou ( = 0.0291) and the stress grou ( < 0.0001).
The number of binucleated he atocytes was significantly increased in the chronic stress grou (15.90 ± 1.67 vs. 9.30 ± 1.14 in control, = 0.0043), but did not differ significantly from control in either the reservative grou (8.70 ± 0.50, = 0.6341) or the combined ex osure grou (9.00 ± 0.70, = 0.8246). A statistically significant difference was found between the stress and combined ex osure grou s ( = 0.0013).
10-12 week-old Male Wistar rats
Group 3.
Sorbic and Benzoic Acids
A single dose of sorbic acid 500 mg/kg b.w. and benzoic acid 100 mg/kg b.w.
Group 4.
Sorbic and Benzoic Acids + Chronic Stress
Histology
28 days of exposure Group 1.
Control
Group 2. Chronic Stress
Modeled according to the protocol described by Matisz et al. (2021)
Liver response
Enzymes
ALT, AST, LDH, ALP, I lipid/protein metabolism parameters in blood serum
Gene expression in liver tissue Sod1, Hmoxl, Nqo1

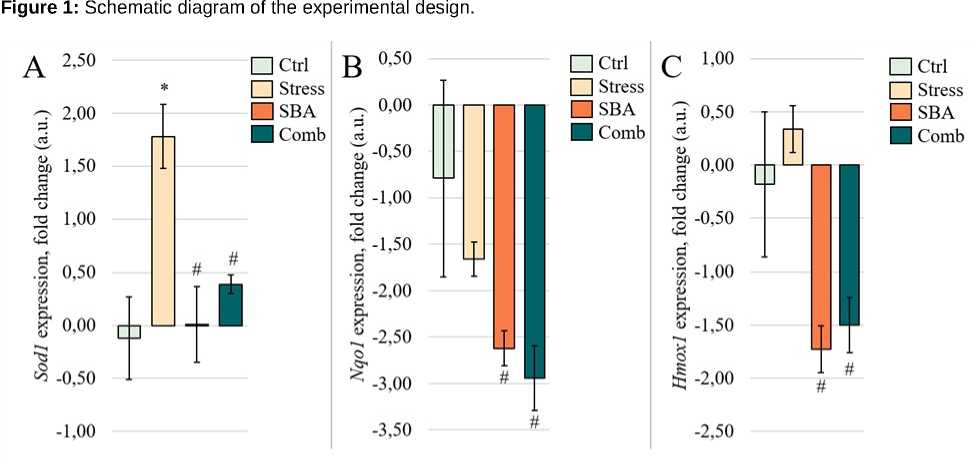
Figure 2: Fold change in ex ression of Sod1 (A), Nqo1 (B), and Hmox1 (C) genes in liver tissue from animals in the Control grou and grou s ex osed to chronic stress (Stress), food reservatives (sorbic and benzoic acids, SBA), or their combination (Comb). The Y-axis shows the fold change relative to control; the X-axis indicates the ex erimental grou s. Asterisks (*) denote significant differences vs. the Control grou ; hash marks (#) vs. the Stress grou .
■ ( t'liih
Activity levels of liver enzymes ALT (A), AST (B), LDH (C), and ALP (D), as
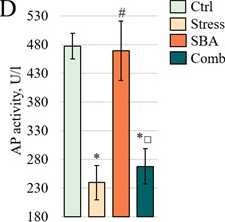
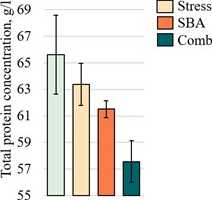
Figure 3:
well as concentrations of total
rotein (E), albumin (F), triglycerides (G), and total cholesterol (H) in the blood serum of animals from the Control grou and those ex osed to chronic stress (Stress), food reservatives (sorbic and benzoic acids, SBA), or their combination (Comb). The Y-axis shows the measured arameter values; the X-axis indicates the ex erimental grou s. Asterisks (*) denote significant differences vs. the Control grou ; hash marks (#) vs. the Stress grou ; and o en squares (□) vs. the SBA grou ( < 0.05).
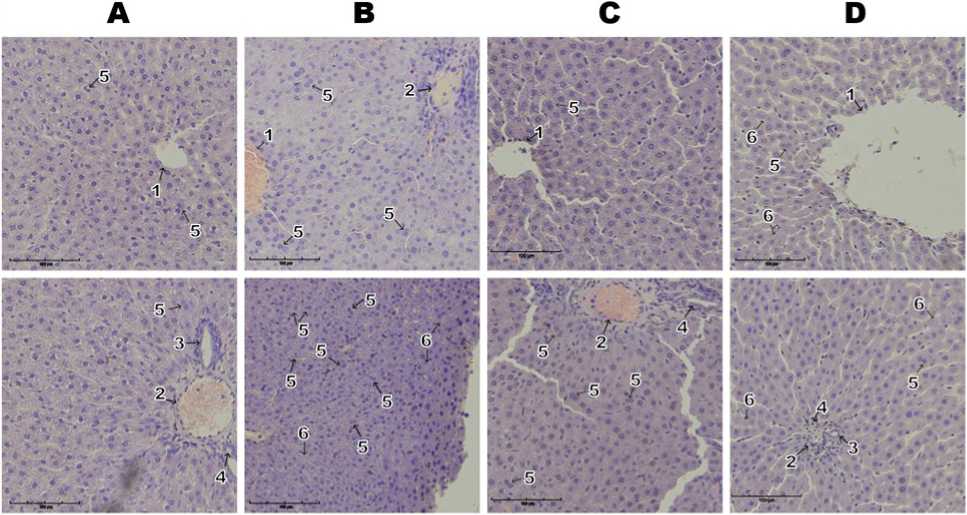
Figure 4: Re resentative microgra hs of liver tissue sections from animals in the Control grou (A) and those ex osed to chronic stress (B), food reservatives – sorbic and benzoic acids (C), or their combination (D). Labeled structures include: central vein (1) in the liver arenchyma; interlobular vein (2), bile duct (3), and interlobular artery (4) within the he atic triad; binucleated young he atocytes (5); and anucleated he atocytes (6). Hematoxylin and eosin staining; original magnification ×200.
Table 1: Characteristics of the Analyzed Genes
|
Gene Name |
NCBI Gene ID |
Reference mRNA Sequence (GenBank) |
Oligonucleotide Primers for PCR Am lification |
|
Superoxide dismutase 1 (Sod1) |
24786 |
NC_086029.1 |
F-5’-CTT-CTG-TCG-TCT-CCT-TGC-TT-3’ R-5’-CTC-CAA-CAT-GCC-TTG-TGT-ATT-G-3’ |
|
NAD(P)H quinone dehydrogenase 1 (Nqo1) |
24314 |
NC_086037.1 |
F-5’-GCT-GCA-GAC-CTG-GTG-ATA-TT-3’ R-5’-AGA-ATC-CTG-CCT-GGA-AGT-TTA-G-3’ |
|
Heme oxygenase 1 (Hmox1) |
24451 |
NM_012580.2 |
F-5’-ATT-TCA-GAA-GGG-CCA-GGT-GA-3’ R-5’-GGA-AGT-AGA-CAG-GGG-CGA-AGA-3’ |
The number of anucleated he atocytes did not differ significantly from control in any ex erimental grou . The values were 3.30 ± 0.37 in the control grou , 3.00 ± 0.39 in the stress grou , 3.00 ± 0.39 in the reservative grou , and 3.60 ± 0.34 in the combined ex osure grou .
DISCUSSION
Our findings are broadly consistent with reviously ublished data demonstrating stress-induced he atic dysfunction and metabolic disru tion. For instance, Xu et al. (2022) showed that a four-week ex osure to chronic sychological stress alone can initiate inflammatory res onses and im air liver function in rats, including he atocyte necrosis. However, the changes observed in our study a eared more com ensatory in nature: in the chronic stress grou , we detected a significant u regulation of Sod1 ex ression, while Nqo1 and Hmox1 ex ression levels remained com arable to the control.
This ex ression attern may indicate an early stage of mitochondrial dysfunction, characterized by activation of the rimary antioxidant defense without the engagement of hase II detoxification mechanisms (Trist et al., 2021; Kaszubowska et al., 2024; Pi er et al., 2017; Preethi et al., 2022). This hy othesis is su orted by the findings of Komar et al. (2021), who re orted that in obesity, he atic SOD1 ex ression and mitochondrial DNA co y number are reduced, correlating with the severity of steatosis. Given the high sensitivity of mitochondria to ROS, chronic stress accom anied by ROS over roduction may selectively induce Sod1 ex ression as a mitochondrial ada tive res onse to su eroxide anions. In contrast, the lack of Nqo1 and Hmox1 induction may result from transcri tional su ression under ersistent hy ercortisolemia, as reviously described by Joëls et al. (2019).
Biochemical data further su ort this inter retation. Elevated LDH activity combined with reduced ALT, AST, and ALP levels may indicate develo ing mitochondrial dysfunction in the absence of overt cytolysis— considering that LDH release is associated with reduced tissue oxygenation and rogressing hy oxia (Kotoh et al., 2011), while ALT and AST are classic markers of he atocellular injury (Bonventre & Yang, 2010; Botros & Sikaris, 2013; Sookoian & Pirola, 2015; de Vos et al., 2019). These findings align with histological observations showing no necrosis but the resence of binucleated he atocytes, which may reflect com ensatory regeneration or a rotective res onse to stress (Darmasa utra et al., 2024). Notably, binucleated he atocytes are the main roliferative cell ty e in regenerating liver, including after toxic injury (Hammad et al., 2014). Total rotein and albumin levels remained unchanged, indicating reserved synthetic liver function. Dysregulated li id metabolism, with reduced triglycerides and elevated cholesterol, is consistent with stress-induced dysli idemia (Dille et al., 2022; Xu et al., 2022). Interestingly, des ite the reservation of the nucleus-to-cyto lasm ratio in the chronic stress grou , a significant reduction in both nuclear and cyto lasmic areas of he atocytes was observed. In combination with an increased number of binucleated cells, this may indicate a redominance of young, functionally active he atocytes involved in regenerative rocesses. It is lausible that by the end of the 28-day ex osure eriod, the mor hological icture reflected not the acute hase of injury, but rather the ongoing recovery hase of the liver.
Under isolated ex osure to reservatives (SBA grou ), we observed signs of xenobiotic-induced metabolic disru tion. The decrease in ALT and AST activity, in the absence of cytolysis, may suggest transaminase su ression (Cornell et al., 1984), although we did not find direct evidence of such effects s ecifically for sorbic and benzoic acids. LDH and ALP levels remained stable, whereas Nqo1 and Hmox1 ex ression was significantly su ressed— ossibly indicating de letion or downregulation of antioxidant systems following an initial activation. These alterations occurred alongside dysli idemia, consistent with toxic liver effects of food reservatives (Taghavi et al., 2016; Chen et al., 2020). Histological examination of the grou ex osed to food reservatives revealed signs of moderate he atocellular hy ertro hy, in contrast to the cellular reduction observed under chronic stress. The simultaneous enlargement of both nuclear and cyto lasmic com artments, while maintaining a stable nucleus-to-cyto lasm ratio, may suggest functional overload of mature he atocytes, articularly in light of the accom anying biochemical alterations. A slight decrease in the number of binucleated cells and the unchanged count of anucleated cells indicate the absence of acute cytotoxic injury. These findings oint to functional strain without overt cellular destruction, though a risk of subsequent decom ensation remains with rolonged xenobiotic ex osure.
The combined ex osure grou deserves articular attention. Here, a marked reduction was observed in all enzyme activities (ALT, AST, ALP, LDH), accom anied by a significant decrease in albumin levels—indicative of im aired energy metabolism and rotein synthesis. Serum triglycerides were also shar ly reduced, otentially reflecting im aired β-oxidation and li id mobilization. At the molecular level, Sod1 ex ression remained unchanged, while Nqo1 and Hmox1 were downregulated, suggesting a breakdown in the antioxidant defense system. In the setting of reserved he atic histoarchitecture and stable numbers of binucleated and anucleated he atocytes, a statistically significant reduction in cyto lasmic area was detected, accom anied by an increased nucleus-to-cyto lasm ratio. This mor hological attern may re resent an early manifestation of cellular stress in the absence of overt tissue damage. The observed cyto lasmic reduction is likely related to diminished cellular energy reserves and contraction of metabolically active organelles, such as mitochondria and the endo lasmic reticulum. Preservation of nuclear size may suggest maintained or even com ensatorily increased transcri tional activity. Collectively, these findings su ort the inter retation of an ada tive remodeling hase receding the onset of functional he atic decom ensation.
Taken together, our findings suggest that chronic stress modifies the he atotoxic effects of food reservatives, intensifying molecular and functional alterations even in the absence of overt structural liver damage. However, definitive conclusions regarding the nature of this interaction—whether additive, synergistic, or antagonistic—require quantitative assessment and the develo ment of mathematical a roaches to combined toxicity evaluation. This re resents an im ortant avenue for future investigation.
Several methodological limitations must be considered in inter reting our results. First, antioxidant gene ex ression (Sod1, Nqo1, Hmox1) was assessed only at the transcri tional level, without corres onding rotein quantification or functional enzymatic activity, and without measurement of ro- and antioxidant metabolites. Second, the 28-day duration of the ex eriment ca tures subacute effects only, leaving otential long-term outcomes unex lored. Third, mitochondrial status was not directly assessed, des ite the ro osed involvement of mitochondrial dysfunction in the mechanisms of stress and toxicant action. Fourth, the absence of a quantitative framework for analyzing the interaction between stress and reservative ex osure (e.g., models of additivity, synergy, or antagonism) limits the inter retation and risk rediction of combined ex osure. These as ects form the basis for future research aimed at in-de th molecular, mor hometric, and com utational validation of our findings.
CONCLUSIONS
Our findings demonstrate that chronic sychological stress can modulate the he atotoxic effects of food reservatives in a 28-day ex erimental model in male rats. The combined ex osure resulted in am lified alterations in he atic molecular and biochemical arameters. Des ite reserved liver histoarchitecture, signs of functional and antioxidant system decom ensation were observed in the grou ex osed to reservatives under chronic stress conditions.
The mechanisms underlying this modification remain insufficiently understood; however, they are likely to involve mitochondrial dysfunction, su ression of hase II detoxification gene ex ression, and im aired li id regulation.
The lack of a quantitative assessment of the nature of interaction between the studied factors limits our ability to accurately redict health risks and inter ret the findings. This highlights the urgent need for the develo ment and validation of a methodological a roach to quantitatively evaluate the ty e of combined action—whether additive, synergistic, or antagonistic— between sychological stress and chemical ex osure.
AUTHOR CONTRIBUTION
Conce tualization, Project administration: D.O. Karimov, Yu.V. Ryabova, E.F. Re ina
Writing – original draft: Yu.V. Ryabova, Ya.V. Valova, A.A. Gizatullina
Investigation: A.A. Gizatullina, T.G. Yaku ova, D. A. Smolyankin, O.A. Khmel, M.V. Kurilov
Visualization: Yu.V. Ryabova, O.A. Khmel, D. A. Smolyankin
Validation, Formal analysis: Yu.V. Ryabova, D.O. Karimov
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.


