Circadian clock precision, health, and longevity
Автор: Gubin D.G., Kolomeichuk S.N., Weinert D.
Журнал: Тюменский медицинский журнал @tmjournal
Статья в выпуске: 1 т.23, 2021 года.
Бесплатный доступ
An accurate circadian clock, associated with the precise intrinsic period, tau, can be linked with a definite chronotype and health status. Exemplified by different animal species, and, as we argue herein, may as well be in humans, endogenous tau close enough to 24 hours is associated with higher life expectancy, lower morbidity, and is possibly adhered to moderate morning chronotype. Accurate circadian tau facilitates maintaining a high amplitude of circadian rhythms, a phenotypic trait that is related to health and longevity. Some genetic factors that coordinate tau and ensure circadian clock precision is considered.
Health, longevity, life expectancy, intrinsic circadian period, chronotype, circadian resonance, amplitude, morbidity, mortality, genetics, genes, polymorphism
Короткий адрес: https://sciup.org/140303371
IDR: 140303371 | DOI: 10.36361/2307-4698-2020-23-1-3-15
Текст научной статьи Circadian clock precision, health, and longevity
The speed of the biological clock and the circadian period. The biological clock (BC) of the can run with different speed, species-specific feature which is determined by the internal circadian period ( tau ) and vary in the range of ~24 ± 4 hours (Dibner et al., 2010). Each organism and every cell have its own tau , which is largely predetermined by individual polymorphism of core clock genes, CG; though some other related genes also matter. Cellular BC is conserved in its basic principles and has common evolutionary prerequisites (Reppert & Weaver, 2002; Partch et al, 2014; Takahashi, 2017). Optimal BC synchronization implies phase adjustment (entrainment) and coordinated circadian rhythms (CR) of metabolism (assimilation and dissimilation phases) (Gubin, 2013; Dibner et al, 2010; Eckel-Mahan & Sassone-Corsi, 2013; Millius & Ueda, 2017; Takahashi, 2017), but also the balance of redox state (Edgar et al, 2012).
The endogenous circadian tau can be accurately determined in the absence of changes in ambient light. Under constant light conditions, BC free-runs with an endogenous period, tau, somewhat different from 24 hours. Deviations of tau are possible in the range of 24 ± 4 hours (defining term circadian = “circa + dies”). There are 2 main models BC synchronization by light (Daan, 2000). According to the parametric model of Jurgen Aschoff, the direction of the shift in one direction or another from 24 hours depends on the level of external illumination and in species with day and night activity, changes in different directions. In diurnally active animals, including humans, constant light speeds up BC, reducing tau. In the dark, tau, on the contrary, increases. In nocturnal species, the situation is the opposite. The nonparametric model of Colin Pittendrigh considers moments of abrupt transition from light to dark and backward in the phase adjustment of the circadian rhythms (CR) and its synchronization with external photoperiodic conditions.
In everyday conditions, the main BC synchronizing factor is light (changes in its physical characteristics – illumination intensity and spectral composition) (Beersma & Daan, 1993). Light enters the retina, mainly through melanopsin receptors, transmitting signal further through the retinohypothalamic tract to the central oscillator of the hypothalamus, paired suprachiasmatic nuclei, SCN (Provencio & Warthen, 2012), indirectly affecting the production of melatonin by the pineal gland (Phillips et al, 2019). Even minor changes in ambient light during the day are enough to synchronize the CR, which ensures their accurate tau = 24 hours.
Tau , phenotypic CRs amplitude and health. ^e amplitude of phenotypic CRs (parameters measured in the clinic, i.e., physiological and biochemical variables) is determined by the phase stability of the underlying BC. Factors that affect BC tau , or synchronicity between coordinated CRs, can thereby reduce the amplitude of the CR and provoke circadian disruption. Thus, CR amplitude is an important integral indicator of synchronization / circadian alignment. CR amplitude may also serve as a non-specific criterion for the robustness of the circadian system and its adaptability, which develops both during evolution and during individual development (Gubin and Gerlovin, 1980). Aging, however, usually associates with a gradual decrease in the CR amplitude (Gubin and Weinert, 1991). Moreover, it was hypothesized that CR amplitude may serve a marker for quantification of “health rating”, which can be used for early diagnostics of pathologies (Gubin and Chesnokov, 1976).
In recent years, many studies have related CR amplitude to comprehensive health assessment, and pointed to benefits of proper / “high enough” CR amplitude (Buhr & Takahashi, 2013; Eckel-Mahan & Sassone-Corsi, 2013; van der Berg et al, 2017; Gubin et al, 2013, 2017; Chen et al, 2013; Dupont Rocher et al, 2016; Maloney et al, 2019; Weinert & Gubin, 2018; Takahashi et al, 2017; Zare et al, 2017). A high CR amplitude of serum cholesterol was associated with human lifespan (van der Berg et al, 2017), and a high CR amplitude of temperature and motor activity was a predictor of the lifespan of laboratory mice (Basso et al, 2016). Higher levels of physical activity and oxygen capacity in the elderly correlate with a high CR amplitude of expression of the clock gene Per3 (Takahashi et al, 2017) and a CR amplitude of oral temperature (Dupont Rocher et al, 2016). Caloric restriction, a proven way to increase life expectancy, is accompanied by an increase in the CR amplitude (Nelson and Halberg, 1986; Escobar et al, 2011). On the contrary, a decrease in the amplitude (absence of significant CR) of the thyroid-stimulating hormone, TSH was detected in Alzheimer’s disease against the background of lower average daily production of this hormone (Chen et al, 2013). A sharp decrease in the CR amplitude, up to the disappearance of the rhythmicity of one of the key clock genes in Drosophila, TIMELESS, and a violation of the CR expression of another key per gene in peripheral tissues is a predictor of death in different age groups (Zhao et al, 2018). A decrease in the amplitude of the CR rhythm of body temperature, heart rate, and fasting glucose levels was found in pre-diabetes and type 2 diabetes among individuals with increased body mass index (Gubin et al, 2017). Pronounced phase and amplitude changes in CR temperature (Gubin et al, 2019) and amplitude-phase changes in intraocular pressure, IOP (Gubin et al, 2018) are observed in the progression of the primary open-angle glaucoma. It is the low CR amplitude, along with a decrease in sleep quality, that was the leading factor associated with the forecast of absence from the workplace due to temporary disability among gas industry workers (Zare et al, 2017). Finally, a decrease in the CR amplitude is a characteristic attribute of the aging process itself (reviews: Gubin and Weinert, 2015; Gubin et al, 2016), and the maintenance of a genetically determined high CR amplitude is associated with a longer life span (Hurd & Ralf, 1998; Froy et al, 2006).
Circadian resonance hypothesis. High CR amplitude may be linked with precise enough endogenous BC, i.e., with tau close enough to exactly 24 hours (Pitterndrigh et al, 1991; Agajanyan and Gubin, 2004). Hypothesis justifying the general biological expediency and adaptability of the exact τau, also known as the “circadian resonance” hypothesis, was first proposed by Pittendrigh and Bruce in 1959 (Pittendrigh & Bruce, 1959). Subsequently, it was shown that the relationship between the internal (biological) time and external entraining factors for different species, including humans, is mainly determined by τau (Wright et al, 2005).
Intrinsic tau and life expectancy. Interestingly, precise endogenous tau can not only be associated with higher and desynchronization-resistant circadian amplitude but with a longer life span, which was shown in studies on various species of not only mammals, in particular, rodents and Primates (Pittendrigh & Minis, 1972; Hurd & Ralf, 1998; Martino et al, 2008; Wyse et al, 2010; Gutman et al, 2011; Libert et al, 2012; Hozer et al., 2020), insects (i. e. Drosophila) (Katewa et al, 2016), but also plants (Dodd et al, 2005) and even cyanobacteria (Ouyang et al, 1998). Mice that have precise tau maintain it stable even during aging (Gutman et al, 2011). In a study on a mouse, an approximate confidence interval of the required tau accuracy was established with a standard deviation within 7 minutes, associated with a significant increase in life expectancy (Libert et al, 2012).
Chronotype and tau . To date, accurate measurement of tau remains a uneasy and time-consuming method, since it is possible only when the subject is located in controlled laboratory conditions for several days under constant conditions (constant route protocol) or under forced desynchrony protocol (Czeisler et al, 1999; Chang et al, 2019), or, with less accuracy, cellular can be assessed by studying fibroblast cell cultures (Pagani et al, 2010; Hida et al, 2013). On the other hand, chronotype score can be obtained by simple questionnaires that can serve as approximate estimate of tau (Duffy et al, 2001; Emens et al., 2009). The chronotype questionnaires using one of the two most common methods, Horne-Ostberg (1976) or Munich questionnaire (Roenneberg et al., 2003), are also widely used and simple ways to evaluate BC phase.
In the representative statistical sample (Knutson & von Shantz, 2018), an analysis of UK Biobank data of almost half a million Englishmen showed a clear relationship between the evening chronotype and an increased risk of morbidity and mortality. In the discussion, the authors of this paper agree with many others that this relationship may be a consequence of the existing social discrimination of evening chronotypes, forced to adapt to the Procrustean bed of standard social working regimes. In the conditions of modern civilization, persons with evening chronotype are the most susceptible to external circadian disruption – namely, social jet lag (misalignment between intrinsic BC and social factors) (Roenneberg et al, 2003). Indeed, the severity of social jet lag correlates with complex metabolic disorders, including age-associated chronic pathology (high-density lipoproteins, increased triglycerides, fasting insulin, insulin resistance, risk of metabolic syndrome, predisposition to type 2 diabetes and atherosclerosis (Wong et al, 2015), as well as a higher prevalence of sleep apnea and higher levels of stress hormones (Lucassen et al, 2013). Many studies also showed relationship between sleep disorders / sleep deficits with changes in lipid metabolism (Aho et al, 2016; Weljie et al, 2015) and carbohydrate metabolism (Maury, 2019; Poggiogalle et al, 2018).
However, beyond social, a biological explanation may exist. Namely, conjunction between certain chronotype and precise intrinsic tau .
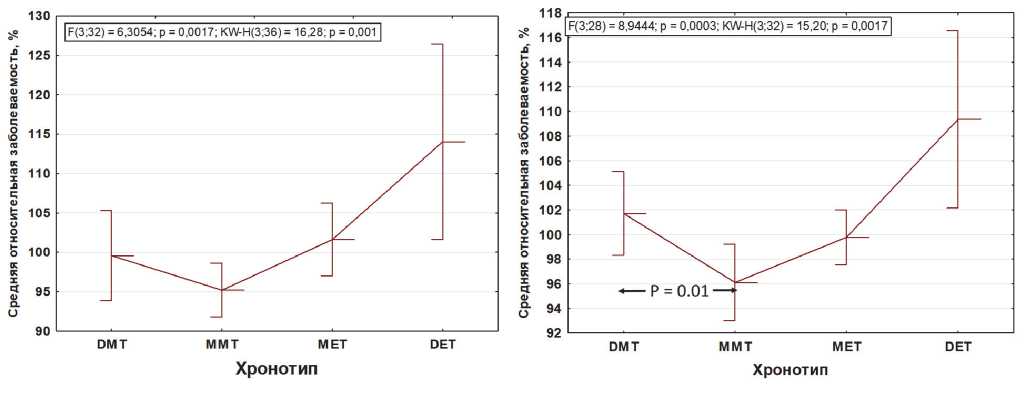
Tau, chronotype and health, or which chronotype is better? It is hardly possible to give a simple answer to this question. However, it is possible to justify the relationship between the precise tau and the chronotype. Based on the original data presented in the referenced paper (Knutson & von Shantz, 2018), we attempted to analyse the association between the 4 variants of the chronotype and the comorbidities. Figure 1 shows our calculations based on the original table data of Knutson & von Shantz, 2018. Data from Table 1 of the original article on comorbid pathology were expressed as a percentage for each of the 9 comorbid pathologies. Then the average relative incidence was calculated for each chronotype. We excluded from the calculations the incidence “psychological disorders» since the distinctive feature of it was a clear linear relationship between chronotypes. Stand alone “somatic pathology” acquired a nonlinear relationship that fits into the concept of “circadian resonance” – the lowest incidence for various somatic pathologies is stably associated not with marginal definite morning, but rather with a moderate morning chronotype (as we will argue below this type is most likely be attributed to precise intrinsic tau ). The differences in pooled somatic comorbidity between definitive and moderate morning chronotype is significant (p = 0.01), Fig.1. This trend is most clearly seen in the case of diabetes and other endocrine pathologies, and persists for respiratory, gastrointestinal, and musculoskeletal pathologies. Notably, cardiovascular, and renal pathology have inverted regularity with the highest incidence in definite morning type. Neurological pathology holds the linear trend, which is most clearly manifested for psychological pathologies, although it is expressed to a lesser extent.
We presume that this observation serves an argument for the biological rather than social justification of the relationship between chronotype and somatic pathologies. It also assumes a diverse nature of the relationship of chronotype features with psychological disorders and somatic pathology.
Interestingly, tau also has significant sex differences (Duffy et al, 2011): in women, the average tau is closer to the exact value of 24 hours, Fig. 2, which is remarkable given the greater average life expectancy of women (Austad
& Fischer, 2019). But if the circadian resonance hypothesis is correct to justify sexual differences in life expectancy, then the quality of sleep in this case does not seem to play a role, since sleep disorders are more typical of the females (Zhang & Wing, 2006;
Table 1 – Relative incidence expressed as a percentage with different prevailing comorbid nosology for 4 chronotypes (additional analysis of UK BioBank data, based on data from Knutson & von Schantz, 2018)
|
Prevalent Comorbidity (PC) |
DMT |
MMT |
MET |
DET |
|
Cardiovascular |
106.0773 |
98.0663 |
97.23757 |
99.72376 |
|
Diabetes |
108 |
88 |
98 |
124 |
|
Neurological |
95.74468 |
96.80851 |
102.1277 |
114.8936 |
|
Endocrine |
101.5873 |
98.4127 |
100 |
106.3492 |
|
Renal |
102.1739 |
100 |
95.65217 |
100 |
|
Respiratory |
99.38272 |
93.82716 |
101.8519 |
116.6667 |
|
Musculoskeletal |
102.7907 |
97.2093 |
100 |
103.7209 |
|
Gastrointestinal |
97.94521 |
96.57534 |
103.4247 |
109.589 |
|
Psychological |
82.43243 |
87.83784 |
116.2162 |
151.3514 |
|
Overall mean |
99.57048 |
95.19302 |
101.6122 |
114.0327 |
|
Somatic PC mean |
101.7127 |
96.11241 |
99.78674 |
109.3679 |
DMT – definite morning type; MMT – moderate morning type; MET – moderate evening type; DET – definite evening type.
Similarly, many studies have found that women are more morning chronotypes than men (Adan & Natale, 2002; Bailey & Silver, 2014; Hu et al, 2016). However, the chronotype, unlike tau , which does not change with age (Duffy et al, 2001), undergoes significant age-related changes towards earlier (morning) types (reviewed in Hood & Amir, 2017). Of note, chronotype changes with age in men and women unequally: in men, the changes are more pronounced. After 40 years, the chronotype of men, being initially more “evening” becomes on average more “morning” (Duarte et al, 2014; Fischer et al, 2017). This pattern is probably due to epigenetic factors, primarily features of age-related hormonal dynamics (Bailey & Silver, 2014). Thus, the relationship between tau and chronotype features (adherence to a particular phase of sleep) is better observed at earlier stages of ontogenesis, decreasing with age, which was shown in the example of other primates (macaques) (Zhdanova et al, 2011). Notably, the highest tau stability is found in mice with a higher CR amplitude and longer life span (Gutman et al, 2011).
Curiously, the relationship between the optimal sleep duration for most people and morbidity, on the one hand, is also U-shaped, increasing in marginal cases – when sleep duration is < 6 and > 9 hours (Ferrie et al, 2007; Yin et al, 2017). On the other hand, U-shaped curve differs from the normal distribution and has marked intersex differences (Ferrie et al, 2007).
Further studies of individual genetic features that determine resistance to desynchronizing factors are important. It is interesting to what extent large individual differences in degree of decrease in melatonin production to light of the same intensity (Phillips et al, 2019) may be related to tau and a specific chronotype.
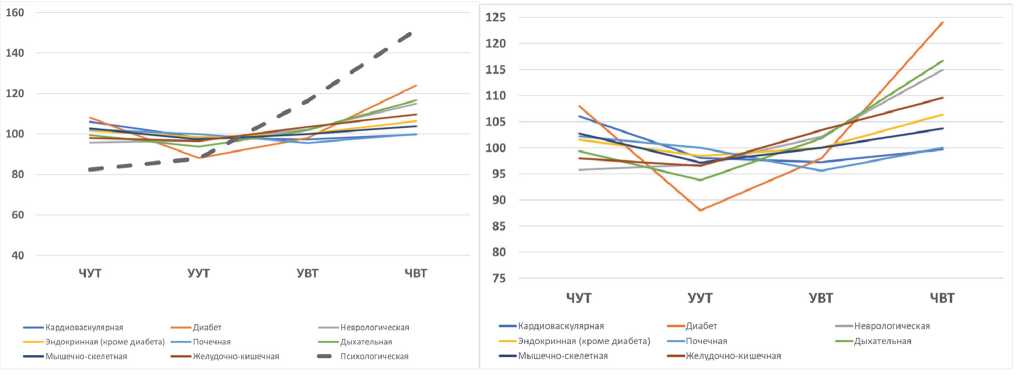
A
B
C
D
Figure 1 – Chronotype and prevalent comorbidities (additional analysis of UK BioBank data, based on original data from Knutson & von Schantz, 2018)
A unidirectional trend of increasing morbidity from morning to evening chronotypes can be traced only for pathology “psychological disorders”, a dotted line (A). Somatic pathology of eight different nosology (B), as well as the overall incidence (C), are devoid of such a linear relationship. After withdrawal of “psychological disorders”, the distinctive feature of which was a linear relationship between chronotypes, all somatic pathology acquires a nonlinear relationship and fits into the concept of “circadian resonance” – the lowest incidence associates with a moderate morning chronotype (likely owners of the precise intrinsic circadian period, tau). Moreover, the differences in the average relative incidence of somatic pathology between the owners of definite and moderate morning chronotype is significant (p=0.01; two-tailed test of student) (D). Abscissa left to right: DMT – definite morning type; MMT – moderate morning type; MET – moderate evening type; DET – definite evening type.
In modern industrial civilization, we actively manipulate the light factor. Typical consequences of such manipulations are a significant shortage of daylight and an excess of night light (Roenneberg et al, 2003; Yetish et al, 2015; Dijk & Skeldon, 2015;
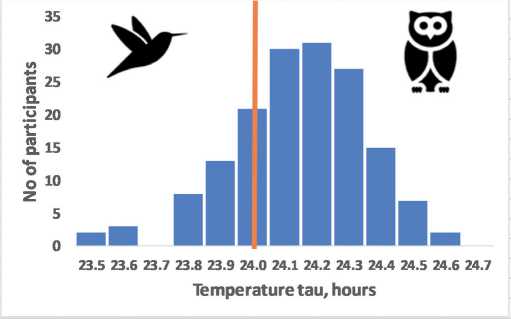
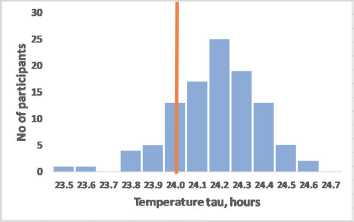
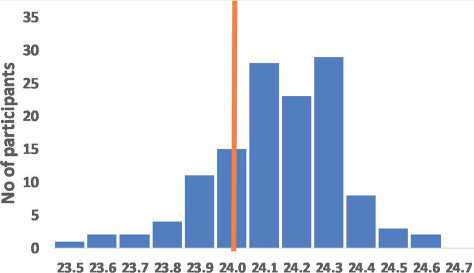
Melatonin tau, hours
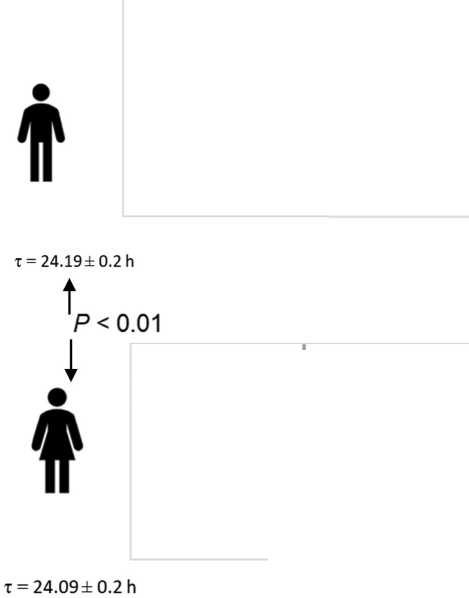
Figure 2 – The assumed relationship of precise circadian tau with moderate morning chronotype exemplified by sex differences
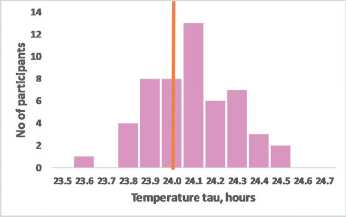
Frequency distribution for the intrinsic tau assumes that precise intrinsic tau most likely associates with moderate morning chronotype (A). Women who have on average a higher average life expectancy also have on average more accurate BC (tau = 24.09±0.2 hours), than men (tau = 24.19±0.2 hours), p<0.01 (B) (data redrawn from Duffy et al, 2011). Th e vertical line indicates the frequency of accurate tau = 24 hours.
spontaneously choose the optimal calorie-restricted diet.
Which chronotype is closer to tau 24 (most accurate biological clock)?
An approximate recalculation of the test scores for the chronotype of the Morning-Evening Questionnaire, MEQ scale accordingly to the average tau can be based on the fact that data obtained in the most representative study (Duffy et al, 2011), the range of actually recorded values of tau in humans is 23.5-25.7 hours, and MEQ chronotype scale (Horne & Ostberg, 1976) varies in the range from 86 (extreme morning) to 16 (extreme evening). Since the later phase position corresponds to slower clock (Toh et al., 2001; Duffy et al, 2001; Merrow et al, 2005), then the hypothetical linear relationship between the chronotype and tau can have the form shown in Fig.3. Frequency distribution of data for the internal tau period assumes that the owners of the accurate tau = 24 hours correspond to a score of MEQ=57. Thus, the deviation from MEQ57 (Δ MEQ57) can to some extent characterize the expected deviation from the precise tau = 24 hours.
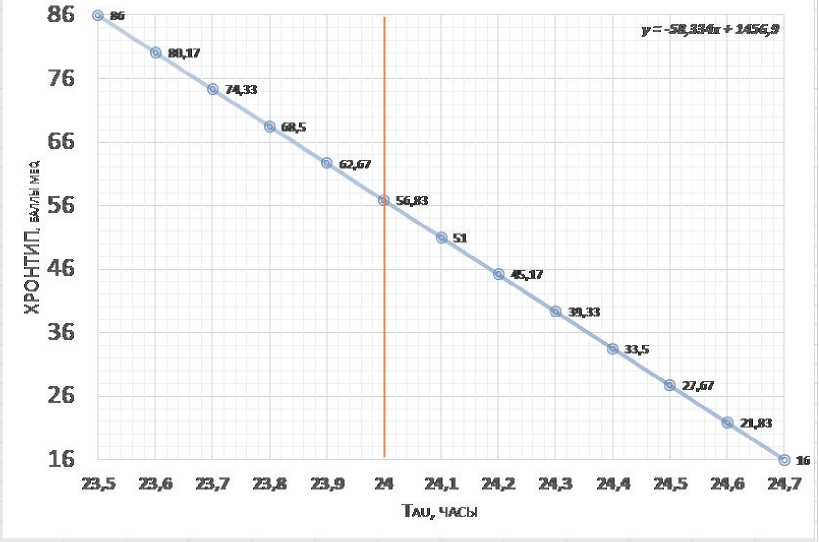
Figure 3 – Expected relation between intrinsic tau and MEQ chronotype score (intrinsic tau range based on data from Duffy et al, 2011, see also Fig.2; and MEQ from Horne & Ostberg, 1976)
Th e frequency distribution of data for the intrinsic tau assumes that precise tau = 24 hours correspond to MEQ57 score (~56.83). Th e vertical line marks tau=24 hours.
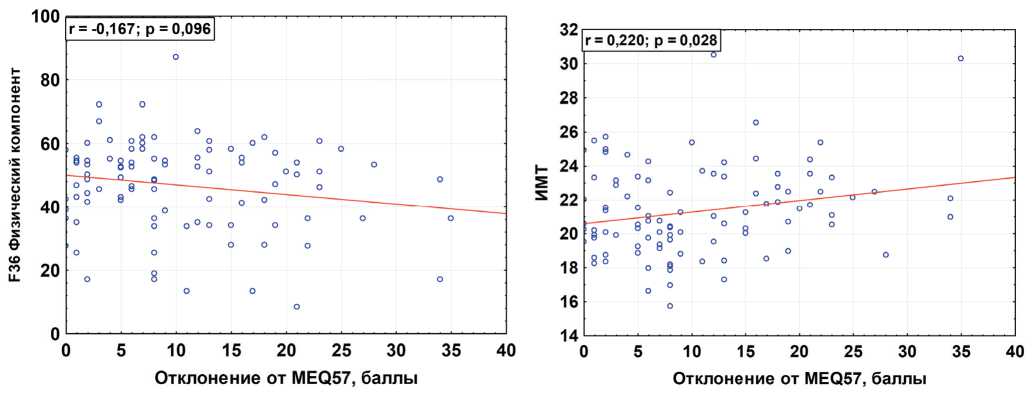
Figure 4 – Correlation between ΔMEQ57 (deviations from the assumed precise tau) with the score of the physical component of health in the SF-36 questionnaire (A) and the body mass index (BMI) (b) (data from the own study of 100 first-year students at the medical University)
Tau genetics. The obvious factors are polymorphic variants of the core clock genes (PER 1,2,3; CRY 1,2; BMAL and CLOCK). The most pronounced influence on the speed BC and the accuracy of tau are proteins of the PER family (which, in fact, is assumed by their name) and, also, CRY. For example, the mouse tau linearly depends on PER/PER2 proteins ratio. At the lowest PER1/ PER2 ratio (knockout genotype PER1 -/ -), tau extends up to the extreme values of the circadian range ~ 28h in constant light mode, while in constant dark mode tau increases slightly (Tamiya et al, 2016). Duration of existence for protein products of PER gene expression is crucial for BC speed, which, in turn, is determined by the dynamics of their stabilization and phosphorylation. The activity of casein kinases, primarily CK1ε, in PER2 phosphorylation determines the mendelian nature of tau inheritance in Syrian hamsters by an autosomal dominant type with incomplete expression in SCN neurons (Lowrey & Takahashi, 2011). A mutation first discovered in 1988 (Ralph & Menaker, 1988), in homozygotes with the highest CK1 activity, causes a change in tau up to 20 hours, in heterozygotes ~ 22 hours, while in wild-type homozygotes tau ~ 24 hours. In another classic paper, the authors showed that the transplantation of a donor SCN allografts determines tau phenotype in the recipient (Ralph et al, 1990). Recent study conducted under controlled laboratory conditions (forced desynchrony protocol) discovered association of T-allele of the SNP rs35333999 polymorphic marker of the human PER2 gene with tau elongation by 11-12 minutes (Chang et al, 2019). Genomewide association studies (GWAS) aimed at searching for associations between genetic polymorphisms and chronotype in the UK Biobank database also confirmed the role of the SNPs for genes PER2 and PER3 (Hu et al, 2016; Lane et al, 2016; Jones et al, 2016; Kalmbach et al, 2017). Expression of another Period gene, PER3, is probably less associated with light information than PER1/2 (Zylka et al., 1998). This gene may be more related to the quality and duration of sleep (Archer et al., 2008, 2018; Viena et al., 2016), or to individual characteristics of the activity and chronotype (Viola et al., 2007; Hida et al., 2014; Turco et al., 2017). Literature data on the role of PER3 polymorphisms (for example, PER3 VNTR) in the regulation of tau are contradictory (Dijk & Archer, 2010; Hasan et al, 2012, 2014; Mansour et al, 2017).
The dominant allele of the CRY1 gene (CRY1 Δ11) was identified as an inherited factor that causes the extreme evening variant of the chronotype (Patke et al, 2017) with a frequency of 0.6%. Individuals heterozygous for this allele may have an alternative phenotype associated with sleep fragmentation.
Since the 24-hour expression cycle of CRY and PER proteins determines the speed of BC, factors affecting the rate of stabilization and phosphorylation of these proteins at the post-translational level should also affect tau (Toh et al, 2001; Meng et al, 2007; Jordan & Lamia, 2013; Takahashi, 2017). AMP-activated protein kinase can serve as a link between energy exchange, nutrition, and the speed of BC. Thus, metabolic factors that can affect AMP-activated protein kinase (Jordan & Lamia, 2013) may also affect tau . The presence of glucose in the extracellular environment inhibits the expression of the PER1 and PER2 genes (Hirota et al, 2012), and accordingly, the rate of its metabolism and entry into the cell, as well as sensitivity to insulin, can modulate BC speed (Nohara et al, 2019), at least in peripheral tissues. Since external desynchronization factors, such as jet lag, are interrelated with dysbiosis, and the microbiota, in turn, participates in the daily dynamics of glucose metabolism (Thaiss et al, 2014), biochemical factors of the intestinal microbiome can also participate in the modulation of tau and BC phase.
Conflicting results have been obtained regarding the role of the CLOCK gene, in particular one of its actively studied polymorphisms, CLOCK3111T/C (rs1801260): a number of studies have shown the relationship of the minor C-allele of the Clock3111t/C polymorphic marker with the evening chronotype, longer tau, or sleep phase delay (Katzenberg et al, 1998; Benedetti et al, 2007; Garaulet et al, 2011; Bandin et al, 2013), while other authors have not confirmed such data (von Schantz & Archer, 2003; Barclay ET al, 2011; Chang et al, 2011). However, the results obtained recently in mice showed that the C-allele of the polymorphic marker CLOCK3111T/C increases the expression of CLOCK protein, as well as the expression of the PER2 gene, which describes putative mechanism by which the C-allele is theoretically able to lengthen tau (Ozburn et al, 2016).
A number of microRNAs are also able to extend tau by modulating the expression of Per (miR-24, miR-132), or Clock (miR-17-5p), particularly in peripheral tissues. Such factors as FBXL3, which regulates the rate of PER/ CRY dimer formation, and GSK3β, which reduces the rate of REV-ERB protein metabolism, also have a similar effect (Gubin, 2013).
Interestingly, according to GWAS (Jones et al, 2019), the genes responsible for the sleep phase and sleep duration differ significantly. Thus, genetics may explain the apparent contradiction between, on the one hand, a higher average life expectancy, a more accurate tau , and a more morning chronotype of women, and, on the other hand, a greater prevalence of insomnia and sleep disorders among women. At the same time, existing data leave open the question of genetic factors of intersex differences tau there is no indication of candidate genes that would be associated with sex chromosomes.
Mechanisms of external synchronization and biological age. Beyond relationship among circadian amplitude, clock accuracy (intrinsic tau ) and life expectancy, we also have previously proposed that circadian amplitudes (Gubin and Weinert, 1991; Durov,
1999) and / or specific spectral transformation (Gubin et al, 1997, 2013; Gubin et al, 1998) can quantify biological age, non-specific resistance, and individual aging rate. Objective analysis of biological age remains a non-trivial and complex task, which uses a variety of approaches (Moskalev et al, 2017); high hopes are placed on the modern capabilities of artificial intelligence (Zhavoronkov et al, 2019). In a recent study, the frailty index (Li et al, 2020) was recognized as one of the most accurate criteria for assessing biological age. We suggest that additional factors contributing to reduced viability are also those that provoke misalignment between fundamental circadian mechanisms. Misalignment can be caused by a) lack of periodicity, b) suboptimal phase, c) insufficient or excessive intensity of the “entrainment” signal (synchronization factor). Such factors are primarily light, food, and temperature. On the example of circadian rhythmicity, periodic changes in light exposure are necessary to increase the stability and “ resilience ” of health (used here as the antonym of “ frailty ”). In addition, the periodic nature of food intake (the well-known synchronizer of the peripheral clock) (intermittent fasting; time-restricted feeding) also provides obvious health benefits (Longo & Panda, 2016; Challet, 2019). However, in both cases (light or food), the question of the optimal duration of the alternation of each phase remains unresolved, which also leaves open the question of the optimal individual “dosing” and “timing”.
A mechanistic explanation of the benefits of intermittent light and food regimens in ensuring health and longevity (or vice versa, the cost of not having such interruptions) may depend on the balanced coordination of expression of light-signal-induced genes. For example, the production of serotonin, vitamin D, etc depend on light, while the synthesis of the nocturnal hormone melatonin depends on serotonin, its chemical precursor. Constant lighting conditions can disrupt the delicate balance between light-dependent factors (i.e., vitamin D, serotonin, etc) and “night” factors (melatonin, magnesium (Feeney et al, 2016), etc.), as well as the balance of cellular redox state (Edgar et al, 2012; Wilking et al, 2013).
What fundamental explanation can be offered to justify why tau compliance with the 24-hour period of the external pacemaker is optimal for health? One of the reasons may be the different sensitivity of single neurons of the brain to light, or individual peripheral clocks to chemical factors of food. As a result, a gradual phase shift of one of the elements of the circadian circuit will lead to internal desynchronization of the circadian system. This misalignment may be mild in comparison with mutation-induced desynchronization, or even comparing to chronic social jet lag. However, in the long term, individuals with a large deviation of endogenous tau from 24 hours, need to make more regular “efforts” to ensure a stable synchronization with the natural status quo.
It should be noted, however, that individuals with greater tau lability may have some advantages relative to those with “rigid” tau. It is known that individuals who do not have a precise tau due to their genetic characteristics can adapt faster to sudden changes in conditions, including factors of acute circadian disruption (Abraham et al, 2010; Yamaguchi et al, 2013; Tamiya et al, 2016). The presence of extreme variants of the chronotype is evolutionarily appropriate, from the point of view of increasing the survival rate of the group when it is necessary to protect the homes around the clock (Ayala, 1974; Samson et al, 2017). Extreme chronotypes, such as owls are better “sprinters”, able to adapt faster and, it would seem, easier, for example, to work in the night shift. However, at the longer runs, they may lose, having a shorter life span.
Health risk factors for evening chronotypes may be partly associated with a shift in the phase of the usual diet to a later time and regular skipping of the morning meal. This type of diet is recognized as non-physiological (Longo & Panda, 2016; Challet, 2019; Kelly et al, 2020), accompanied by a high risk of metabolic disorders and associated pathologies (Gouda et al, 2018; Kolbe and Oster, 2019; Vieira Musse et al., 2019). There are still open questions about the optimal interval between meals, their relationship to the sleep phase and light mode. In addition, the optimal multiplicity of food intake is debatable, for which individualization may require a more complete understanding of the mechanisms of ultradian rhythmicity.
Practical guidelines:
-
1. The key synchronization factors of the circadian biological clock (light, nutrition, temperature) must have an optimal frequency and phase sequence.
-
2. Constant light conditions (both constant light and constant darkness) are suboptimal for health and well-being.
-
3. Factors that artificially reduce the amplitude of the circadian rhythm (light-at-night, night food, etc) are deleterious.
-
4. Core principle of “default” chronotherapy should be based on choosing the time to increase the amplitude of the circadian rhythm, unless proven otherwise.
-
5. It should be expected that staying for a long time in conditions of increasing the dark phase (for example, polar night) is more unfavourable for people with a long tau (evening owls) as it causes further “slowdown” of the slow clock. The reverse might be true for morning chronotypes.
Список литературы Circadian clock precision, health, and longevity
- Abraham U, Granada AE, Westermark PO, et al. Coupling governs entrainment range of circadian clocks. Mol Syst Biol. 2010;6:438. doi:10.1038/msb.2010.92.
- Adan A, Natale V. Gender differences in morningnesseveningness preference. Chronobiology International. 2002;19 (4):709-720.
- Agadzhanian NA, Gubin DG. Desinkhronoz: mekhanizmy razvitiia ot molekuliarno-geneticheskogo do organizmennogo urovnia [Desynchronization: mechanisms of development from molecular to systemic levels]. Usp Fiziol Nauk. 2004;35 (2):57-72.
- Aho V, Ollila HM, Kronholm E, et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep. 2016; 6:24828.
- Amirdzhanova VN, Goryachev DV, Korshunov NI, Rebrov AP, Sorotskaya VN. Populyatsionnye pokazateli kachestva zhizni po oprosniku SF-36 (rezul’taty mnogotsentrovogo issledovaniya kachestva zhizni «MIRAZH»). Nauchnoprakticheskaya revmatologiya. 2008; 1.
- Archer SN, Schmidt C, Vandewalle G, Dijk DJ. Phenotyping of PER3 variants reveals widespread effects on circadian preference, sleep regulation, and health. Sleep Med Rev. 2018;40:109-126. doi:10.1016/j.smrv.2017.10.008.
- Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31 (5):608-617. doi:10.1093/sleep/31.5.608.
- Austad SN, Fischer KE. Sex Differences in Lifespan. Cell Metab. 2016;23 (6):1022-1033. doi:10.1016/j.cmet.2016.05.019.
- Ayala FJ, Campbell CA. 1974. Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5, 115-138. (10.1146/annurev.es.05.110174.000555).
- Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35 (1):111-139. doi:10.1016/j.yfrne.2013.11.003.
- Bandín C, Martinez-Nicolas A, Ordovás JM, et al. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes (Lond). 2013;37 (8):1044-50. doi: 10.1038/ijo.2012.180.
- Barclay NL, Eley TC, Mill J, et al. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3, and CLOCK 3111. Am J Med Genet B Neuropsychiatr Genet. 2011;156B (6):681-90. doi: 10.1002/ajmg.b.31210.
- Basso A, Del Bello G, Piacenza F, Giacconi R, Costarelli L, Malavolta M. Circadian rhythms of body temperature and locomotor activity in aging BALB/c mice: early and late life span predictors. Biogerontology. 2016;17 (4):703-714. doi:10.1007/s10522-016-9635-y.
- Beersma DGM and Daan S. Strong or weak phase resetting by light pulses in humans? J Biol Rhythms. 1993;8:340-347.
- Bell-Pedersen D, Cassone VM, Earnest DJ, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6 (7):544-556. doi:10.1038/nrg1633.
- Benedetti F, Dallaspezia S, Fulgosi MC, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007 Jul 5;144B (5):631-5.
- Bordyugov G, Abraham U, Granada A, et al. Tuning the phase of circadian entrainment. J R Soc Interface. 2015;12 (108):20150282. doi:10.1098/rsif.2015.0282.
- Buhr ED, Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol. 2013; (217):3-27. doi:10.1007/978-3-642-25950-0_1.
- Challet E. The circadian regulation of food intake. Nat Rev Endocrinol. 2019;15 (7):393-405. doi:10.1038/s41574-019-0210-x.
- Chang AM, Buch AM, Bradstreet DS, Klements DJ, Duffy JF. Human diurnal preference and circadian rhythmicity are not associated with the CLOCK 3111C/T gene polymorphism. J Biol Rhythms. 2011;26 (3):276-279. doi:10.1177/0748730411402026.
- Chang AM, Duffy JF, Buxton OM, et al. Chronotype Genetic Variant in PER2 is Associated with Intrinsic Circadian Period in Humans. Sci Rep. 2019;9 (1):5350. doi:10.1038/s41598-019-41712-1.
- Chen JM, Huang CQ, Ai M, Kuang L. Circadian rhythm of TSH levels in subjects with Alzheimer’s disease (AD). Aging Clin Exp Res. 2013;25 (2):153-7. doi: 10.1007/s40520-013-0025-x.
- Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284 (5423):2177-2181. doi:10.1126/science.284.5423.2177.
- Daan S. Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J Biol. Rhythms. 2000;15:195-207.
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517-49.
- Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14 (3):151-160. doi:10.1016/j.smrv.2009.07.002.
- Dijk DJ, Skeldon AC. Biological rhythms: Human sleep before the industrial era. Nature. 2015;527 (7577):176-177. doi:10.1038/527176a.
- Dodd AN, Salathia N, Hall A, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309 (5734):630-633. doi:10.1126/science.1115581.
- Doi M, Ishida A, Miyake A, et al. Circadian regulation of intracellular G-protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat Commun. 2011;2:327. doi:10.1038/ncomms1316.
- Duarte LL, Menna-Barreto L, Miguel MA, et al. Chronotype ontogeny related to gender. Braz J Med Biol Res. 2014;47 (4):316-320. doi:10.1590/1414-431x20143001.
- Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108 Suppl 3:15602-15608.
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115 (4):895-899. doi:10.1037//0735-7044.115.4.895.
- Dupont Rocher S, Bessot N, Sesboüé B, Bulla J, Davenne D. Circadian Characteristics of Older Adults and Aerobic Capacity. J Gerontol A Biol Sci Med Sci. 2016;71 (6):817-822. doi:10.1093/gerona/glv195.
- Durov AM. Biological age of man (Chronobiological aspects). Tyumen State University, 1999.
- Eckel-Mahan K, Sassone-Corsi P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013;93 (1):107-135.
- Edgar RS, Green EW, Zhao Y, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459-464.
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol Int. 2009;26 (3):474-493. doi:10.1080/07420520902821077.
- Escobar C, Salgado-Delgado R, Gonzalez-Guerra E, et al. Circadian disruption leads to loss of homeostasis and disease. Sleep Disord. 2011;2011:964510. doi: 10.1155/2011/964510.
- Feeney KA, Hansen LL, Putker M, et al. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532 (7599):375-379.
- Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30 (12):1659-1666. doi:10.1093/sleep/30.12.1659.
- Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US – Influence of age and sex. PLoS One. 2017;12 (6): e0178782. doi:10.1371/journal.pone.0178782.
- Froy O, Chapnik N, Miskin R. Long-lived alphaMUPA transgenic mice exhibit pronounced circadian rhythms. Am J Physiol Endocrinol Metab. 2006;291: E1017–E1024.
- Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY). 2010;2 (1):7-27. doi:10.18632/aging.100116.
- Garaulet M, Sánchez-Moreno C, Smith CE, Lee YC, Nicolás F, Ordovás JM. Ghrelin, sleep reduction and evening preference: relationships to CLOCK 3111 T/C SNP and weight loss. PLoS One. 2011;6 (2): e17435. Published 2011 Feb 28. doi:10.1371/journal.pone.0017435.
- Gouda M, Matsukawa M, Iijima H. Associations between eating habits and glycemic control and obesity in Japanese workers with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2018; 11:647-658. doi: 10.2147/DMSO.S176749.eCollection 2018.
- Granada AE, Bordyugov G, Kramer A, Herzel H. Human chronotypes from a theoretical perspective. PLoS One. 2013;8 (3): e59464. doi:10.1371/journal.pone.0059464.
- Gubin D, Cornélissen G, Halberg F, et al. The human blood pressure chronome: a biological gauge of aging. In Vivo. 1997;11:485-494.
- Gubin D, Cornelissen G, Weinert D. et al. Circadian disruption and Vascular Variability Disorders (VVD) – mechanisms linking aging, disease state and Arctic shiftwork: applications for chronotherapy. World Heart Journal. 2013;5 (4): 285-306.
- Gubin D, Weinert D, Solovieva SV, et al. Melatonin attenuates light-at-night effects on systolic blood pressure and body temperature but does not affect diastolic blood pressure and heart rate circadian rhythms. Biol Rhythm Res. 2019. https://doi.org/10.1080/09291016.2018.1564586.
- Gubin D. G. Chronodiagnostics and Chronotherapy – Frontiers for Personalized Clinical Medicine. Tyumen Medical Journal. 2019;21 (1):20-40.
- Gubin DG, Malishevskaya TN, Astakhov YS, et al. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol Int. 2019; 36 (4):564-577. doi: 10.1080/07420528.2019.1566741.
- Gubin DG, Malishevskaya TN, Weinert D, et al. Intraocular Pressure Circadian Rhythm in Stable and Advanced Primary Open-Angle Glaucoma. Tyumen Medical Journal. 2018;20 (3):3-9.
- Gubin DG, Nelaeva AA, Uzhakova AE, et al. Disrupted circadian rhythms of body temperature, heart rate and fasting blood glucose in prediabetes and type 2 diabetes mellitus. Chronobiol Int. 2017;34 (8):1136-1148. doi: 10.1080/07420528.2017.1347670.
- Gubin DG, Weinert D, Bolotnova TV. Age-Dependent Changes of the Temporal Order – Causes and Treatment. Curr Aging Sci. 2016;9 (1):14-25.
- Gubin DG. [Molecular Basis of Circadian Rhythms and Principles of Circadian Disruption]. Usp Fiziol Nauk. 2013;44 (4):65-87.
- Gubin GD, Chesnokov AA. Bioritm kak diagnosticheskiĭ test normy i patologii [Biorhythm as a diagnostic test of normal and pathological conditions]. Lab Delo. 1976; (7):430-432.
- Gubin, D.G., Weinert, D. Deterioration of temporal order and circadian disruption with age 2: Systemic mechanisms of aging-related circadian disruption and approaches to its correction. Adv Gerontol 6, 10-20 (2016). https://doi.org/10.1134/S2079057016010057.
- Gubin, D.G., Weinert, D. Temporal order deterioration and circadian disruption with age 1. Central and peripheral mechanisms. Adv Gerontol 5, 209-218 (2015). https://doi.org/10.1134/S2079057015040086.
- Gubin, G.D. and Gerlovin, A. Sh., Sutochnye ritmy biologicheskikh protsessov i ikh adaptivnoe znachenie v ontoi filogeneze pozvonochnykh (Diurnal Rhythms of Biological Processes and Their Adaptive Role in Ontoand Phylogenesis of Vertebrates), Novosibirsk: Nauka, 1980.
- Gubin, G.D., Gubin, D.G., and Komarov, P.I., Aging in terms of time organization of biological systems, Usp. Gerontol., 1998, vol. 2, pp. 67-73.
- Gutman R, Genzer Y, Chapnik N, et al. Long-lived mice exhibit 24 h locomotor circadian rhythms at young and old age. Exp Gerontol. 2011;46 (7):606-9. doi: 10.1016/j.exger.2011.02.015.
- Hasan S, Santhi N, Lazar AS, et al. Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB J. 2012;26 (6):2414-2423. doi:10.1096/fj.11-201699.
- Hasan S, van der Veen DR, Winsky-Sommerer R, et al. A human sleep homeostasis phenotype in mice expressing a primate-specific PER3 variable-number tandem-repeat coding-region polymorphism. FASEB J. 2014;28 (6):2441-2454. doi:10.1096/fj.13-240135.
- Herzog ED, Hermanstyne T, Smyllie NJ, Hastings MH. Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harb Perspect Biol. 2017;9 (1): a027706. doi:10.1101/cshperspect.a027706.
- Hida A, Kitamura S, Katayose Y, et al. Screening of clock gene polymorphisms demonstrates association of a PER3 polymorphism with morningness-eveningness preference and circadian rhythm sleep disorder. Sci Rep. 2014;4:6309. doi:10.1038/srep06309.
- Hida A, Kitamura S, Ohsawa Y, et al. In vitro circadian period is associated with circadian/sleep preference. Sci Rep. 2013;3:2074. doi:10.1038/srep02074.
- Hirota T, Okano T, Kokame K, et al. Glucose downregulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. 2002 Nov 15;277 (46):44244-51. Epub 2002 Sep 3.
- Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127 (2):437-446. doi:10.1172/JCI90328.
- Horne JA, Ostberg OA. self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4 (2):97-110.
- Hozer C, Perret M, Pavard S, Pifferi F. Survival is reduced when endogenous period deviates from 24 h in a nonhuman primate, supporting the circadian resonance theory. Sci Rep. 2020;10 (1):18002. doi:10.1038/s41598-020-75068-8.
- Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun. 2016;7:10448. doi:10.1038/ncomms10448.
- Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430-436.
- Jones SE, van Hees VT, Mazzotti DR, et al. Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat Commun. 2019;10 (1):1585. doi:10.1038/s41467-019-09576-1.
- Jordan SD, Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol. 2013;366 (2):163-169. doi:10.1016/j.mce.2012.06.017.
- Kalmbach DA, Schneider LD, Cheung J, et al. Genetic Basis of Chronotype in Humans: Insights From Three Landmark GWAS. Sleep. 2017;40 (2): zsw048. doi:10.1093/sleep/zsw048.
- Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, Hall D, Davis S, Nelson CS, Brem RB, Ramanathan A, Sehgal A, Giebultowicz JM, Kapahi P. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2016;23 (1):143-54.
- Katzenberg D, Young T, Finn L, et al. A clock polymorphism associated with human diurnal preference. Sleep. 1998;21:569-576.
- Kelly KP, McGuinness OP, Buchowski M, et al. Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biol. 2020;18 (2): e3000622. doi:10.1371/journal.pbio.3000622.
- Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int. 2018;35 (8):1045-1053. doi:10.1080/07420528.2018.1454458.
- Kolbe I, Oster H. Chronodisruption, Metabolic Homeostasis, and the Regulation of Inflammation in Adipose Tissues. Yale J Biol Med. 2019;92 (2):317-325.
- Lane JM, Vlasac I, Anderson SG, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun. 2016;7:10889. doi:10.1038/ncomms10889.
- Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife. 2020;9: e51507. doi:10.7554/eLife.51507.
- Libert S, Bonkowski MS, Pointer K, Pletcher SD, Guarente L. Deviation of innate circadian period from 24 h reduces longevity in mice. Aging Cell. 2012;11 (5):794-800. doi:10.1111/j.1474-9726.2012.00846.x.
- Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23 (6):1048-1059. doi: 10.1016/j.cmet.2016.06.001.
- Lowrey PL, Takahashi JS. Genetics of Circadian Rhythms in Mammalian Model Organisms. Advances in Genetics. 2011; 74:175-230.
- Lucassen EA, Zhao X, Rother KI, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8 (3): e56519. doi:10.1371/journal.pone.0056519.
- Maloney SK, Meyer LC, Blache D, Fuller A. Energy intake and the circadian rhythm of core body temperature in sheep. Physiol Rep. 2013;1 (5): e00118. doi:10.1002/phy2.118.
- Mansour HA, Wood J, Chowdari KV, et al. Associations between period 3 gene polymorphisms and sleep- / chronotype-related variables in patients with late-life insomnia. Chronobiol Int. 2017;34 (5):624-631. doi:10.1080/07420528.2017.1287083.
- Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294 (5): R1675–R1683. doi:10.1152/ajpregu.00829.2007.
- Maury E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int J Mol Sci. 2019;20 (7):1597. doi:10.3390/ijms20071597.
- Meng Q-J, Logunova L, Maywood ES, et al. Setting clock speed in mammals: The CK1ε tau mutation in mice accelerates the circadian pacemaker by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78-88. doi: 10.1016/j.neuron.2008.01.019.
- Merrow M, Spoelstra K, Roenneberg T. The circadian cycle: daily rhythms from behaviour to genes. EMBO Rep. 2005;6 (10):930-935. doi:10.1038/sj.embor.7400541.
- Mihalcescu I, Hsing W, Leibler S. Resilient circadian oscillator revealed in individual cyanobacteria. Nature. 2004;430 (6995):81-85. doi:10.1038/nature02533.
- Millius A, Ueda HR. Systems Biology-Derived Discoveries of Intrinsic Clocks. Frontiers in Neurology. 2017;8:25.
- Moskalev A, Anisimov V, Aliper A, et al. A review of the biomedical innovations for healthy longevity. Aging (Albany NY). 2017;9 (1):7-25. Published 2017 Jan 29. doi:10.18632/aging.101163.
- Münch M, Wirz-Justice A, Brown SA, et al. The Role of Daylight for Humans: Gaps in Current Knowledge. Clocks & Sleep. 2020;2:61-85.
- Nelson W, Halberg F. Schedule-shifts, circadian rhythms and lifespan of freely-feeding and meal-fed mice. Physiol. Behav. 1986;38:781-788. doi: 10.1016/0031-9384 (86) 90043-0.
- Nohara K, Mallampalli V, Nemkov T, et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun. 2019;10 (1):3923. doi:10.1038/s41467-019-11926-y.
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95:8660-8664.
- Ozburn AR, Purohit K, Parekh PK, et al. Functional Implications of the CLOCK 3111T/C Single-Nucleotide Polymorphism. Front Psychiatry. 2016;7:67. doi:10.3389/fpsyt.2016.00067.
- Pagani L, Semenova EA, Moriggi E, et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS One. 2010;5 (10): e13376. doi:10.1371/journal.pone.0013376.
- Partch C, Green C, Takahashi J. Molecular Architecture of the Mammalian Circadian Clock. Trends in cell biology. 2014;24 (2):90-99.
- Patke A, Murphy PJ, Onat OE, et al. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell. 2017;169 (2):203-215.e13. doi:10.1016/j.cell.2017.03.027.
- Phillips AJK, Vidafar P, Burns AC, et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci U S A. 2019;116 (24):12019-12024.
- Pittendrigh C, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 1972;69:1537-1539. 10.1073/pnas.69.6.1537.
- Pittendrigh CS, Bruce VG. 1959. In Photoperiodism and related phenomena in plants and animals (ed. Withrow R. B., editor.), pp. 475-505 Washington, DC: American Association for the Advancement of Science.
- Pittendrigh CS, Kyner WT, Takamura T. 1991. The amplitude of circadian oscillations: temperature dependence, latitudinal clines, and the photoperiodic time measurement. J. Biol. Rhythms 6, 299-313. (10.1177/074873049100600402).
- Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11-27. doi: 10.1016/j.metabol.2017.11.017.
- Provencio I, Warthen DM. Melanopsin, the photopigment of intrinsically photosensitive retinal ganglion cells. Wiley Interdiscip Rev Membr Transp Signal. 2012;1 (2): 228-23.
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975-978.
- Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225-1227.
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935-941 (doi:10.1038/ nature00965).
- Riddle M, Mezias E, Foley D, LeSauter J, Silver R. Differential localization of PER1 and PER2 in the brain master circadian clock. Eur J Neurosci. 2017;45 (11):1357-1367. doi:10.1111/ejn.13441.
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003; 18:80-90.
- Samson DR, Crittenden AN, Mabulla IA, et al. Chronotype variation drives night-time sentinel-like behaviour in hunter-gatherers. Proc Biol Sci. 2017;284 (1858):20170967. doi:10.1098/rspb.2017.0967.
- Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18 (3):164-179.
- Tamiya H, Ogawa S, Ouchi Y, Akishita M. Rigid Cooperation of Per1 and Per2 proteins. Scientific Reports. 2016;6: 32769.
- Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159 (3):514-529. doi:10.1016/j.cell.2014.09.048.
- Toh KL, Jones CR, He Y, Eide EJ, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291 (5506): 1040-3.
- Turco M, Biscontin A, Corrias M, et al. Diurnal preference, mood and the response to morning light in relation to polymorphisms in the human clock gene PER3. Sci Rep. 2017;7 (1):6967. doi:10.1038/s41598-017-06769-w.
- van den Berg R, Noordam R, Kooijman S, et al. Familial longevity is characterized by high circadian rhythmicity of serum cholesterol in healthy elderly individuals. Aging Cell. 2017;16 (2):237-243. doi:10.1111/acel.12547.
- Vieira Musse GN, Moreira T, Ayumi Kimura M, et al. Skipping breakfast concomitant with late-night dinner eating is associated with worse outcomes following ST-segment elevation myocardial infarction. Eur J Prev Cardiol. 2019; Apr 17:2047487319839546. doi: 10.1177/2047487319839546.
- Viena TD, Gobin CM, Fins AI, et al. A PER3 Polymorphism Interacts with Sleep Duration to Influence Transient Mood States in Women. J Circadian Rhythms. 2016;14:3. doi:10.5334/jcr.135.
- Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17 (7):613-618. doi:10.1016/j.cub.2007.01.073.
- von Schantz M, Archer SN. Clocks, genes and sleep. J R Soc Med. 2003;96 (10):486-489. doi:10.1258/jrsm.96.10.486.
- Weinert D, Gubin D. The Circadian Body Temperature Rhythm – Origin and Implications for Health and Wellbeing. Тюменский медицинский журнал. 2018;20 (2):6-14.
- Weljie AM, Meerlo P, Goel N, et al. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc Natl Acad Sci USA. 2015;112 (8):2569-2574.
- Wilking M, Ndiaye M, Mukhtar H, Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal. 2013;19 (2):192-208. doi:10.1089/ars.2012.4889.
- Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100 (12):4612-4620.
- Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20 (2):168-177. doi:10.1177/0748730404274265.
- Wyse CA, Coogan AN, Selman C, Hazlerigg DG, Speakman JR. Association between mammalian lifespan and circadian free-running period: the circadian resonance hypothesis revisited. Biol Lett. 2010;6 (5):696-698. doi:10.1098/rsbl.2010.0152.
- Yamaguchi Y, Suzuki T, Mizoro Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85-90.
- Yetish G, Kaplan H, Gurven M, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25 (21):2862-2868. doi:10.1016/j.cub.2015.09.046.
- Yin J, Jin X, Shan Z, et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc. 2017;6 (9): e005947. doi:10.1161/JAHA.117.005947.
- Zare R, Choobineh A, Keshavarzi S. Association of Amplitude and Stability of Circadian Rhythm, Sleep Quality, and Occupational Stress with Sickness Absence among a Gas Company Employees-A Cross Sectional Study from Iran. Saf Health Work. 2017;8 (3):276-281. doi:10.1016/j.shaw.2016.09.007.
- Zhang B, Wing YK. Sex differences in insomnia: a metaanalysis. Sleep. 2006;29 (1):85-93. doi:10.1093/sleep/29.1.85.
- Zhao J, Warman GR, Cheeseman JF. Clock gene expression and locomotor activity predict death in the last days of life in Drosophila melanogaster. Sci Rep. 2018;8 (1):11923. doi:10.1038/s41598-018-30323-x.
- Zhavoronkov A, Mamoshina P, Vanhaelen Q, Scheibye- Knudsen M, Moskalev A, Aliper A. Artificial intelligence for aging and longevity research: recent advances and perspectives. Ageing Research Reviews. 2019;49:49-66. doi: 10.1016/j.arr.2018.11.003.
- Zhdanova IV, Masuda K, Quasarano-Kourkoulis C, Rosene DL, Killiany RJ, Wang S. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythms. 2011;26 (2):149-159. doi:10.1177/0748730410395849.
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998; 20 (6):1103-10


