Coal based carbon materials for adsorption of heavy metal ions from solution
Автор: Ariunaa A., Narangerel J., Erdenechimeg R., Bazarova Zh.G.
Журнал: Вестник Бурятского государственного университета. Философия @vestnik-bsu
Рубрика: Химия
Статья в выпуске: 3, 2010 года.
Бесплатный доступ
The presence of contaminant heavy metal ions like cadmium, mercury and lead in the environment is the major problem due to their high toxicities. The use of activated carbon for the removal of the toxic metal ionic pollutants present in low concentration in solution is of considerable importance. Mongolian bituminous and subbituminous coals were used as raw material to prepare activated carbons by CO2. The coal derived carbons were oxidized with nitric acid in order to introduce surface oxygen groups. The ultimate elemental analysis, Fourier-transform infrared spectroscopy (FTIR), temperature programmed desorption (TPD), and selective neutralization were used to characterize the surface oxygen functional groups of oxidized carbons and naturally oxidized low rank coals. The effect of oxidation and the surface oxygen functional groups on the adsorption properties of the carbon was studied. The remove of heavy metal ions from solution by oxidized carbon and low rank coal was compared. It is observed that the use of lignite could be considerably effective in removing Hg, Cd and Pb cations from solution.
Coal, activated carbon, oxidation, temperature programmed desorption, functional groups
Короткий адрес: https://sciup.org/148179508
IDR: 148179508 | УДК: 544.723
Текст научной статьи Coal based carbon materials for adsorption of heavy metal ions from solution
Activated carbons are usually produced from natural precursors e.g. fruit stones, coconut shells, coal etc. Among them coal is the most commonly used precursor due to its low cost and large supply. The most important applications of activated carbon to the adsorption of heavy metal species from solution are for the recovery of precious metals from gold plant liqours, slurries and the treatment of wastewater containing trace toxic metals [ 1 ] . The interaction between the surface functional groups of active carbons and trace metal ions in aqueous media has been subject of many research works. It has been shown that in the case of the adsorption of inorganic compounds on activated carbons from aqueous solutions, the chemical nature of the adsorbent determined by the amount and nature of the surface functional groups has in general more influence than the surface area and the porosity of the adsorbent [ 2 ] . Oxygen functional groups on the surface of carbon can be formed by chemisorption at room temperature, oxidation at high temperature or can originate from the precursor material [ 3 ] . In most of the research on the carbon oxidation are used active carbon derived from biomass. Therefore, the removal of heavy metal ions from solution by oxidized carbon derived from bituminous coal is an interesting subject.
The ability of low rank coals to form stable complexes with metal ions has long been recognized. Although the adsorption capacities of low rank coals are lower in comparison to those of activated carbons, the substantially lower cost of the bulk material shows great potential for the usage of low rank coals. The high oxygen content of low rank coals allow the unique capability for lignite to remove cations from solution via ion exchange with carboxylic acid and phenolic hydroxyl functional groups on the coal surface
The aim of the study is to demonstrate the potential of Mongolian coals as a low cost raw material for environmental protection application, with the ability to adsorb toxic heavy metals (Cd, Hg and Pb) from waste water. Carbon dioxide activated carbon derived from the coals was used in the present study as a precursor for oxidized carbons. The adsorption capacities of the oxidized carbons are compared with those of low rank coals. The subject is also to gain an understanding of how the metal ions and carbon surface interaction operates with the view of using coals, modified coal adsorbents for the removal of heavy metals from solution.
Materials and elemental analysis
Bituminous and low rank coals from some main deposits of Mongolia were used as a precursor in this study. The elemental and proximate analysis of the coals are summarized in Table 1. The coals were selected samples with low to medium ash content from their respective deposits. Before use, the coals were ground under nitrogen. Coal fractions with a particle size range between 0.6-1.2 mm were selected for the study.
The adsorption of heavy metal ions were conducted on three different types of oxidized carbons. Oxidized carbon named SGACO (Sharyn Gol coal-activated carbon-oxidized), prepared from Sharyn Gol subbituminous coal. The coal was carbonized in a vertical quartz reactor at 800oC (heating rate 5oC/min) in an N2 flow (rate 160 cm3/min) for 1 h and activated at 800oC (heating rate 5oC/min) in an CO2 flow (rate 160 cm3/min) for 3 h. The resultant carbons from the carbonization and activation were designated SGC (Sharyn gol coal-carbon) and SGAC (Sharyn Gol coal-activated carbon), respectively. A portion of the SGAC was oxidized. It was boiled at 95oC with 7.5N HNO3 (solid:liquid-1:10) for 24 h in a flask fitted with a reflux condenser.
Table 1.
Elemental and proximate analysis of coals
Oxidized carbon named ASGACO (Acid treated Sharyn Gol coal-activated carbon-oxidized), prepared also from Sharyn Gol subbituminous coal. This sample was treated with 2.3N HCl at 25oC for 1 h before carbonization to remove exchangeable cations and carbonates. The product was washed thoroughly with hot distilled water until it is free of chlorine ions. Acid treated coal named as ASG (Acid treated Sharyn Gol coal) was carbonized at 800oC (heating rate 10oC/min) in an N 2 flow (rate 160 cm3/min) for 1 h and activated at 800oC in an CO 2 flow (rate 160 cm3/min) for 5 h. The sample obtained by activation was labeled as ASGAC (Acid treated Sharyn Gol coal-Activated carbon). Portion of ASGAC was boiled as SGAC with 7.5N HNO3 (solid:liquid-1:10) for 48 h.
Oxidized carbon named NSACO (Naryn Suhait coal-activated carbon-oxidized) was prepared from Naryn Suhait bituminous coal. The coal was carbonized at 900oC (heating rate 10oC/min) in an N2 flow (rate 160 cm3/min) for 1 h and activated at 900oC in an CO2 flow (rate 160 cm3/min) for 3 h. The sample obtained by activation was labeled as NSAC (Naryn Suhait coal-Activated carbon). Portion of NSAC was boiled as SGAC with 7.5N HNO 3 (solid:liquid-1:10) for 24 h. After oxidation, the samples was recovered and washed thoroughly with hot distilled water until they are free of nitrate ions.
FTIR
The FTIR spectra for different carbons were obtained on a Nicolet 20-PC FTIR Spectrophotometer with CsI optics and a DTGS detector. The KBr disc contained 0.5% finely ground carbon sample.
Temperature Programmed Desorption
A Thermal Science STA 1500 thermo gravimetric analyzer (TGA) was used to do TPD analysis of carbons. It connects with a VG Quadruple 300 AMU mass spectrometer by a heated stainless steel capillary lined with deactivated fused silica. About 5 mg of carbon was put into a sample bucket sealed in an oven and was not heated until the density of the species desired and sample mass displaying became stable. The temperature programmer controlled furnace heating from room temperature to 1200oC at rate of 15K/min in argon gas of flow rate 50 ml/min. The gas evolved from carbon were sampled and analyzed through gas sample probe and a heated stainless steel capillary into mass spectrometer. When furnace temperature reached to 1200oC, sample heating was stopped. The sample was cooled to room temperature in argon. The typical m/z values of 28, 30, 44 and 46 were detected.
Titration
The selective neutralization method used to evaluate the carbon surface acidity was the scheme suggested by Boehm [ 4 ] . The amounts of the various acidic functional groups can be measured by selective neutralization using NaHCO 3 , Na 2 CO 3 , and NaOH solutions, respectively. About 0.2 g of carbon was placed in 25 cm3 of each 0.1 N of solution and mixtures were allowed to stand for 72 h at room temperature. The mixtures were separated by filtration. The amount of each base neutralized by the carbon was determined by back-titration using 0.1 N HCl solution.
Adsorption of metal ions in solution
About 0.1 g of carbon was doped into 50 ml of solution having the known initial concentration (0-2 mM) containing metal ions Pb, Cd and Hg. Adsorption experiments were conducted at 25oC for 48 hours. A Unicam 701 Inductively Coupled Plasma (ICP) atomic emission spectrometer was employed to determine the concentration of stock solution and the equilibrium concentration of metal ions in solutions after adsorption. The metal ions adsorbed on active carbon were calculated as following;
Ma=(Co-Ce)*V/1000*Wc where Co and Ce refer to initial and equilibrium concentration of metal ion in solution individually, mM; V is the volume of solution. For all experiments, the volume V is equal to 50 ml; Wc, the weight of carbon sample, g; Ma, the amount of metal ions adsorbed by carbons, mmol/g.
Results
Characterization of carbon and coals
The characteristics of the carbons from Sharyn gol coal are summarized in Table 2. It is well known that essentially all oxygen containing groups are removed during the carbonization process in a nitrogen atmosphere. But, the resultant carbon surface adsorbs relative high quantities of oxygen upon re-exposure to air at room temperature. The oxidation with HNO3 reduced app. 2 times the ash content of the activated carbons and added significant amount of oxygen to the activated carbons both for SGAC and ASGAC. A small amount of nitrogen is introduced to the oxidized carbons.
Table 2.
The characteristics of the carbons from Sharyn gol coal
FTIR
Intensive bands near 1600 cm-1 (1635 cm-1 and 1627 cm-1) have been explained by stretching vibrations of aromatic C=C bonds which are polarized by oxygen atoms bound near one of the carbon atoms. The new bands at 1576 cm-1 and 1717 cm-1 were observed in the IR spectrum of oxidized carbons. The band at 1576 cm-1 can be assigned to carboxylate groups. The 1717 cm-1 band is characteristic of stretching vibrations of carbonyl groups C=O in carboxylic acid groups. Intensive band near 1100 cm-1 (1087-1097 cm-1) is characteristic band for C=C groups in aromatic structure.
TPD
Temperature Programmed Desorption (TPD) experiments were carried out to characterize surface oxygen functionality from their thermal stability by monitoring evolved CO2 (pic. 1-5). TPD profiles of the original coals differ from each other much. In the coalification row from SO to NS coals decreases total content of oxygen functionalities and increases relative content of temperature stabile oxygen, which is consistent with the results from titration studies (pic. 1).
Two well-resolved peaks were observed in the TPD for SG coal at about 420 and 600oC, respectively. At about 420oC begins also cleavage of many weak bonds in the internal structure of coal. Second decomposition begins at about 600oC. Oxidation of carbons results in composition of the surface oxygen functional groups and significant changes in the TPD profiles (pic. 2).
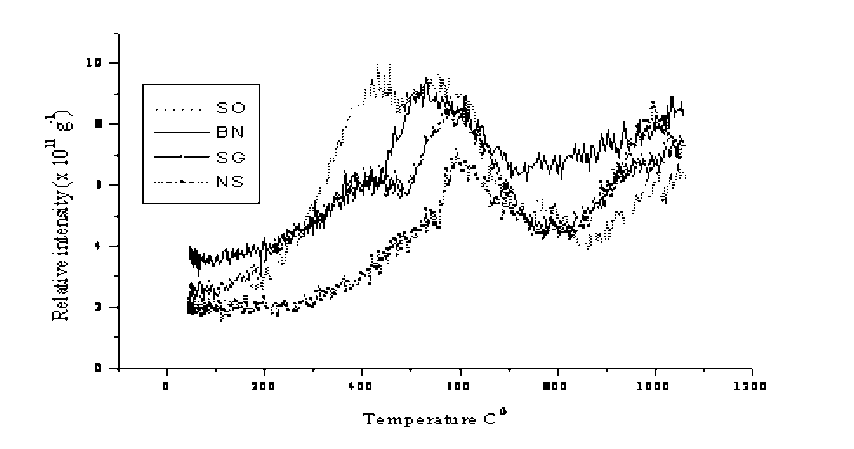
Pic. 1. TPD CO 2 profiles for the original coals
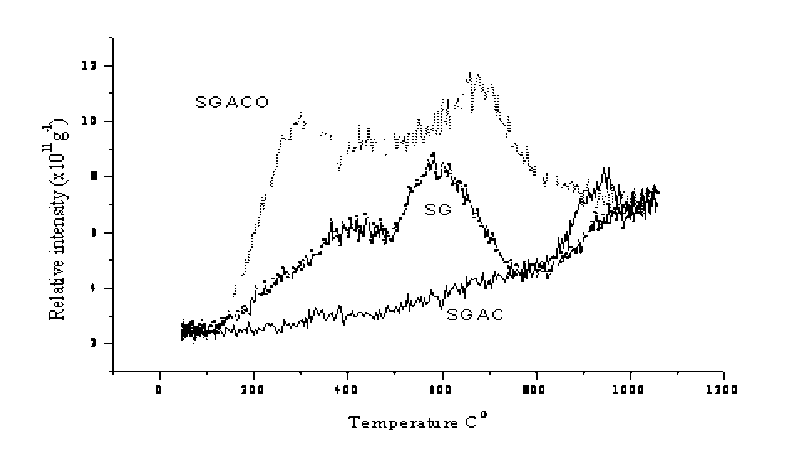
Pic. 2. TPD CO 2 profiles for the SG coal and coal derived oxidized (24 h) carbons
The proposal of the implication of various decomposition steps in the TPD profiles of oxygen functional groups is not new [ 5 ] . The CO 2 evolution of oxidized carbons starts at lower temperatures and ends at higher temperatures than that of the original coals (pic. 3, 4). Oxidation of active carbons for 48 h increases the relative intensity of the CO2 evolution at 300oC if compare the profiles with that of active carbons oxidized for 24 h. Also with the increase of the oxidation time increases content of “most reactive oxygen group”.
The oxidized carbon from NS coal (NSACO) evolves little more CO 2 at 300oC than at 650oC (pic. 4). It is known that carboxylic groups are less stable than phenolic groups when exposed to heat treatment. In contrast, phenolic and carbonyl groups have been found to remain stable up to about 800oC.
Difference between oxidized carbon and naturally oxidized brown coal in relation to oxygen functional groups could be seen from the pic. 5. Oxygen functionalities in oxidized carbon from coal have much broader thermal stability than that of SO lignite.
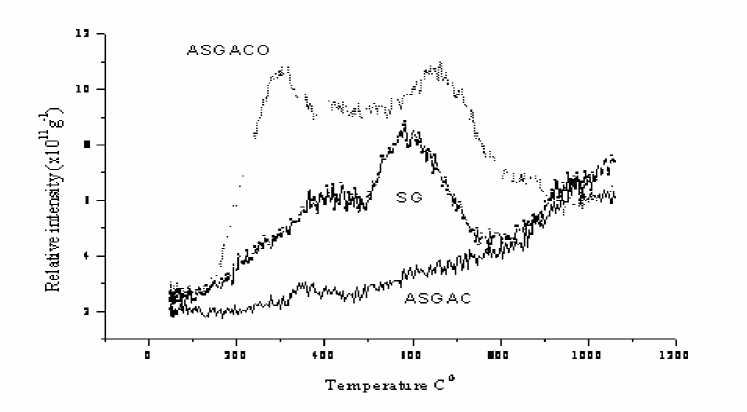
Pic. 3. TPD CO2 profiles for the SG coal and coal derived oxidized (48h) carbons
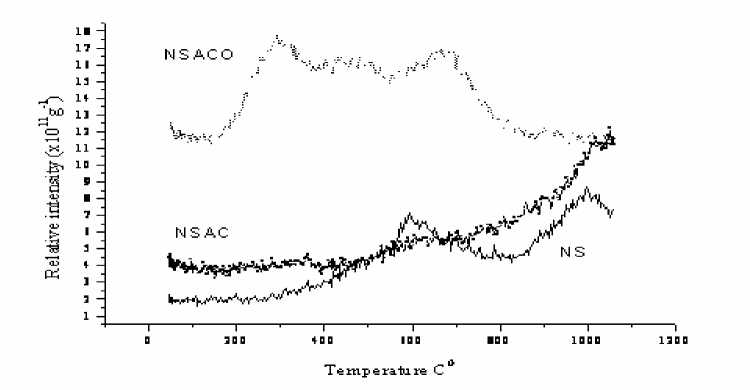
Pic. 4. TPD CO 2 profiles for the NS coal and coal derived carbons
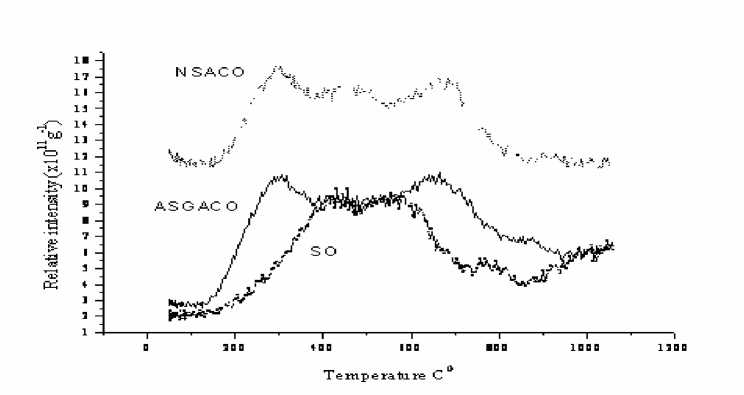
Pic. 5. TPD CO 2 profiles for the SO coal and coal derived oxidized carbons
It is proposed that, oxygen functional groups in oxidized carbons, COOH, lactone-lactole and phenol are corresponding mainly to the desorption steps at 200-370oC, 370-450oC and 450-800oC. Oxygen desorbed above 900oC could be corresponded to decomposition of basic pyrone and chromene groups [ 6 ] .
Titration.
Titration results for the original coals and the oxidized carbons are shown in Table 3. According to Boehm [ 4 ] the amount of NaHCO 3 reacted is equivalent to the amount of strongly acidic carboxylic groups, while Na2CO3 reacted with both carboxylic and lactone-lactole groups.
Table 3.
Selective Neutralization Results of Oxidized Carbons and Coals
|
Sample code |
mequiv g |
Acidic groups, % |
|||||
|
HCl |
NaHCO 3 |
Na 2 CO 3 |
NaOH |
Carboxylic |
Lactone-lactole |
Phenolic |
|
|
ASGACO |
0.15 |
2.73 |
3.34 |
5.13 |
53.22 |
11.89 |
34.89 |
|
SGACO |
0.15 |
0.79 |
2.08 |
3.53 |
22.38 |
36.54 |
41.08 |
|
SG |
0.30 |
0.80 |
0.00 |
3.85 |
20.78 |
0.0 |
79.22 |
|
BN |
0.54 |
0.83 |
1.58 |
4.43 |
18.80 |
16.95 |
64.24 |
|
SO |
0.76 |
1.01 |
2.27 |
3.85 |
26.37 |
32.52 |
41.11 |
The amount of NaOH reacted minus the Na 2 CO 3 value gives the amount of weakly acidic phenols present on carbon. It is assumed that neutralization with HCl characterizes the amounts of surface basic groups. The basic properties are ascribed to surface basic groups and the n electron system of carbon basal planes.
Low rank coals had relative similar amounts of total acidic oxygen functional groups, while with increasing coalification, was increased the relative concentration of phenolic groups. If compare ASGACO and SGACO, the former has 45% higher amount of total acidic functional groups. Also ASGACO has almost 3 fold higher amount of carboxylic group. Relative long activation (5 h) and oxidation (48 h) times for ASCACO might be reason for such higher amount of acidic oxygen groups, compared with SGACO, which had activation and oxidation times 3 h and 24 h respectively.
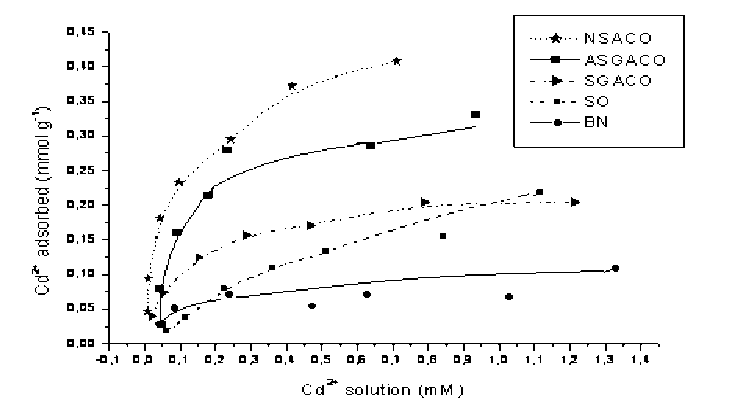
Pic. 6. Adsorption isotherms of Cd on oxidized carbons and low rank coals
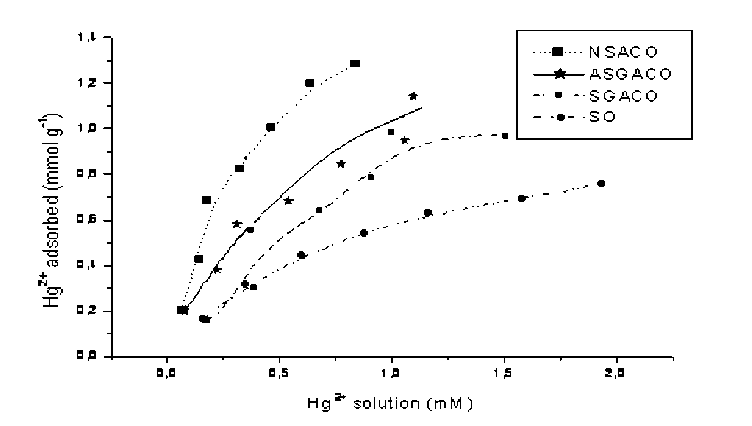
Pic. 7. Adsorption isotherms of Hg on oxidized carbons and SO coal
Adsorption of metal cations from solution.
Adsorption isotherms of metal ions on low rank coals and oxidized carbons from coal are shown in pic. 8-10. It is apparent that the adsorption capacities were dramatically enhanced after the coal carbon was oxidized with HNO 3 .
Oxidized carbon from bituminous coal (NSACO) adsorbs more metal ions than that of from subbituminous coal (ASGACO) although, oxidation time for NSACO was 2 times less than that for ASGACO. The reason is probably in the structure of original carbons from coal. SO lignite has high adsorption capacity for metal ions comparable with oxidized carbon SGACO (pic. 8, 9). It is known that the cation exchange property of low rank coal is dominated by the chemical factor such as affinity between metal ions and carboxylic groups and by the physical factor such as steric arrangement of carboxylic groups [ 7 ] .
0,65
0,60
0,55
0,50
0,45
0,40
0,35
0,30
0,25
0,20
0,15
0,10
0,05
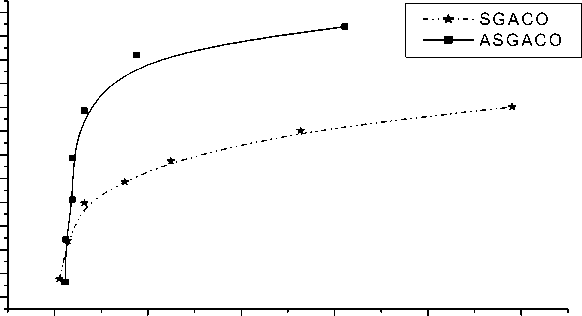
0,0 0,2 0,4 0,6 0,8 1,0
Pb2+ solution (mM)
Pic. 8. Adsorption isotherms of Pb on oxidized carbons
Comparison of the adsorption isotherms of metal ions on ASGACO and SGACO shows that ASGACO has a higher adsorption capacity. This can be explained by higher extent of oxidation treatment of carbons by HNO 3 (pic. 6-8). As mentioned above, ASGACO has more acidic oxygen groups compared with SGACO. It was observed that, the adsorption capacity for metal ions is correlating with low temperature (~300oC) CO 2 evolution presented by carbon and coal.
The adsorption of heavy metals by the oxidized carbons derived from various coal and low rank coals have been studied.
The oxidation with HNO3 added significant amount of oxygen to the activated carbons from coal. Two well-resolved peaks were observed in the TPD for the studied coals at about 420 and 600oC, respectively. It is proposed that, oxygen functional groups in oxidized carbons; carboxylic, lactone-lactole and phenol are corresponding mainly to the desorption steps at 200-370oC, 370-450oC and 450-800oC. Oxygen functionalities in oxidized carbon from coal have much broader thermal stability than that of low rank coals. The amounts of adsorbed metal ions were in agreement with the amount of ‘most reactive oxygen containing groups’ on the surface of carbon.
High uptake of Cd ion (~0.4 mmol/g) was observed by adsorption of oxidized carbons, derived from bituminous coal. Naturally oxidized Shivee ovoo coal has high adsorption capacity for metal ions comparable with oxidized carbons and could be used as a cheap raw adsorbent material for removing heavy metals from waste water.


