Comparative analysis of neem extract and chemical pesticides on physiological and biochemical responses in maize seedlings
Автор: Ralh P., Singh G.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Chemical pesticides are an essential component of modern agriculture practice but they mitigate with various metabolic pathways of crops. Biopesticides including botanical extracts are plant-based flavonoids and polyphenolic compounds which has the potential of both the pesticides as well as phyto-protection. The present study compares the effect of chemical pesticide viz-a-viz neem based biopesticides on various physiological, biochemical and antioxidative properties in the developing maize seedlings. We observed that different conc. of profenofos and malathion resulted in decreased shoot and root length, interfered with photosynthetic components such as Chl a, chl b , chl a+b, carotenoid content and also resulted in decreased activity of total phenol, starch and MDA content. The MDA content, FRAP assay and various antioxidant enzyme assays show differential behaviour. The total antioxidative activity (FRAP assay) and antioxidant enzyme activities of APX, CAT, SOD showed enhanced activities vis-à-vis differential expression under chemical pesticide stress conditions, thus suggests that the pesticide produces the oxidative stress in plant and triggers the antioxidant defence system to counter the stress. Different conc. of Neem based biopesticide, exhibited an increase in shoot and root length, chlorophyll and carotenoid pigments, phenol, sugar content. This study thus suggests the biopesticide alleviates the physiological and biochemical activities thereby increasing the productivity compared to chemical pesticides and thus further supports that organic pesticides can be used as alternative pesticide.
Neem extract, profenofos, malathion, antioxidative enzymes, maize seedling, lipid peroxidation
Короткий адрес: https://sciup.org/143184706
IDR: 143184706
Текст научной статьи Comparative analysis of neem extract and chemical pesticides on physiological and biochemical responses in maize seedlings
Organophosphate pesticides (OPs) class of pesticides which are known as organic ester derivatives of phosphorous, generally thiol or amide derivatives of thiophosphoric, phosphinic, phosphonic, phosphoric acids with additional side chains of phenoxy, cyanide and thiocyanate group (Kumar et al., 2016). Profenofos, also known as O-(4-bromo-2-chlorophenyl) O-ethyl S- propyl phosphorothioate, is a widely utilized non-systemic organophosphate insecticide with broadspectrum effectiveness. It is commonly applied to field crops, vegetables, and fruit crops (Khushwaha et al., 2016). Malathion [S-(1, 2-dicarbethoxyethyl)-O, O-dimethyl dithio-phosphate] is a member of organophosphates (OP), is a non-systemic, wide-spectrum insecticide which is known to induce toxicity to plants and animals by altering the cell division and antioxidant potential (Badr, 2020; Singh and Roy, 2017).
Biopesticides including the Botanical extracts are naturally occurring substances that are derived from various sources such as animals, plants, bacteria, cyanobacteria, and microalgae. They are utilized in agricultural practices to effectively manage and control pests and pathogens (Kumar et al., 2021). Azadirachta indica A. Juss, aka. Neem (Meliaceae), has been widely known to be a very popular potent biopesticide due to their various active ingredients (Baby et al., 2022). Studies has shown that various biopesticides are able to confer tolerance to the developing plants by preventing the damage induced under abiotic stresses including pesticide stress (Ngegba et al., 2022).
Universally maize is called as sovereign of cereals since it has the most elevated hereditary yield potential among the grains. Maize is the third most important cereal after rice and wheat for human food because of its contribution of about 9 % to India’s food basket and 5 % to World’s dietary energy supply (Erenstein et al., 2022). The present study reports the comparative analysis of pesticides and biopesticide on growth, photosynthetic pigments and antioxidative enzymes in the developing seedling of maize.
MATERIALS AND METHODS
Pesticide treatment and growth conditions
Healthy seeds of Zea mays L. (maize) were procured from local market. Dry seeds were rinsed and presoaked in distilled water overnight. Different pesticides were procured from local market with higher (more than 95%) of purity. Stress was imposed by incubating seeds (30 each) in different conc. (0.10%, 0.15%, 0.20%) of Profenofos and malathion and neem extract. The seeds were dipped in distilled water to serve as control sample.
Post stress-imposition incubated seeds were grown in incubator chamber at 25 ºC ± 1 ºC with regular supply of water. Seedlings were harvested at 7-days and 14 days and stored at -20 °C for further analysis.
Estimation of Physiological parameters (Shoot and root length measurements)
Shoot and root length were measured with the help of thread and ruler. A set of 5 seedlings were taken and shoot and root length were measured with thread and ruler.
Estimation of Photosynthetic pigments
Chlorophylls (Chl a, Chl b, Chl a+b)
Fresh leaf discs (50mg) were cut and placed in a test tube containing 10ml of N, N-dimethyl formamide (DMF) and stored for 24 hours at 4C. The absorbance of the colored supernatant was read at 647 nm and 666 nm in a spectrophotometer (Systronics, India) by taking DMF as blank. Chl a, b and total Chl a+b was calculated (Inskeep and Bloom, 1985).
Chl a (mgg-1FW) = (12.7(A 666 ) –
2.799(A 647 ))/(100×W×a) ×V
Chl b (mgg-1 FW) = (20.7 x (A 647 ) – 4.62 x (A 666 ))/(100×W×a) ×V
Chl a+b (mgg-1 FW) = (17.9 x (A 647 ) + 8.08 x (A 666 ))/(100×W×a) ×V
Where, A = absorbance, a = length of light path in the cell (1cm), V = volume of extract in ml and W = fresh weight of the sample in g.
Carotenoid content
Fresh leaf discs (50mg) were cut and placed in a test tube containing 10ml of N, N-dimethyl formamide (DMF) and stored for 24 hours at 4C. The absorbance of the colored supernatant was read at 647, 666 and 480 nm in a spectrophotometer with DMF as blank. Absorbance values of DMF pigment extract at 480 nm, 647 nm, and 666 nm were used to find the corrected optical density (OD) for carotenoids which was further used for calculations.
Corrected OD = A 480 + [(0.114 × A 666 ) – (0.638 × A 647 )]
Carotenoid (mgg-1 FW) = Corrected OD × 1/100 ×V/W
Where, A = absorbance, V = volume of extract in ml and W = fresh weight of the sample in g.
Preparation of alcoholic extract for estimations of Total sugars, Total phenols, Total starch content.
Fresh leaves were oven dried (80C, 48 hr.) and powdered using pestle and mortar. Dried leaf powder (50 mg) was boiled in 5ml of 80% ethyl alcohol on a water bath. The homogenate was first cooled and then centrifuged at 6000 rpm for 15 minutes at room temperature. The supernatant was diluted to 10ml with 80% ethyl alcohol. The extract was the further used for quantitative estimations of starch, total sugars and total phenols. The residue was also saved for the starch estimation.
Estimation of Total starch content
The estimation of starch was done using the method of (McCready et al ., 1950). 5ml of 52% perchloric acid was added to residues of each stress and left for 1hour. After 1 hour the mixture is filtered using Whatman filter paper. To 1ml perchloric extract added 4ml of distilled water and then 5 ml of cold Anthrone reagent. Reaction mixture was boiled for 7.5 minutes in water bath. Cooled the tubes under running tap water and then taken reading under spectrophotometer at 630 nm.Total starch content was estimated using glucose as standard.
Lipid peroxidation estimation
MDA content was determined with Thiobarbituric acid (TBA) reaction (Del Rio et al ., 2005). 300 mg of tissue was weighed (each stressed conditions) and homogenized in 1.5 ml of 0.1% TCA. The homogenate was centrifuged at 10,000 rpm, 10 minutes and 4C. To 1ml of supernatant, 4ml of TBA solution was added (Blank tubes containing 4ml TBA solution and 1ml water). The mixture was then incubated at 95C for 30minutes in water bath. The reaction was stopped by putting the reaction tubes in ice for 5minutes. Absorbance was recorded at 532nm and 600nm using spectrophotometer. The amount of MDA present was calculated from the extinction coefficient of 155mM-1 cm-1.
MDA = (A 532 -A 600 )/155
Estimation of Total phenol content
The estimation of phenolic content was carried out using reaction mixture contained alcoholic extract
(100µl), Folin-Ciocalteu reagent (10%), sodium carbonate (7%) which was incubated at 40 °C for 30 min. The total phenolic contents were determined by recording the absorbance at 760 nm and gallic acid was taken as standard as per the protocol described (Phuyal et al ., 2020).
Analysis of antioxidant enzyme assays
Estimation of FRAP assay
FRAP assay is used to estimate the total antioxidant activity. In ferric reducing antioxidant assay the reaction mixture consisted of sodium phosphate buffer (20mM), potassium ferricyanide (1%), TCA 10%, Ferric chloride (0.1%), 100 µg of enzyme. The absorbance was recorded at 700nm.
Guaiacol peroxidase (GPX) activity
The guaiacol peroxidase activity was measured according to the method described (Egley et al ., 1983). The reaction mixture consists of 100µg of enzyme extract, 200µl of H 2 O 2 , 400µl of guaiacol, 1.5ml of sodium phosphate buffer (50mM) and 700µl of distilled water. The absorbance was recorded at 420 nm at the time of H 2 O 2 addition and 2 min later. The difference in absorbance was divided by molar extinction coefficient (26.6 mM-1 cm).
Catalase (CAT) activity
The catalase activity was determined using the procedure described (Aebi, 1984). Assay mixture consisted of 100µg of enzyme extract, 1500µl of Sodium phosphate buffer (50mM), 500µl of H 2 O 2, 1800µl of distilled water. The decrease in H 2 O 2 was monitored by reading the absorbance at 240nm at the time of H 2 O 2 addition and 1 min later. The difference in absorbance was divided by molar extinction coefficient (36 mM-1cm).
Superoxide dismutase (SOD) activity
The superoxide dismutase activity was measured according to procedure as described (Roth and Gilbert, 1984). Reaction mixture consisted of 100µg of enzyme extract, 500µl of sodium phosphate buffer (50mM), 300µl of pyrogallol (10mM) and 2ml of distilled water. The enzyme activity was monitored at 420 nm for 3 min.
Data and statistical analysis.
Experiments were conducted in triplicate for measurement of physiological and biochemical
parameters. The results of Chl a, Chl b, Chl a + b, carotenoid, total phenolic and starch content and antioxidant activity are presented as mean ± standard deviation (SD) with SE± 5% of control sample.
RESULTS
Effects of various pesticides on morphological parameters
In this study we observed that there was significant inhibition of shoot length with the increase of pesticide conc. The increasing conc. of pesticides show differential pattern of shoot length in the seedlings. At 7 and 14-day old seedling stage, the shoot growth gradually decreased by 17.97%, 43.77% and 5.52 % and 1.21%, 33.84% and 31.70 % at 0.10%, 0.15% and 0.20% of Profenofos stress respectively. Whereas the shoot length decreased by 13.05%, 17.5%, 13.05% and at 0.10%, 0.15%, and 0.20% of Malathion stress respectively. The neem Biopesticide treatment leads to an increase in shoot length by 0.4%, 6.02%, and 4.417% and 10.9%, 10.30%, and 15.50% at 0.10%, 0.15%, 0.20% for 7-D and 14-D stages of seed development (table 1). A similar pattern of effect of these pesticides on root development was observed under perfenofos and malathion stress. A maximum reduction of 19.58% and 21.56% at 0.15% for perfenofos and 48.25% (0.20%) and 56.8% (0.15%) under Malathion stress was observed at 7 and 14 day of root length was observed. In contrast to decrease, a significant increase of 10-49% was observed for neem biopesticides (Table 1).
Effects of various pesticides on the Chl a, Chl b , Chl a+b and carotenoid Content
Reduction in chl a content under the stress of Profenofos and malathion was observed both at 7-D and 14-D seedling stage. While the Chl a content decreased by 0.31%, 43.9%, 34.57% and 9.52%, 9.52% and 55.4 % at 0.10%, 0.15%, 0.20% conc. of Profenofos stress., the decrease of 35.19%, 30.3%, 67.09% and 52.8%, 2.37%, 52.8% at 0.10%, 0.15%, 0.20% under malathion stress was observed for both 7 and 14 day of seedling stage. Seed treated with 0.10%, 0.15%, 0.20% of neem biopesticide revealed that the Chl a content increased by 37.6%, 38.8%, 6.21% and 41.4%, 28.2% at 7-D and
14-D except for 0.2% at 14-D seedling stage where a reduction of 29.6% was observed (Fig. 1a). The increasing conc. of pesticides show differential pattern of Chl b content in the seedlings. In case of Profenofos, the Chl b content decreased by 47.68%, 33.6% and 35.4%, 18.1% and 77.5 % at 0.1%, 0.15%, 0.20% conc. the Chl b content decreased by 50.9%, conc. Chl b content showed a differential behavior at 7-D and 14-D seedling stage under malathion stress. At 7-D and 14-D seedling stage a decrease of 32.0%, 21.07%, 11.07% and 6% at 0.10%, 0.15%, 0.20% was observed, however an increase of 35.5%, 2.68% at 0.10%, 0.15% was observed (Fig. 1b). It was observed that there was a consistent decease in the content of Chl a+b at both 7-d and 14-D by 2%, 45.47%, 32.76 % and 26.4%, 15.10% and 17.2 % at 0.10%, 0.15%, 0.20% of profenofos stress. Similar results were also obtained for the malathion stress where a decrease of 41.60%, 39.50%, 51.60% and 19.7%, 25.1%, 63.3% at 0.10%, 0.15%, 0.20% was observed. Chl a+b content was observed to be alleviated by 26.4%, 15.10% 17.2 % and 37.4%, 3.54%, 6.4% at 0.10%, 0.15%, and 0.20% conc of neem biopesticide at 7-D and 14-d seedling stage (Fig. 1c).
The increasing conc. of pesticide showed differential pattern of carotenoid content of seedlings. In case of Profenofos, the carotenoid content decreased by 18.7%, 44.8 % and 14.34% and 16.6%, 2.9% and 12.2 % at 0.10% 0.15% and 0.20% conc at 7-D and 14-D seedling stage. Under the influence of 0.10%, 0.15%, and 0.20% malathion conc., the carotenoid content increased slightly by 17.15% at 0.10% and decreased by 40.82%, 52.77% at 0.15%, 0.20% at 7-D seedling stage, whereas 56.4%, 11.96%, 5.98% decrease was observed at 14-D seedling stage. Preincubation of seeds with neem biopesticide with 0.10%, 0.15%, and 0.20% conc. resulted in an increased seedling growth by 5.22%, 42.01% at 0.10%, 0.15% and decreased by 18.32% at 0.20% at 7-D seedling stage whereas a decrease of 22.80% at 0.10%, remained same at 0.15%, increased by 17.54% at 0.20% conc at 14-D seedling stage (Fig.1d).
Effects of various pesticides on starch content
In present study it was observed that, the starch content was reduced on imposition of Profenofos, malathion stress. In case of Profenofos, at 7th stage the starch content decreased, maximum decrease(70%) was observed at 0.10% conc. of Profenofos and at 14th day stage maximum decrease (34%)was at 0.20% conc. of pesticide. In case of malathion, the starch content also decreased maximum inhibitory effect at 0.10% conc. of pesticide stress at 7th day stage, at the 14th day seedling stage the content decrease maximally (25.7%) at 0.10% conc. of pesticide stress. the starch content presented differential pattern under increasing conc. of Biopesticide, Content remained same at 0.10%, decreased by 5.7% at 0.15% and increased by 4.8% at 0.20% conc. of pesticide stress at 7th day and at 14th day the starch content increased with increasing conc. of pesticide stress (Fig. 2a)
Effects of various pesticides on total phenol content
It was observed that total phenol content declined under different conc. of Profenofos and malathion stress, In case of Profenofos at 7th day stage phenol content decreased under stress, with maximum reduction at 0.10% conc. of pesticide stress and at 14th day seedling stage the content decreased at 0.10%, 0.20% conc. of pesticide stress but increased by 1% at 0.15% conc. of pesticide stress as compared to the control. In case of malathion the phenol content decreased both at 7th day (except, increased at 0.10% by 5.2%) and 14th day seedling stage maximum decrease at 0.10% conc. of pesticide stress. The phenolic content increased with increasing conc. of pesticide , with maximum phenol content at 0.20% conc. of Biopesticide stress both at 7th and 14th day seedling stage under the different conc. of neem biopesticide stress (Fig. 2b)
Effects of various pesticides on Lipid Peroxidation
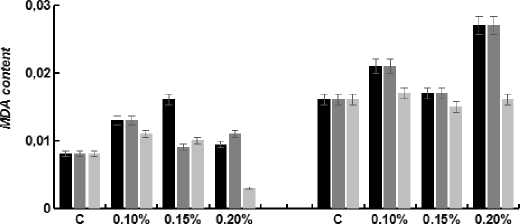
After imposition of different conc. of pesticide stress on seedlings, the seedlings show significant difference in MDA content (between the different treatments groups and the control seedlings) with increasing conc. of stress. At 7-D and 14-D old seedling stage, the MDA content increased by 64.7%, 8.82% and 23.5%, 52.9%, 5.8% under 0.10%, 0.15%, 0.20% conc. of Profenofos stress. Increased conc. malathion stress resulted in an increased MDA content by 55.5%, 11.1%, 33.33% and by 38.8%, 1.8%, 94.4% at 0.10%, 0.15%, 0.20% conc (Fig.3). In case of Biopesticide, the MDA content increased by 33.3%, 22.2% at 0.10%, 0.15% and decreased by 55.5% at 0.20%. conc. at 7-D seedling stage whereas MDA content increased by 7.69% at 0.10% and decreased by 7.6% and 9.6% at 0.15% and 0.20% conc. respectively (Fig. 3).
Effects of various pesticides on total antioxidant enzyme activity and Antioxidant Enzyme Activities
The effect of different conc. of various pesticides on the total antioxidant enzyme activity was recorded. An increased FRAP activity of 6%, 13.8%, 33.28% and 22.3 %, 16.5 % and 24.1 % at 0.10%, 0.15%, 0.20% of was observed for the samples incubated with Perfenofos pesticide at both the 7-D and 14-D of stress conditions. Similar results were obtained for Malathion stress where the FRAP activity get induced by 35.05%, 43.29%, 20.6% and 31.2%, 32.2%, 0.6% at 0.10%, 0.15%, 0.20%. contrary to an induced activity of stress and in case of Biopesticide, FRAP decreased by 46%, 34.1%, 30.7% at 0.10%, 0.15%, 0.20% respectively (Fig.4a).
In case of Profenofos the guaiacol peroxidase (GP ) activity increased by 36.3%%, 63.6%, 27.2 % and 7.5%, 44%, 55.5 % at 0.10%, 0.15%, 0.20% of pesticide stress respectively. In case of Malathion, the GP activity increased by 33.3%, 25% and 7.5%, 44%, 55.5 % at 0.10%, 0.15%, 0.20% conc except for an decreased by 15% at 0.20% at 7-D stage of development. In case of Biopesticide the enzyme activity decreased by 41.7% at 0.10%, and by 12.6% at 0.20% the activity increased slightly by 6.7% at 0.15% conc. at 7-D stage of development, however an overall decrease of 3-16% was observed under different conc of biopesticide stress at 14-D of seed development (Fig.4b). Similar pattern of enzyme activity was observed for the catalase enzyme. Under the influence of different conc of profenofos the activity increased by 11.1%, 67%, 52.6 % and 44.4%, 75%, 68.7 % at 0.10%, 0.15%, 0.20% of pesticide stress respectively. Whereas different conc. of Malathion resulted in an increased catalase activity by 76%, 62.5%, 66% and 25%, 56% at 0.10%, 0.15%, 0.20% except a decrease of 37% at 0.1% at 7-D stage of seed development. Non-significant decrease was observed for the biopesticides at 7-D stage of development whereas 25% and 37 % decrease was observed at 0.10% and 0.15% conc at 14-D stage of seed development (Fig.4c). The increasing conc. of pesticides show differential pattern of SOD activity in the seedlings. In case of Profenofos stress, SOD activity increased by 70.1%, 72.1% and 1.32% and 28.3%, 33.1% and 2.48% at 0.10%, 0.15% and 0.20% at 7-D and 14-D stages of seed development. Imposition of the different conc of Malathion stress resulted in an increased activity of 13.2%, 35.7%, 15.2% and 68.0%, 55.6%, 82.2% at 0.10%, 0.15%, 0.20% conc. respectively. In case of Biopesticide, the SOD activity decreased marginally by 3-36% at 0.10%, 0.15%,0.20% conc at both 7-D and 14-D stages of seed development except for at 0.15% conc where an increased SOD activity by11.8% was observed (Fig. 4d).
Table 1. Comparative analysis of the effect of different pesticide concentrations on the root and shoot length in the developing maize seedlings
|
Pesticide |
Profenofos |
||||
|
Conc. of stress |
Control |
0.10% |
0.15% |
0.20% |
|
|
Root length |
7-D |
11.6 ± 0.13 |
10.5 ± 0.11 |
9.7 ± 0.15 |
11.4 ± 0.12 |
|
14-D |
15 ± 0.07 |
12.2 ± 0.08 |
10.8 ± 0.09 |
11.7 ± 0.1 |
|
|
Shoot length |
7-D |
25.2 ± 0.23 |
21.3 ± 0.19 |
15.7 ± 0.15 |
24 ± 0.12 |
|
14-D |
38.7 ± 0.14 |
38.3 ± 0.09 |
27.6 ± 0.12 |
28.3 ± 0.14 |
|
|
Pesticide |
Malathion |
||||
|
Root length |
7-D |
11.6 ± 0.09 |
7.1 ± 0.04 |
6.1 ± 0.05 |
5.7 ± 0.07 |
|
14-D |
15 ± 0.09 |
11.4 ± 0.06 |
9.2 ± 0.08 |
7.2 ± 0.07 |
|
|
Shoot length |
7-D |
25.2 ± 0.15 |
21.4 ± 0.09 |
20.1 ± 0.06 |
21.8 ± 0.12 |
|
14-D |
38.7 ± 0.12 |
35.6 ± 0.1 |
30 ± 0.14 |
20.8 ± 0.16 |
|
|
Pesticide |
Biopesticide |
||||
|
Root length |
7-D |
11.6 ± 0.11 |
14.83 ± 0.14 |
13.5 ± 0.09 |
15.6 ± 0.15 |
|
14-D |
15 ± 0.06 |
16.4 ± 0.06 |
15.5 ± 0.07 |
16.3 ± 0.06 |
|
|
Shoot length |
7-D |
25.2 ± 0.1 |
25.4 ± 0.14 |
27 ± 0.17 |
26.6 ± 0.15 |
|
14-D |
38.7 ± 0.12 |
42.9 ± 0.11 |
42.7 ± 0.13 |
44.7 ± 0.11 |
|
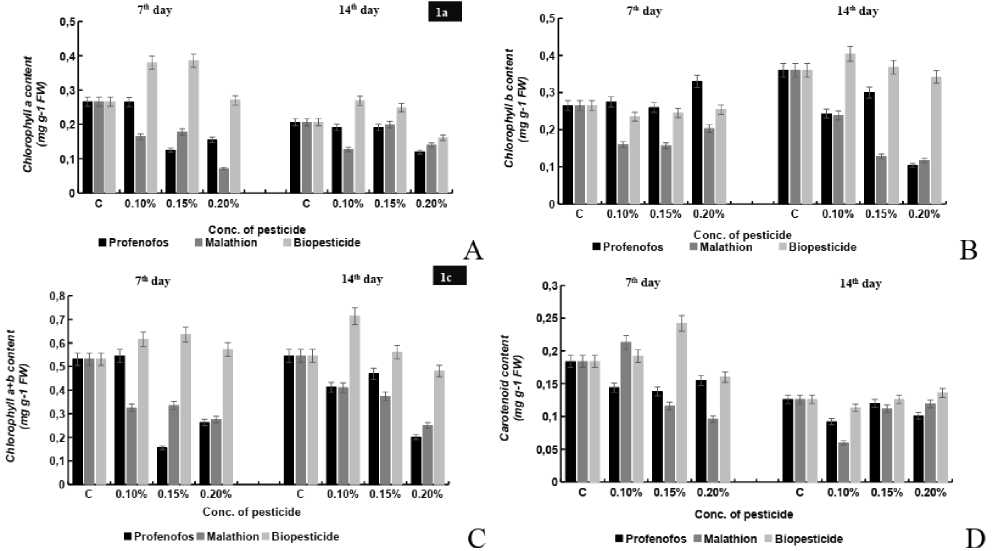
Figure 1. Effects of different concentrations (0.10%, 0.15%, 0.20%) of profenofos, malathion and Biopesticide on (A) Chl a, (B) Chl b, (C) Chl a+b and (D) carotenoid content in seedlings of maize were analyzed. Error bars indicate mean of independent replicate of 10 seedlings ( in triplicate ) each with SE± 5% of control sample.
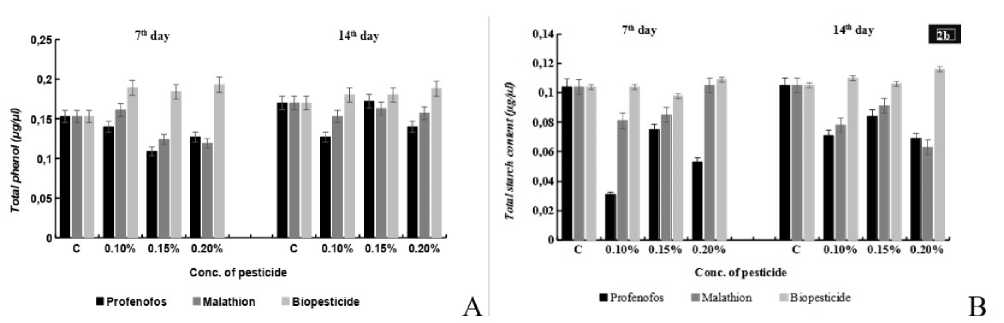
Figure 2 . Effects of different concentrations (0.10%, 0.15%, 0.20%) of Profenofos and malathion on (A) total phenol and (B) total starch content in seedlings of maize were analyzed. Error bars indicate mean of independent replicate of 10 seedlings (in triplicate) each with SE± 5% of control sample.
7“ day 14'“ day
Cone, of pesticide
■ Profenofos □ Malathion ■Biopesticide
Figure 3 . Effects of different concentrations (0.10%, 0.15%, 0.20%) of Profenofos and malathion on MDA content in seedlings of maize were analyzed. Error bars indicate mean of independent replicate of 10 seedlings (in triplicate) each with SE± 5% of control sample.
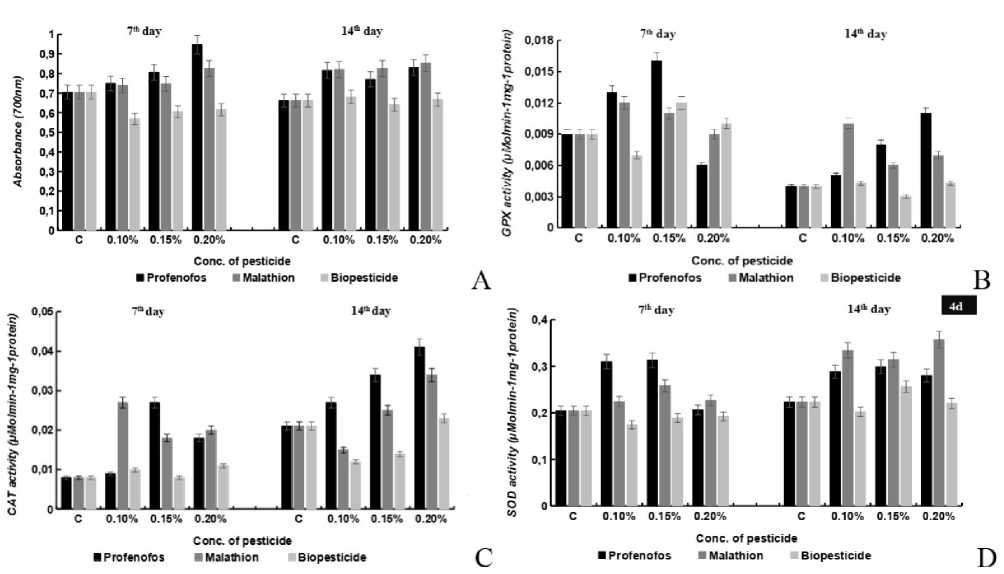
Figure 4. Effects of different concentrations (0.10%, 0.15%, 0.20%) of profenofos, malathion and Biopesticide on (A) total antioxidant activity, (B) GP , (C) CAT and (D) SOD activity in seedlings of maize were analyzed. Error bars indicate mean of independent replicate of 10 seedlings ( in triplicate ) each with SE± 5% of control sample.
DISCUSSION
Seed treatment with systemic fungicides is a routine integrated crop management practice for crops. The usage of these pesticides is thought to be important in vegetable cropping systems to avoid disease and insect damage. Various pesticides significantly harm the developmental and reproductive systems of non-target species such as plants by causing cellular oxidative damage in plants besides disrupting the growth and yield, retarding the germination rate and disturbing the morphological and physiological parameters (Shahid et al., 2018). We observed that there is a considerable decrease in the root and shoot length under various pesticide conc., however an increase in shoot/root length was observed for neem based biopesticide. Similar decrease was also observed under various chemical pesticides in vigna and wheat (Li et al., 2023; Mishra et al., 2015) whereas an increased seed germination parameters were observed under neem extract treatment in quinoa seedlings (Kilani-Morakchi et al., 2021). It may be interpreted that delayed in germination due to the various pesticide stress can be attributed to the interference in germination metabolism as theses chemical pesticides effect on cell division and elongation (Hatamleh et al., 2022). However, the enhanced germination activity under neem extract could be due to the presence of high amount of ascorbic acid besides the polyphenolic and tetranortriterpenoid compounds which has antibacterial and insecticidal activities (Kilani-Morakchi et al., 2021).
Various pesticides are known to impact the photosynthetic machinery via uncoupling of photophosphorylation and inhibition of the electron transport system (Giménez–Moolhuyzen et al., 2020), leading to decrease in chlorophyll and carotenoid content. We observed that increased conc. of Profenofos and malathion pesticides resulted in a substantial decrease in the activity of Chl a, Chl b, Chl a+b and carotenoid content. These results are further supported by our previous studies on chickpea where a significant decrease in various chlorophyll components was detected when subjected to various fungicide stress (Singh and Sahota, 2018). The decrease in chlorophyll activity may be attributed to the breakdown of thylakoids and chloroplast envelopes besides the inactivation of the enzymes responsible for the formation of chlorophyll molecules (Lei et al., 2020; Stenbaek and Jensen, 2010). The impairment of the photosynthetic apparatus due to pesticide stress through decrease photoprotection may be attributed to the decrease carotenoid activity (Hashimoto et al., 2016). Contrary to the lower activity of chlorophyll content under pesticides stress, an increased chlorophyll content was observed under neem biopesticide stress which may be attributed to the increased activity of photosynthetic machinery (Mkindi et al., 2020).
Carbohydrates encompass a wide range of substances, including sugars, starches, gums, and celluloses. Carbohydrate contents were reported to be affected by the treatment of herbicides (Orcaray et al ., 2012; Shivani et al ., 2023) and pesticides (Zhang et al ., 2022). The principal storage carbohydrate in plants is the polysaccharide, starch. We observed that the level of total starch content declined under different conc of pesticide stress but a marginal increase was observed under the biopesticide stress. This may be due to the fact that pesticide might had obstructed the biosynthetic pathway or altered the breakdown of starch (Zobiole et al ., 2012). Results were in accordance to the study which showed that the metalaxyl cause decline in starch content at its higher concentration on the gradual days of germination. The breakdown of starch with metalaxyl treatment may be because of increased amylase activity during seed germination which correlates that amylase activity was influenced by metalaxyl which cause decrease in starch content (Li et al ., 2020). Increased activity of the starch content may be due to the resistance provided by the various phytoconstituents of the neem extract against various pathogens (Godlewska et al ., 2021).
Lipid peroxidation is a process in which oxidants such as free radicals damage lipids containing carboncarbon double bond(s), and it is a key biomarker in understanding the oxidative stress caused by various abiotic stimuli such as pesticide stress (Ayala et al., 2014). Pesticides' mechanisms of cellular and tissue damage typically entail oxidative stress events, specifically the formation of free radicals, lipid peroxides, oxidised proteins, and oxidised carbohydrates (Wang et al., 2016). Because of the high reactivity of ROS (Reactive Oxygen Species), cellular enzymes, structural proteins, membrane components, carbohydrates, and nucleic acids are vulnerable to damage (Jabłońska – Trypuć and Wiater, 2022; Zarkovic, 2018). In this study we find that the level of MDA has increased substantially in response to the pesticide stress thus suggesting the interference of these pesticides. However, the MDA response was decreased in response to the different conc. of neem biopesticide thus suggesting that neem extract prevents the cytoplasmic leakage and alleviates the integrity of the membrane. Similar observations of reduced lipid peroxidation levels on tomato leaves under botanical pesticides Sophora flavescens alkaloids (SFA) has been recorded ( iong et al., 2016).
Plants are known to produce various secondary metabolites such as flavonoids and phenolic content such as salicylic acid, gallic acid etc. under various abiotic stress conditions (Chowdhary et al ., 2022) and the these phenolic compounds provide protection by stabilizing membranes against antioxidant molecules via reducing the peroxidation and decreasing the fluidity of the membranes (Foti, 2010, Sadžak et al ., 2020). We also observed the declining rate of phenolic content in response to the different conc. of pesticide stress while a marginal increase was observed under varied neem biopesticide conc. since neem extract already contains various polyphenolic phytoconstituents which are likely to provide protection thus also contribute to the increase conc. of phenolic content (Kilani-Morakchi et al ., 2021).
Antioxidant capacity and differential regulation of various antioxidative enzymes have been widely used as biomarkers for various abiotic stresses including chemical and pesticides (Dias, 2012; Sachdev et al., 2021). The enzymatic defence system includes generation of ROS scavenging enzymes – Catalase, Superoxide dismutase, Ascorbate peroxidase, Guaiacol peroxidase. The neutralisation or detoxification of harmful ROS molecules is significantly aided by antioxidant enzymes including SOD, CAT, GP , and GR, which are present in nearly all cellular compartments. SOD is an enzyme triggered by ROS and the breakdown of singlet oxygen radicals (1O2-) or superoxide radicals (*O2) into H2O2 and O2. The H2O2 produced as a result of SOD still has the ability to cause cell damage since it can be converted into the hydroxyl radical (fotOH), which can cause damage to cells. Consequently, CAT, AP , and GP and detoxify H2O2 into H2O and O2 while also protecting cells from injury (Hasanuzzaman et al., 2017). We observed an overall increase in the ferric reducing antioxidant potential (FRAP) activity under pesticide stress whereas substantial decrease in total antioxidant capacity was observed for the seedling grown under the neem biopesticide stress. Further it was observed there is an induced activity of AP , CAT and SOD enzyme under both the pesticide stress conditions which may attributed to the plant defence mechanism to nullify the effect of ROS derived due to pesticide stress. Marginal decrease in the activity of various antioxidative enzymes under biopesticide stress could be an effect of various polyphenolic compounds of neem extract in providing the negative correlation. the decrease in the antioxidative enzyme activity is likely to be due to protection provided by various phenolic compounds present in neem extract to the developing maize seedlings. It has been reported that the botanical pesticides has synergistic effect in antioxidant enzyme protection (Ayilara et al., 2023; Kumar et al., 2021; Lengai et al., 2020; Li et al., 2023).
CONCLUSION
Biopesticides including the botanical pesticides consisting of various phytoconstituents viz polyphenols and flavonoids are an alternative route to plant protection. In this study we find that chemical pesticides induce various deleterious metabolic changes which hinders the overall growth and development of the plants whereas the neem biopesticides has been playing a protective role thus indicating the efficacy of these pesticides over the chemical pesticide in improving the crop productivity.
ACKNOWLEDGEMENTS:


