Comparative analysis of osmotin-like PR5 and PA13 proteins in potato infected by late blight (Phytophthora infestans)
Автор: Al-daoude A., Shoaib A., Jawhar M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.21, 2025 года.
Бесплатный доступ
Induction and accumulation of osmotin-like proteins are crucial components of innate immune responses in plants challenged with pathogens. One of these proteins, PR5 and PA13 , are abundantly secreted and are able to elicit plant defenses, however, their native function in potato plants infected with Phytophthora infestans remains unknown. Here, the pathway signaling of the two genes were monitored in potato plants at early points of infection with P. infestans using quantitative real-time PCR (qPCR). Our data demonstrated significant gene expression variance of the two genes in infected plants as compared to the non-infected controls. It is also notable that PA13 gene had higher expression than PR5 in the resistant cultivar 'Sponta' as compared to the susceptible one 'Draga' with a maximum expression for PR5 (3.5 and 1.2-fold) and PA13 (8.2 and 2.7-fold) respectively, at 48 hours post infection. The obtained results suggested that PR5 and PA13 genes, positively regulate P. infestans -resistance in potato plants during disease progress, which can offer testable hypotheses that will need straight upcoming experiments to define how the PR5 and PA13 pathway signaling may be specified in potato defense system.
Potato, defense response, phytophthora infestans, pr5, pa13, pcr (qpcr)
Короткий адрес: https://sciup.org/143183774
IDR: 143183774
Текст научной статьи Comparative analysis of osmotin-like PR5 and PA13 proteins in potato infected by late blight (Phytophthora infestans)
Late blight (LB), caused by the fungus Phytophthora infestans Mont.) de Bary , is a dangerous disease of potato ( Solanum tuberosum L. ) causing significant yield losses worldwide (Dong and Zhou 2022; Angmo et al., 2023). The complicated systemic responses of potato to fight LB necessitate attention due to the potential for yield protection in field, and the immune responses during these interactions, however, are highly complex and often differ between genotypes (Salima 20 5; Al-Daoude et al., 2023). Therefore, it is highly challenging to control LB due to a poor understanding of the resistance mechanisms since plants resist this disease through initiation of diverse signaling pathways (Paluchowska et al., 2022).
P. infestans begins potato infection through a biotrophic phase which needs living cells to get the nutrition through the haustoria (Kagda et al., 2020), and they respond by activating different signaling pathways including osmotin-like proteins. However, many of their specific functions still remain unknown. The proteins members of the PR5 and PA13 family play vital functions in plant defense responses (Liu et al., 994). PR5 family includes basic and acidic members according to their isoelectric points, although they show similar activity, however, PR5 proteins had an antifungal activities in rice and orange plants after inoculation with to the two fungal pathogens Rhizoctonia solani and Phytophthora infestans (Bachmann et al ., 998).
In addition, osmotin-like proteins have in vitro antifungal activity against the P. infestans (Woloshuk et al., 99 ). Zhu et al. ( 995) found high levels of the PA13 osmotin-like protein in transgenic potato plants that infected with this pathogen at different stages of infection. However, relatively little is known about gene expression of PR5 and PA13 during potato interaction with P. infestans.
Quantitative PCR (qPCR) is an accurate technique for assessing plant gene expression levels under various biotic and abiotic stresses (Bates et al., 200 ). In previous work, the potato resistant cv. Sponta was exhibited a lower level of LB symptoms progress comparing with the susceptible cv. Draga (Salima 20 5). We accordingly hypothesized that PR5 and PA13 genes could drive differentiated levels of resistance in these two cultivars, inoculated by the same isolate of P. infestans.
Hence, the objective of the present work was to evaluate the changes in induction of the two osmotinLike PR 5 and PA13 proteins in the two selected potato cultivars with different resistance levels against P. infestans using PCR (qPCR) technique.
MATERIALS AND METHODS
Host Plant
The resistant potato (Sponta) and susceptible (Draga) cultivars from Netherlands grown commonly in Syria were used. A single tuber was planted in plastic pot filled with sterilized peatmoss with three replicates. Pots were placed under a growth chamber conditions at 20° ( 6h light/ 8h dark) and 85-90 % relative humidity.
Inoculation with P. infestans
The Syrian P. infestans virulent isolate PiSYR (Salima 20 5) was used in the experiments. It was isolated from small infected potato leaves and grown in Petri dishes under disinfected tuber slices for 7 days at 20 ± 2 °C under 6 h/8h light/dark photoperiod. Once mycelium was growing on the surface of the potato slice, the mycelium was transferred to fresh rye agar (Caten and Jinks 968). A conidial suspension with 5 × 04 spores/mL were sprayed onto the potato seedlings, whereas, control plants were sprayed with fungus-free water.
RNA isolation and cDNA synthesis
RNA was isolated from Potato leaves after 24, 48, 72 and 96 hours post infection (hpi) using Trizol Reagent (Macherey-Nagel, Germany). Controls were collected at the similar time points. cDNA was generated using the QuantiTect Reverse Transcription Kit (Qiagen) due to the manufacturer’s instructions.
RT-qPCR analysis
PR5 and PA 3 genes were assessed during the four time points by RT-qPCR assays using SYBR Green Master kit (Roche, USA). The used primers are shown in Table . PCR conditions were 95° for 5 min, followed by 40 cycles of 95° for 0 s, 60° for 20 s, and 72° for 20 s. Gene expression was calculated by the 2-ΔΔ C t method using EF1α as a reference gene (Livak and Schmittgen
200 ). Means comparisons were carried out using Tukey’s test. All experiments were repeated three times.
RESULTS AND DISCUSSION
In this investigation, two potato cultivars with different resistance levels towards P. infestans were used. Four time points 24, 48, 72 and 96 hpi, were chosen as being illustrative of biotrophy and switch to necrotrophy phases (Xiao et al., 20 9). L B symptoms were more severe on the susceptible cultivar as compared to the resistant one (data not shown).
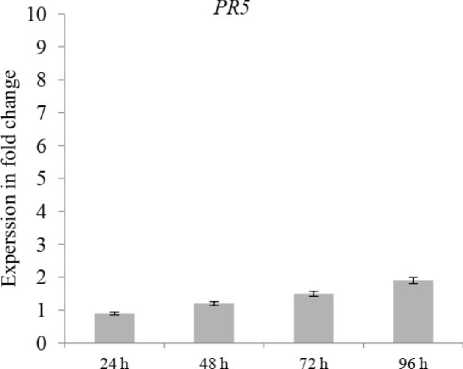
To increase our understanding to these interactions, PR5 and PA13 expressions were evaluated at the four early time points of potato infection by P. infestans. Our results presented that at 24 hpi, the two genes were significantly upregulated after infection in both resistant and susceptible cultivars (Figs. and 2), proposing that distinct defense responses were early activated, and the expressed gene patterns were recorded cooperative functions which occur after 24, 48, 72 and 96 hpi infecting by P. infestans. It is also remarkable that PA13 gene had higher expression than PR5 and that maximum expressions in resistant and susceptible were 48 hpi for PR5 (3.5 and .2-fold) and PA13 (8.2 and 2.7-fold), respectively (Figs. and 2).
However, considering that the two used potato cultivars had high variant resistance levels to LB (Salima 20 5), the both PR5 and PA13 genes were up-regulated 24 hpi in infected plants comparing to the non - infected ones, which might indicate that their functions are associated to the LB severity symptoms rather than to resistance as it obvious in the resistant cultivar than in the susceptible one (Figs. and 2).
T able 1. List of genes studied with accession number and conesponding primers used for RT -qPCR
|
Gene |
Accession No. |
Sequence |
|
EFla |
AT1G07920 |
TGGATTTGAGGGTGACAACA CCGTTCCAATACCACCAATC |
|
PA 13 |
P5O7O1 |
CATGGGTTATCAATGCGCCA GTTGCTGAACTGGTCCAAGG |
|
PR5 |
AT1G75O4O |
GGGGCTACTGTTTCAAGCAA GCAGACTGTGGCGGTCTAAG |
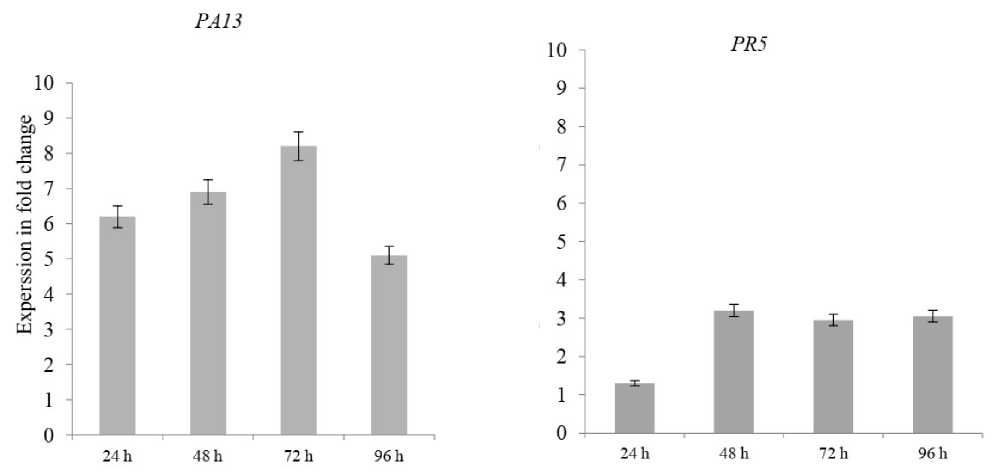
Figure 1 . Relative expression profiles of PR5 and PA13 in resistant cv. Sponta cultivar during the time course of LB infections. Error bars are representative of the standard error (mean ± SD, n = 3). Data are normalized to Elongation factor α (EF- α) gene expression level (to the calibrator, Control 0 h, taken as 0).
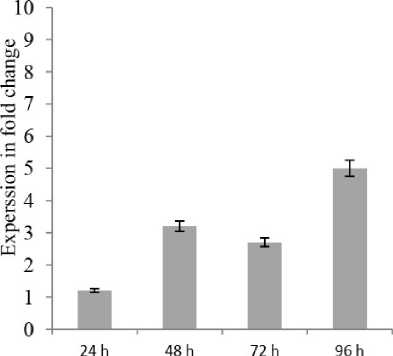
Figure 2. Relative expression profiles of PR5 and PA13 in susceptible cv. Draga cultivar during the time course of LB infections. Error bars are representative of the standard error (mean ± SD, n = 3). Data are normalized to Elongation factor α (EF- α) gene expression level (to the calibrator, Control 0 h, taken as 0).
It has been reported that the osmotin-like proteins are cysteine-rich proteins that could increase protein stability under various conditions (Zhao et al., 2020). Therefore, they play a vital role in plant defense through signal transduction pathways for inhibiting the defensive cell wall barriers and rises its cytotoxic efficiency. This might elucidate the changes of the both genes PR5 and PA13 during P. infestans –potato interaction. Zhu et al. ( 995) and Sanju et al. (20 5) reported overexpression of osmotin like proteins in potato response under biotic and abiotic stresses. In addition, de Freitas et al. (20 ) found that osmotin like protein purified from Calotropis procera latex had a strong antifungal activity against Fusarium solani and Colletotrichum gloeosporioides .
CONCLUSION
Our data showed that significant increases in PR5 and PA13 expressions were found upon potato challenged with the P. infestans, which can contribute to LB resistance, because these signaling responses induced together in each resistant and susceptible cultivars. It is also notable that PA13 had higher expressions than PR5 in the resistant cultivar as compared to the susceptible one with a maximum for PR5 (3.5 and .2-fold) and PA13 (8.2 and 2.7-fold) respectively, at 48 hpi. These results could be in line with the well-accepted concept that defense responses are very intense in potato resistant plants. Furthermore, we painted the fact that the osmotin-like PR5 and PA13 proteins signaling pathways might be activated in response to the same isolate of P. infestans in different potato cultivars.
ACKNOWLEDGMENT
The authors thank the Director General of AECS and the Head of Biotechnology Department for their help throughout the period of this research.
CONFLICTS OF INTEREST
The author declare that he has no potential conflicts of interest.
Список литературы Comparative analysis of osmotin-like PR5 and PA13 proteins in potato infected by late blight (Phytophthora infestans)
- Al-Daoude A, Shoiab A, Jawhar M. 2023. Increased expression of beta-tubulin in potato plants challenged with Phytophthora infestans. J. Stress Physiol. Biochem., 19: 105-110.
- Angmo D, Sharma SP, Kalia A. 2023. Breeding strategies for late blight resistance in potato crop: recent developments. Mol Biol Rep., 50: 78797891.
- Bachmann D, Rezzonico E, Retelska D, Chetelat A, Schaerer S, Beffa R. 1998. Improvement of potato resistance to Phytophtora infestans by overexpressing antifungal hydrolases. 5th International Workshop on pathogenesis-related proteins. Signaling pathways and biological activities. 1998, Aussois, France. Abstr. P-57.
- Bates J, Taylor E, Kenyon D, Thomas J. 2001. The application of real-time PCR to the identiication, detection and quantification of Pyrenophora species in barley seed. Mol, Plant Pathol., 2: 4957
- Caten CE, Jinks JL. 1968. Spontaneous variability of single isolates of Phytophthora infestans. I. Cultural variation. Can. J. Bot., 46: 329-347.
- de Freitas, C. D., Nogueira, F. C., Vasconcelos, I. M., Oliveira, J. T., Domont, G. B., and Ramos, M. V. 2011. Osmotin purified from the latex of Calotropis procera: Biochemical characterization, biological activity and role in plant defense. Plant Physiol. Biochem., 49:738-743.
- Dong S, Zhou S, 2022. Potato late blight caused by Phytophthora infestans: From molecular interactions to integrated management strategies, J. Integr. Agric., 21: 3456-3466,
- Kagda MS, Martínez-Soto D, Ah-Fong AMV, Judelson HS. 2020. Invertases in Phytophthora infestans localize to haustoria and are programmed for infection-specific expression. mBio., 11: e01251-20.
- Liu D, Raghothama KG, Hasegawa PM, Bressan RA. 1994. Osmotin overexpression in potato delays development of disease symptoms. Proc. Natl. Acad. Sci. USA., 91: 1888-1892.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods, 25: 402-408.
- Paluchowska P, Sliwka J. Yin Z. 2022. Late blight resistance genes in potato breeding. Planta, 255: 127.
- Salima NI. 2015. Surveying the distribution of late blight Phytophthora infestans on potato in Syria and Studying the physiological and molecular variability of the pathogen. Thesis, University of Damascus, Faculty of Agriculture, pp.61.
- Sanju, S., Thakur, A., Siddappa, S., Sreevathsa, R., Srivastava, N., Shukla, P., et al. 2015. Retraction note to: Pathogen virulence of Phytophthora infestans: From gene to functional genomics. Physiol. Mol. Biol. Plants., 21:167.
- Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, van den Elzen PJM, Cornelissen BJC. 1991. Pathogen-induced proteins with inhibitory activity toward Phytophthoru infestuns. Plant Cell., 3: 619628.
- Xiao C, Gao J, Zhang Y, Wang Z, Zhang D, Chen Q, Ye X, Xu Y, Yang G, Yan L, Cheng Q, Chen J, Shen Y. 2019. Quantitative proteomics of potato leaves infected with Phytophthora infestans provides insights into coordinated and altered protein expression during early and late disease stages. Int. J. Mol. Sci., 20: 136.
- Zhao Q, Qiu B, Li S, Zhang Y, Cui X, Liu D. 2020. Osmotin-like protein gene from Panax notoginseng is regulated by jasmonic acid and involved in defense responses to Fusarium solani. Phytopathology., 110: 1419-1427.
- Zhu B, Chen THH, Li PH. 1995. Expression of three osmotin-like protein genes in response to osmotic stress and fungal infection in potato. Plant Mol Biol., 28: 17-26.


