Comparative analysis of theoretical and experimental UV-spectra of 2- and 8-thioquinoline
Автор: Matveychuk Yu.V., Ilkaeva M.V., Krivtsov I.V., Bartashevich E.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 2 т.7, 2015 года.
Бесплатный доступ
The comparative analysis of the calculated electronic absorption spectra of various forms of 2- and 8-thioquinoline and the experimental UV spectra of their solutions in various solvents has been carried out. Two conformational isomers of SH-forms have been analyzed for each compound. Calculations of other tautomeric forms of these compounds, namely NH-forms of 2-thioquinoline (2-thioquinolone) and 8-thioquinoline (zwitter-ion), and S-forms (anions) and 1-H-2-thioquinolinium cation have been analyzed additionally. It is shown that the absorption bands in the spectrum of 8-thioquinoline solution correspond to the electronic transitions in the SH-tautomer. On the contrary, the absorption bands in a spectrum of 2-thioquinoline solution correspond to the electronic transitions in the NH-tautomer and S-anion. The spreading of absorption bands in the experimental spectra is associated with conformational and tautomeric equilibrium which is established in the solutions of the considered compounds.
Thioquinoline, electronic absorption spectra, solvatochromic effect, td-dft
Короткий адрес: https://sciup.org/147160314
IDR: 147160314 | УДК: 544.18,
Текст научной статьи Comparative analysis of theoretical and experimental UV-spectra of 2- and 8-thioquinoline
Oxyquinolines and oxypyridines are the objects of long-term examinations, including their electronic spectra, which are influenced by such factors as solvents, position of OH-groups in a ring, environment conditions, etc. [1-3]. Such interest is associated with the application of the given substances as chelates in analytical chemistry, and as intermediate products for synthesis of new biologically active compounds. Such feature of the compounds, as the existence of various tautomeric forms in solutions, depending on environment conditions, has been noticed in experiments. Nowadays the attention is turned to the similar thio-substituted compounds, which are used for the synthesis of new substances, especially in pharmaceutics [4-7]. The active reaction centers and the mechanisms of their interaction with various reagents in directional synthesis are the most important aspects of the precise examination of the substances. The special attention is paid to the study of the interactions of the substances with halogens and organohalogen compounds. Thus, the interaction of 8-thioquinoline and pyrrolidine-2-thione with molecular iodine, the structural changing of complexes during interaction, and its influence on electronic spectra, are presented in the research works [8, 9]. It has been noticed, that it is possible to trace the conversion degree and change of reagent concentration, to determine the stability constant of complexes with the use of UV spectra.
The investigation of the theoretical electronic spectra of organic compounds, including heterocycles, with the evaluation of the energy characteristics within the ab initio TD-DFT method [10, 11] has been widely applied for the detailed interpretation of the observed experimental data. Thus, in the study [12], alongside with the comprehensive experimental research of benzodithiazole derivatives, the calculations of electronic density, spectra and the reaction activity of the synthesized substances, consistent with the experimental data, have been carried out. In the study [13], the changing of the fluorescence ability of the phosphonate derivatives of 8-oxyquinoline and their complexes with zinc has been explained with the help of calculations. In [3], the comparison of NMR, IR and UV spectra of 5,7-dihalogen-8-oxyquinolines with the calculated spectra, obtained by the TD-DFT method has been performed. It is noticed, that the calculations have allowed to describe correctly a pattern of absorption maxima on UV spectra and to carry the assignment of the electronic transitions. Also, the results of the experimental research of 2-amino-5-brombensoic acid were in good accordance with the calculations of the Raman activities and UV spectra [14], and the authors explained some features in view of the existence of conformers and difference of their energy characteristics.
For the correction of medium (solvent) influence on the explored substances, the improving models are involved, in which the different types of interactions of the solvate and the solvent are considered: dipole-dipole, dipole-induced, dispersion, etc . In particular, during the last decade the precise numerical continuum polarization model (PCM) [15, 16] has been advanced. Within the PCM model the solvent is considered as an isotropic medium, described by some physical constants; still the specific interactions are not taken into account in an explicit form. The solvate molecule is placed in a cavity that is formed in this continuous medium. All the solvate atoms are surrounded by spheres of Van-der-Waals radius. To build up the smooth surface, which is necessary for convergence of the method, the secondary environment by spheres of small radii with the subsequent triangulation is made for the formation of the surface elements. By several iterations, the surface charge field of the formed cavity and the free energy of a molecule in the solvent are estimated. The popularity of the PCM model is associated with the rapid calculations of the electronic states in the solvent environment, only a little longer compared to the calculations for a gaseous phase. Still, theoretical results and tendencies of the change of the compound spectra, obtained with the use of the given model, adequately correlate with the dependences of the experimental spectra of the synthesized compounds in solutions and explain their features. It has been shown, for azo-compounds, for example [17].
In turn, the electronic spectroscopy is the available and safe technique of the reaction path study, in which 2- and 8-thioquinoline and their derivatives are the initial components [8, 9].
The aim of this study is the determination of the similarity and the basic differences in the spectral behavior of 2- and 8-thioquinoline that act as the efficient reagents in the synthesis of new heterocyclic systems. It is obviously important to study the influence of the medium polarity on the electronic spectra of 2- and 8-thioquinoline and to estimate the opportunities to forecast the spectral behavior of their derivatives according to the theoretical calculations. For this purpose, we set the following tasks:
-
– to compare the calculated absorption characteristics of the fundamental electronic transitions with experimental UV spectra of 2- and 8-thioquinolines in various solutions;
-
– to trace how the changing basic contributions of the molecular orbitals to oscillators of electronic transitions vary in different compound forms, and what changes of these contributions occur through variation of the functional thionic group position in the quinoline ring;
-
– to find out, how the conformational and tautomeric variety of the investigated thioquinolines affects the position of the calculated absorption bands and, as the result, the interpretation of their experimental spectra in solvents.
Experimental
We used the reactive 2- and 8-thioquinoline (97 %, Sigma-Aldrich) for studying their UV absorption spectra characteristics. Dichloromethane and ethanol of chemical purity were used as solvents for the investigated compounds. The concentration of the compound solution was 10-4 M. The spectra of solutions were obtained with the help of the UV-Vis spectrophotometer Shimadzu UV-2700. The halogen and deuterium lamps were used as radiation sources with changing of one lamp for another at 323 nm. The spectra were obtained in the range from 220 up to 850 nm with shooting speed 450 nm per minute.
Calculations
We have carried out the optimization of structure geometry of various tautomeric forms of 2- and 8-thioquinoline with the subsequent calculation of the energy characteristics of the excited states and the electronic spectra, taking the influence of solvents (benzene, ethanol and dichloromethane) into account. The initial SH-forms are represented in calculations as a pair of conformational isomers. The optimization has been carried out for SH-forms 1-4 and NH-forms of 2-thioquinoline (2-thioquinolone 9 ) and 8-thioquinoline (zwitter-ion 5 ), S-forms (anions 6, 8 ); and 1-H-2-thioquinolinium cation 7 (Fig. 1). For the comparison, the optimized structures and electronic spectra of free molecules have been also considered in a gaseous phase. The optimization of structure geometry was carried out using necessary iteration number to the stationary point with the largest component of the gradient to be less than 1E–5 Hart-ree/Bohr. Then the obtained Hessians of all structures were without imaginary frequencies.
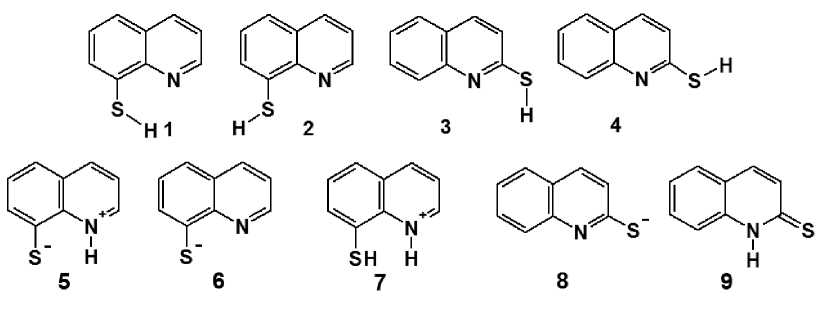
Fig. 1. The analyzed forms of compounds: SH-conformers of 8-thioquinoline (1, 2) and 2-thioquinoline (3, 4); NH-form (zwitter-ion 5), S-form (anion 6) and NH-cation (7) of 8-thioquinoline; S-form (anion 8) and NH-form (2-thioquinolone, 9) of 2-thioquinoline
It is known that PBE0 [18] and B3LYP [19, 20] functionals and basic set 6-311G(d, p) [21, 22] are most commonly used now, as they are the most precise for determination of the molecule characteristics of organic compounds. Therefore optimization of geometry of the studied molecules has been carried out by the Kohn-Sham method (DFT) with the use of functionals PBE0 and B3LYP on a base of fullelectron basic set 6-311G(d, p). The influence of a solvent has been taken into account in model D-PCM (dielectric PCM, base version) [15, 16] with the following parameters: using the same factor for all tesserae, without calculation of cavitation, dispersion and repulsion free energy, at the absolute temperature 298 K. Van-der-Waals radius was taken from [23]. Electronic spectra of the optimized structures are obtained by method TD-DFT [10, 11], also taking into account the influence of solvents. Such calculations have been carried out for 10 excited states using necessary iteration number and convergence criterion on energy of all states to be less than 3E–5 Hartree/Bohr. All calculations of energy and orbital characteristics were performed using software package Firefly 8.0.1 [24].
Discussion
Spectral behaviour of 2- and 8-thioquinoline solutions
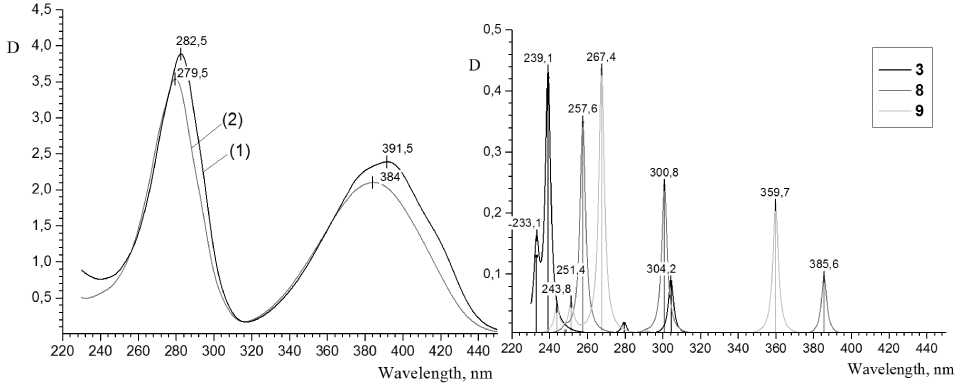
It has been established (see Table 1), that absorption lines in the calculated spectra of different forms of both compounds are shifted towards larger wavelengths with the decrease of the solvent polarity – from ethanol to benzene and further to the gaseous phase state. The experimental interaction of studied compounds with the solvents of different polarity also is impacted; in particular, in shifting of absorption maximums in the spectra of 2-thioquinoline solutions towards larger wave-lengths with changing ethanol to dichloromethane. The experimental spectra of 2-thioquinoline in different solvents are displayed on Fig. 2a, the data of calculations are on Fig. 2b (line spectrum is approximated by Lorentz function); the corresponding data of 8-thioquinoline are shown on Fig. 3a-b. In the experimental spectrum of 2-thioquinoline solution the largest shift of λ max , varying in the range from 310 up to 390 nm, amounts to 8 nm; for other maxima which are varying in the range from 230 up to 300 nm, it is about 4 nm. The experimental spectra of 8-thioquinoline solutions have not shown any similar bathochromic shift. In other words, the negative solvatochromic effect is observed, which is confirmed by calculation results. Such effect was considered earlier in details in the review [25] for comprehensive series of organic compounds.
Our calculations show that for SH-forms of studied compounds 1-4 the calculated dipole moments in solvent medium are increased with transferring a molecule from the basic to the first excited state (see Table 2), and such effect gains in a non-polar solvent. Hence, displaying of the positive solvatochromic effect, associated with the increasing polarity at excitation of a molecule, which should be observed theoretically, at least, for 8-thioquinoline (which is mainly in the SH-forms in the solution), is smoothed out by the influence of other factors, whereby it is small enough. The following can affect it more efficiently than that: the effect of stabilization of the basic state at the solvation by polar solvents, including the one caused by steric difficulties or by the direction change of electrical dipole moment of a solvate; the effect of induced polarization of solvent molecules; also the essential change of the electronic molecule structure is possible. For S- and NH-forms 5, 6, 8, 9 the negative solvatochromic effect dominates in the spectra, the effect is associated with decreasing of molecule polarity in the excited state. This effect, probably, appears in the experimental spectra of solutions of 2-thioquinoline, which is mainly in NH-form 9 in the solution.
Table 1 The position shift of absorption lines in calculated spectra depending on medium, nm (oscillator strength f is in the brackets)
|
Solvent Compound |
Ethanol |
Dichloromethane |
Benzene |
Gas phase |
|
8-thioquinoline (SH-form, conformer 1 ) |
341.9 ( 0.075 ) 240.7 ( 0.221 ) 237.1 ( 0.082 ) |
343.5 ( 0.074 ) 241.1 ( 0.228 ) 237.5 ( 0.070 ) |
348.8 ( 0.073 ) 242.6 ( 0.242 ) 239.0 ( 0.041 ) |
354.8 ( 0.073 ) 244.3 ( 0.244 ) 240.4 ( 0.021 ) |
|
8-thioquinoline (SH-form, conformer 2 ) |
337.0 ( 0.076 ) 239.9 ( 0.176 ) 236.3 ( 0.129 ) |
337.5 ( 0.076 ) 240.1 ( 0.182 ) 236.6 ( 0.119 ) |
339.6 ( 0.078 ) 240.9 ( 0.204 ) 237.6 ( 0.082 ) |
342.2 ( 0.079 ) 241.8 ( 0.221 ) 238.5 ( 0.049 ) |
|
8-thioquinoline (NH-form 5 ) |
622.9 ( 0.031 ) 370.1 ( 0.022 ) 271.8 ( 0.208 ) 262.5 ( 0.210 ) |
– |
714.5 ( 0.029 ) 399.1 ( 0.025 ) 277.4 ( 0.200 ) 268.0 ( 0.190 ) |
783.6 ( 0.029 ) 420.8 ( 0.027 ) 281.4 ( 0.188 ) 271.2 ( 0.175 ) |
|
8-thioquinoline (S-form 6 ) |
457.1 ( 0.076 ) 353.0 ( 0.029 ) 261.9 ( 0.199 ) 257.1 ( 0.090 ) 253.0 ( 0.058 ) |
465.7 ( 0.076 ) 358.6 ( 0.030 ) 263.8 ( 0.211 ) 257.7 ( 0.087 ) 254.2 ( 0.046 ) |
– |
– |
|
8-thioquinoline (NH-cation 7 ) |
418.5 ( 0.033 ) 262.0 ( 0.013 ) 250.3 ( 0.356 ) |
– |
– |
– |
|
2-thioquinoline (SH-form, conformer 3 ) |
304.3 ( 0.088 ) 239.1 ( 0.422 ) 233.0 ( 0.129 ) |
304.7 ( 0.087 ) 239.3 ( 0.420 ) 233.1 ( 0.130 ) |
306.1 ( 0.086 ) 239.8 ( 0.412 ) 233.7 ( 0.133 ) |
307.7 ( 0.085 ) 240.5 ( 0.406 ) 234.4 ( 0.134 ) |
|
2-thioquinoline (SH-form, conformer 4 ) |
305.6 ( 0.085 ) 240.0 ( 0.409 ) 232.7 ( 0.137 ) |
306.0 ( 0.083 ) 240.2 ( 0.406 ) 232.8 ( 0.139 ) |
307.5 ( 0.080 ) 240.8 ( 0.398 ) 233.4 ( 0.144 ) |
309.2 ( 0.078 ) 241.6 ( 0.390 ) 234.2 ( 0.145 ) |
|
2-thioquinoline (S-form 8 ) |
385.5 ( 0.096 ) 300.9 ( 0.245 ) 257.5 ( 0.349 ) |
391.8 ( 0.093 ) 304.0 ( 0.260 ) 257.1 ( 0.319 ) |
– |
– |
|
2-thioquinoline (NH-form 9 ) |
359.8 ( 0.214 ) 267.5 ( 0.435 ) |
361.6 ( 0.209 ) 267.8 ( 0.440 ) |
368.2 ( 0.194 ) 267.8 ( 0.440 ) |
375.2 ( 0.177 ) 266.2 ( 0.432 ) |
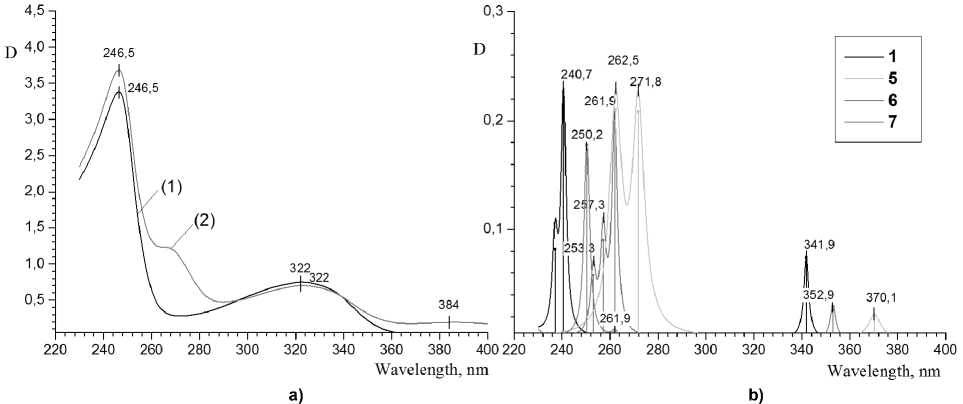
Fig. 2. a) Experimental UV spectra of the solution of 8-thioquinoline in dichloromethane (1) and ethanol (2); b) Calculated spectra of 8-thioquinoline in ethanol medium: SH-form (conformers 1, 2), zwitter-ion 5, S-form 6 and NH-cation 7
а)
b)
Fig. 3. a) Experimental UV spectra of the solution of 2-thioquinoline in dichloromethane (1) and ethanol (2); b) Calculated spectra of 2-thioquinoline in ethanol medium: SH-form (conformer 3), S-form 8 and NH-form 9
Table 2
The calculated dipole moments of the basic and the first excited state depending on medium, D
|
^~^^^ Solvent Compound ^'^^ |
Ethanol |
Dichloromethane |
Benzene |
Gas phase |
||||
|
Do |
Di |
Do |
Di |
Do |
Di |
Do |
Di |
|
|
8-thioquinoline (SH-form, conformer 1) |
4.234 |
7.299 |
4.086 |
7.456 |
3.577 |
7.964 |
3.059 |
8.545 |
|
8-thioquinoline (NH-form 5) |
11.701 |
2.429 |
— |
— |
9.487 |
4.238 |
7.728 |
5.595 |
|
8-thioquinoline (S-form 6) |
33.152 |
3.517 |
32.909 |
3.930 |
— |
— |
— |
— |
|
8-thioquinoline (NH-cation 7) |
25.189 |
8.748 |
— |
— |
— |
— |
— |
— |
|
2-thioquinoline (SH-form, conformer 3) |
2.762 |
2.850 |
2.667 |
3.004 |
2.342 |
3.595 |
2.027 |
4.263 |
|
2-thioquinoline (S-form 8) |
17.147 |
4.023 |
17.288 |
4.536 |
— |
— |
— |
— |
|
2-thioquinoline (NH-form 9) |
8.457 |
5.249 |
8.134 |
5.498 |
6.963 |
6.442 |
5.747 |
7.476 |
The calculations of the spectrum characteristics of the various forms of 2- and 8-thioquinoline (without taking solvent influence into account) have shown the shift of absorption lines λ max from 11 nm (SH-form 1-4 ) up to 51 nm (S-forms 6, 8 ) with respect to the spectrum in a non-polar solvent (benzene, Table 1). It is supposed, that here the elimination of solvent influence on the studied molecules reduces energies of n →π * electronic transitions relevant to λ max , moreover, to a much greater extent, than the influence of solvent polarity (Table 3). The given phenomenon is also the manifestation of solvatoch-romic and other abovementioned effects, which are extrapolated to a gaseous phase. On the other hand, the bathochromic shift of other absorption lines is much less in magnitude or tends to zero.
To estimate spectra accordance, values of calculated absorption lines λ max have been compared with the rightmost maxima on the experimental spectra of solutions. Based on such comparison, it has been established that the difference of λ max for SH-forms of 2-thioquinoline and 8-thioquinoline in the calculated spectra, that equals 25–45 nm, is impossible to find in the experimental spectra of the solutions, as experimental λ max agrees with different tautomeric forms of these compounds. In this case, the calculated absorption lines, including λ max , are in the range of the absorption bandwidth of 330–430 and 290– 350 nm observed in the experimental spectra. Absorption at the transition, corresponding to λ max of "ionic" forms of 8-thioquinoline 5-7 , is not observed in the range of experimental spectra that is larger than 400 nm, under our conditions: pH value 7–8 for the ethanol solution. It can indicate the absence of appreciable amount of these forms of 8-thioquinoline in the solution.
Table 3
The calculated energy characteristics of the electron transitions HOMO-LUMO depending on medium, eV
|
Compound |
E HOMO |
E LUMO |
Δ E |
E HOMO |
E LUMO |
Δ E |
|
Ethanol |
Ethanol |
|||||
|
8-thioquinoline (SH-form, conformer 1 ) |
–6.125 |
–1.970 |
4.155 |
–6.095 |
–1.957 |
4.138 |
|
8-thioquinoline (NH-form 5 ) |
–5.385 |
–2.808 |
2.577 |
– |
||
|
8-thioquinoline (NH-cation 7 ) |
–6.931 |
–3.352 |
3.578 |
– |
||
|
2-thioquinoline (S-form 8 ) |
–5.031 |
–1.344 |
3.687 |
–4.694 |
–1.061 |
3.633 |
|
2-thioquinoline (NH-form 9 ) |
–6.123 |
–2.357 |
3.766 |
–6.090 |
–2.343 |
3.747 |
|
Benzene |
Gas phase |
|||||
|
8-thioquinoline (SH-form, conformer 1 ) |
–5.992 |
–1.905 |
4.087 |
–5.889 |
–1.859 |
4.030 |
|
8-thioquinoline (NH-form 5 ) |
–5.124 |
–2.819 |
2.305 |
–4.947 |
–2.816 |
2.131 |
|
2-thioquinoline (NH-form 9 ) |
–5.976 |
–2.291 |
3.684 |
–5.864 |
–2.240 |
3.624 |
It is worth noting that the represented earlier data have shown the calculation accuracy of absorption maximum values and electron transition energies on the level, similar to our results. In particular, in [13] the differences of the calculated and experimental energies of HOMO-LUMO transition were from 0.1 to 0.4 eV depending on the compound (without taking solution influence into account). Taking the solvent (water) into account at the calculation of the anion forms of Alizarin Red S in model IEF-PCM [26] showed the typical difference of excitation energies in calculation vs. experiment to be from 1% to 15% depending on compound form and functional used. The theoretical reviewing of spectral behavior of various azo-alkanes in model PCM [17] showed the difference of calculated absorption bandwidth values at the transition n →π * in comparison with the experiment to be from 1 to 21 nm (about 0.01 – 0.2 eV). Our data show the similar difference in absorption maxima position. For example, the difference for λ max is in 1–20 nm interval.
The influence of proton solvents and of probable formation of hydrogen bonding and cationic forms, presumably, appears on the experimental spectrum of 8-thioquinoline ethanol solution. In this spectrum there are mean absorption bands in the range corresponding to 272 and 370-nm spectral lines of the zwitter-ionic form 5 (Fig. 2a-b). That is, under our conditions, ethanol will slightly shift the tautomeric equilibrium “SH-form ↔ zwitter-ion” to the right side. In the 2-thioquinoline solution, such influence is inconsiderable because of the dominance of NH-form 9 under experiment conditions (see below). But at the same time, it is not possible to establish the fact of the presence of a probable cationic form 7 in ethanol solution of 8-thioquinoline by the analysis of electronic spectra, as the wavelength value of the single intensive absorption line (250 nm) and also the line (262 nm) for the form 7 are in the range of intensive absorption lines of other forms.
The calculated spectra of 1-2 and 3-4 pairs of SH-form conformational isomers are quite identical, with small shift of absorption lines to the right or to the left. Thus, the bathochromic shift of 5–6 nm for the absorption line λ max of conformer 1 of 8-thioquinoline has the smaller value of total free energy in a solvent in relation to conformer 2 . On the contrary, for 2-thioquinoline calculations, the λ max hypsoch-romic shift of 1-1.5 nm is shown for the conformer 3 with the smaller value of total free energy in a solvent relative to conformer 4 . Such shift depends on the ratio of energy values of the basic and the excited states. This tendency is revealed only in the calculated spectra and may not be used for the estimation of the content of one or another conformational structure in a solution, due to a wide width of absorption bands in the experimental spectra of solutions. However, at the same time, such shift is one of the cases of the absorption bands spreading.
We have noted that the symbasis pattern of absorption lines is observed on the spectra obtained with using different methods – PBE0 and B3LYP (Fig. 4), at that the spectral lines of all forms of compounds are shifted to the left in PBE0 case. The discrepancy between the wavelengths of absorption maxima on the experimental spectra of solutions and the wavelengths of absorption lines on the calculated spectra is increased by 10–18 nm. Thus, somewhat better data consistency is observed in the case of using B3LYP functional.
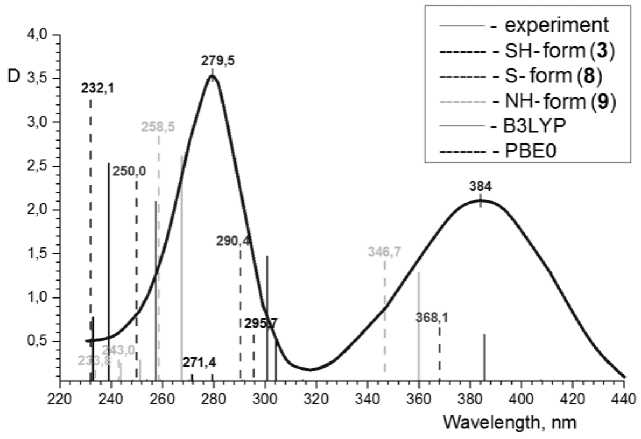
Fig. 4. The comparison of experimental spectrum of 2-thioquinoline in ethanol solution with calculated absorption lines of 2-thioquinoline forms 3, 8 and 9 in ethanol medium, obtained with using the functionals B3LYP and PBE0
It is known, that λ max in th e spec t r um of wate r so lu tio n of 8-oxyquinoline at neutral рН is about 318 n m , while in the s pe ctr um of e t ha nol solution it is about 310 nm [1]. Under these condi tion s c on f or m er s 1, 2 of the SH-form appe a r a s th e pre suma ble ta u tom e r i c f o r m of 8-thioquinoline. The presence of the zwitter-ionic form 5 i s proba bl e to a lesser degree. Consideration of the expe r ime ntal s pe ctr a o f 8-t hioquino lin e i n e t ha no l a t рН v a lue 7 –8, that we have recorded, has confirmed the presence of the absorption bands, co rre spo nding to li nes of t he c a l c ul a te d sp e c t r a of S H -forms (Fig. 2). Thus, the wide bands 230–250 nm (t he most in te ns iv e ) a nd 290–345 nm are observed. Calculations have shown the presence of lines 236–237, 240– 241 ( t he most in te n siv e ) a nd 33 7 –343 nm with ratio of intensities, quite compa r a ble to th e e xp e rim e ntal data. The space parts of molecular orbitals (MO) of the S H -form 1 , zwitter-ion 5 and a ss ig nment of these transitions to the absorption lines in the spec t r um with its c on tr ibutions represented by SAP-coe f ficients (squared SAP is the contribution of the giv e n tra ns it ion to th e exc i te d st a te) , ar e g r a p hically shown in Fig. 5a-b. Note that the electron transitions between this MO d e ter mine th e e xcit e d s tate s of mole c ul e s.
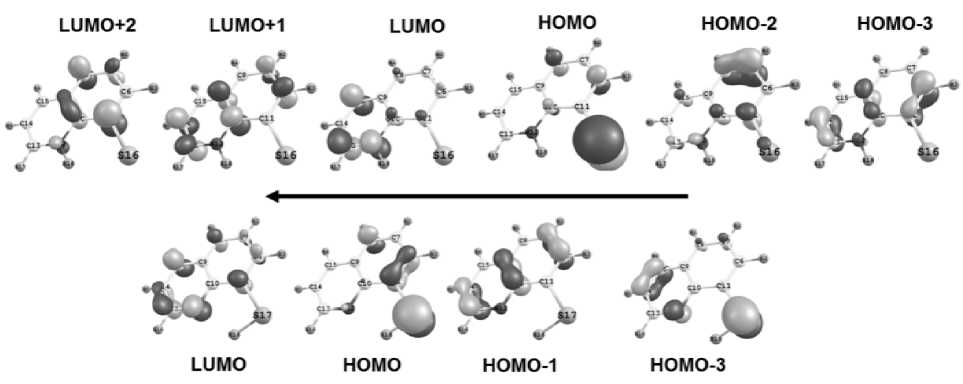
Fig. 5. MO graphical representation of a) NH-form of 8-thioquinoline (zwitter-ion 5) in ethanol medium. 623 nm: HOMO → LUMO (-0.99), 370 nm: HOMO → LUMO+1 (-0.98), 272 nm: HOMO-3 → LUMO (-0.69), HOMO-2 → LUMO (-0.48), 262 nm: HOMO → LUMO+2 (0.86); b) SH-form of 8-thioquinoline (conformer 1) in ethanol medium. 342 nm: HOMO → LUMO (0.98), 241 nm: HOMO-1 → LUMO (-0.58); HOMO-3 → L (-0.54)
The e xp e rime nta l spec t r a o f 2 -thioquinoline solutions have shown the presence of wide bands 250– 310 and 350– 4 25 nm ( F ig . 3a ) . In the range of these bands, there are the lines from c a lc ul a ted sp e ctr a o f the NH-form 9 : 268 and 360–3 62 nm, a nd S-form 8 : intensive lines 257–258 and 301–304 nm, and also 386– 392 nm ( F ig . 3b) . Graphic al r e p r e s e nt a t ion o f M O space parts of these forms is shown on Fig. 6a-b. The p a t ter n of t he a ctiv e MO in the spectra slightly changes with transferring to the io nic s tate , b ut the s tr o ng ba th oc hromic sh ift of the a bso r p tion ba n ds ( 25 –30 nm) is observed at the negative charge distrib ute d on a toms of py r id ine r ing . There are only the lines of small intensity 279 a n d 304–305 nm among all of lin e s o f th e c alc u lat e d spec t ra o f S H-forms 3, 4 in the indicated range of experimental spectra. These fa c t s show th e pr ef er e nt ial pr ese n c e of t he NH-form 9 in the ethanol 2-thioquinoline solution at рН value 7– 8 . T he s imilar f a c t wa s s hown e a rli e r for 2 -oxypyridine, which preferentially exists in the p yridone f or m in a p olar so lv e nt [ 2 ] .
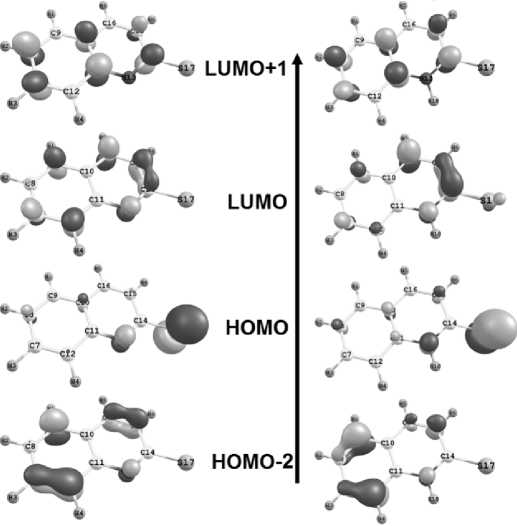
a) b)
Fig. 6. MO graphical representation of a) S-form 8 of 2-thioquinoline in ethanol medium:
385 nm: HOMO → LUMO (0.96), 301 nm: HOMO → LUMO+1 (0.91), 258 nm: HOMO-2 → LUMO (0.87);
b) NH-form 9 of 2-thioquinoline in ethanol medium: 360 nm: HOMO → LUMO (-0.97),
268 nm: HOMO → LUMO+1 (-0.80), HOMO-2 → L (0.51)
Conclusion
The c ompa r ativ e ana ly si s of the e xper ime ntal and calcul a t e d elec t r onic absorption spectra of solutions of 2-and 8- t hioquin ol i ne in various solvents has been carried out. For each c ompound t w o c onformational isomers of SH-f or ms, ta ut ome r ic NH-forms of 2-thioquinoline (2-thioquinolone) and 8-thioquinoline (zwitter-ion), an d a ls o S -forms (anions) and 1-H-2-thioquinolinium cation have been analyzed.
It has been shown that wide absorption bands in the range 250–310, 350–425 and 230–250, 290– 345 nm correspond to the experimental spectra of 2- and 8-thioquinoline in ethanol and dichloromethane solutions, respectively. It has been established that the absorption band in the range 350–425 nm in a spectrum of a solution of 2-thioquinoline, corresponds to λmax in the calculated spectra of its NH-form and S-anion, which is matched by the electronic transition n→π*. As the contributions of sulfur atomic orbitals in HOMO and of atoms of quinoline cycles in LUMO are involved in this transition, therefore its energy characteristics essentially vary for different tautomeric forms of 2-thioquinoline. This fact basically explains the main cause of absorption band spreading in the observed spectrum of 2-thioquinoline, whereas the maximum position of a given absorption band does not depend on the conformational variety.
Also it has been established, that the presence of the absorption lines of the ionic forms is not evident in the spectrum of the neutral solution of 8-thioquinoline. The absorption bands correspond to the electronic transitions of SH-forms. Conformational variety of SH-forms is one of the reasons of the spreading of absorption bands in the solution spectrum of 8-thioquinoline.
On the basis of the comparative analysis of electronic spectra, it has been shown that the basic forms of existence of 2-thioquinoline in the neutral solution are the NH-form and S-anion; while for 8-thioquinoline in the neutral solution they are the conformers of SH-form.
Acknowledgements
This work was supported by the Russian Ministry for Education and Science.
Список литературы Comparative analysis of theoretical and experimental UV-spectra of 2- and 8-thioquinoline
- Chatterjee K.K. U.V. Absorption Spectra of 8-oxyquinoline and its Copper Chelate. Anal. Chim. Acta, 1959, vol. 20, pp. 232-235.
- Meislich H. Pyridinols and Pyridones, in: Klingsberg E. Pyridine and its Derivatives Pt. 3. NY London, Interscience Publishers, a division of John Wiley & Sons, 1962. pp. 619 630.
- Prabavathi N., Nilufer A., Krishnakumar V. Spectroscopic (FT-IR, FT-Raman, UV and NMR) Investigation, Conformational Stability, NLO Properties, HOMO-LUMO and NBO Analysis of Hydroxyquinoline Derivatives by Density Functional Theory Calculations. Spectrochim. Acta Pt. A: Mol. Biomol. Spectroscopy, 2013, vol. 114, pp. 449-474.
- Sharba A. H., Hassan H. A., Hassan D. F. Synthesis of Heterocyclic Compounds Derived from 2 Mercapto Quinoline. Ibn Al-Haitham J. Pure Appl. Sci., 2012, vol. 25, pp. 242-252.
- Obot I.B. Quantum Chemical Assessment of the Interaction of Potential Anticorrosion Additives with Steel Surface. Innov. Corr. Mater. Sci., 2014, vol. 4, pp. 107-117.
- Bartashevich E.V., Yushina I.D., Vershinina E.A., Slepukhin P.A., Kim D.G. Complex Structure Tri-and Polyiodides of Iodocyclization Products of 2-Allylthioquinoline. J. Struct. Chem., 2014, vol. 55, pp. 112 119.
- Kim D.G., Vershinina E.A. ChemInform Abstract: Synthesis and Properties of Thiazolo-and Oxazoloquinolinium Systems and Their Hydrogenated Derivatives (Review). ChemInform, 2015, vol. 46, in press.
- Chernov'yants M.S., Burykin I.V., Starikova Z.A., Tereznikov A.Yu., Kolesnikova T.S. Spectroscopic and Structural Study of Novel Interaction Product of Pyrrolidine-2-thione with Molecular Iodine. Presumable Mechanisms of Oxidation. J. Mol. Struct., 2013, vol. 1047, pp. 204-208.
- Chernov'yants M.S., Starikova Z.A., Karginova A.O., Kolesnikova T.S., Tereznikov A.Yu. Spectroscopic and Structural Investigation of Interaction Product of 8 Mercaptoquinoline with Molecular Iodine. Spectrochim. Acta Pt. A: Mol. Biomol. Spectroscopy, 2013, vol. 115, pp. 861-865.
- Runge E., Gross E. K. U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett., 1984, vol. 52, pp. 997-1000.
- Dreuw A., Head-Gordon M. Single-Reference ab Initio Methods for the Calculation of Excited States of Large Molecules. Chem. Rev., 2005, vol. 105, pp. 4009-4037.
- Makarov A. Yu., Chulanova E.A., Semenov N.A., Pushkarevsky N.A., Lonchakov A.V., Bogomyakov A.S., Irtegova I.G., Vasilieva N.V., Lork E., Gritsan N.P., Konchenko S.N., Ovcharenko V.I., Zibarev A.V. A Novel Sulfur-Nitrogen -Heterocyclic Radical Anion, (6H-1,2,3 benzodithiazol-6-ylidene)malononitrilidyl, and its Homo-and Heterospin Salts. Polyhedron, 2014, vol. 72, pp. 43-49.
- Siud Pui Man, Benoit D.M., Buchaca E., Esan F., Motevalli M., Wilson J., Sullivan A. Synthesis, Structural Characterization, Experimental, and Computational Spectrophotometric Studies of 8-Quinolinyloxymethyphosphonate. Inorg.Chem., 2006, vol. 45, pp. 5328-5337.
- Karabacak M., Cinar M. FT-IR, FT-Raman, UV Spectra and DFT Calculations on Monomeric and Dimeric Structure of 2-Amino-5-bromobenzoic Acid. Spectrochim. Acta Pt. A: Mol. Biomol. Spectroscopy, 2012, vol. 86, pp. 590-599.
- Mennucci B., Tomasi J., Cammi R., Cheeseman J.R., Frisch M.J., Devlin F.J., Gabriel S., Stephens P.J. Polarizable Continuum Model (PCM) Calculations of Solvent Effects on Optical Rotations of Chiral Molecules. J. Phys. Chem. A, 2002, vol. 106, pp. 6102-6113.
- Tomasi J., Mennucci B., Cammi R. Quantum Mechanical Continuum Solvation Models. Chem. Rev., 2005, vol. 105, pp. 2999-3094.
- Jacquemin D., Perpète E.A., Ciofini I., Adamo C. On the TD-DFT UV/vis Spectra Accuracy: the Azoalkanes. Theor. Chem. Account, 2008, vol. 120, pp. 405-410.
- Adamo C., Barone V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys., 1999, vol. 110, pp. 6158-6170.
- Becke A.D. Density-functional Thermochemistry. 3. The Role of Exact Exchange. J. Chem. Phys., 1993, vol. 98, pp. 5648-5652.
- Chengteh Lee, Weitao Yang, Parr R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B, 1988, vol. 37, pp. 785-789.
- Krishnan R., Binkley J. S., Seeger R., Pople J. A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys., 1980, vol. 72, no. 1, pp. 650-654.
- McLean A. D., Chandler G. S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z=11-18. J. Chem. Phys., 1980, vol. 72, no. 10, pp. 5639-5648.
- Emsley John. The Elements (3rd Ed.). Oxford, Oxford University Press, 1998. 300 p.
- Granovsky A.A. Firefly version 8. Available at: http://classic.chem.msu.su/gran/firefly/index.html (accessed 28 February 2015).
- Reichardt C. Solvents and Solvent Effects in Organic Chemistry (2nd Ed.). Weinheim, VCH Verlagsgesellschaft mbH, D-6940, 1988. 750 p.
- Feher P.P., Purgel M., Joo F. Performance of Exchange-Correlation Functionals on Describing Ground State Geometries and Excitations of Alizarin Red S: Effect of Complexation and Degree of Deprotonation. Comput. Theor. Chem., 2014, vol. 1045, pp. 113-122.


