Comparative morpho-biochemical responses of wheat cultivars sensitive and tolerant to water stress
Автор: Aldesuquy Heshmat S., Ibraheem Farag I., Gahnem Hanan E.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.10, 2014 года.
Бесплатный доступ
Water stress is likely the most important factor that adversely affects plant growth and development. In this study two wheat cultivars Gemmieza-7 (sensitive) and Sahel-1 (tolerant) were subjected to water stress and compared in terms of growth parameters (growth vigor of root and shoot), water relations (relative water content and saturation water deficit ) and protein as well as nucleic acids (DNA and RNA) content in flag leaves of both cultivars. In general, water stress caused noticeable reduction in almost all growth criteria of root, shoot and flag leaf which was consistent with the progressive alteration in water relations, protein and nucleic acids content of both cultivars during grain filling. Furthermore, degree of leaf succulence and degree of leaf sclerophylly were severely affected by water stress in both wheat cultivars. In relation to wheat cultivar, the sensitive was more affected by water stress than the tolerant one. Generally, the application of salicylic acid, trehalose or their interaction induced marked increase in growth vigor of root and shoot, water relations and protein as well as nucleic acids in flag leaves of both wheat cultivars in compare with control and water stressed plants. In conclusion, Sahel-1 has suitable mechanisms to enable it to respond more effectively to water stress than Gemmieza-7.
Wheat, growth vigor, water relations, protein, salicylic acid, trehalose
Короткий адрес: https://sciup.org/14323857
IDR: 14323857
Текст научной статьи Comparative morpho-biochemical responses of wheat cultivars sensitive and tolerant to water stress
List of abbreviations: Control = Cont, Least significant difference = LSD, Relative water content =RWC, Sensitive = S, Water stress = WS, Saturation water deficit = SWD, Tolerant = T.
Drought is one of the most important abiotic stress factors that limit plant growth and yield of plants around the world (Akıncı and Lösel, 2012). A drop in water potential induces a variety of metabolic, morphological, and/or physiological responses, including reduction in the vegetative growth (Mahajan and Tuteja 2005). Analysis of morphological traits is useful for studying of plant adaptations to environmental stresses such as water deficit. Morphologically the most typical symptom of water stress injury to plant is reduction of growth (Jaleel et al., 2008b). Usually, under water stress conditions, increasing stress level is detrimental to plant growth and may result in marked alteration in its morphological features including significantly reduction in shoot and root lengths, leaf area and total biomass production (Aldesuquy et al., 2012).
Plant growth is one of the most droughtsensitive physiological processes due to the reduction of turgor pressure (Shao et al., 2008). Cell expansion can only occur when turgor pressure is greater than the cell wall yield threshold. Water stress greatly suppresses cell expansion and cell growth due to the low turgor pressure (Jaleel et al ., 2007a). Many authors have observed that when wheat plants were exposed to water stress, many growth parameters, including plant height, fresh and dry weights of shoot and root, the relative growth rate as well as leaf area tended to decrease (Shao et al., 2008; Aldesuquy et al., 2014).
Salicylic acid could counteract the adverse effects of wheat plants subjected to drought by improve of growth vigor of root and shoot, leaf area as well as water relations (Aldesuquy et al ., 2012).
Trehalose is a non-reducing disaccharide in which the two glucose units are linked in an alpha, alpha-1,1-glycosidic linkage. This sugar is present in a wide variety of organisms, including bacteria, yeast, fungi, insects, invertebrates, and lower and higher plants, where it may serve as a source of energy and carbon. In yeast and plants, it may also serve as a signaling molecule to direct or control certain metabolic pathways or even to affect growth. In addition, it has been shown that trehalose can protect proteins and cellular membranes from inactivation or denaturation caused by a variety of stress conditions, including desiccation, dehydration, heat, cold, and oxidation (Elbein et al., 2003).
The present study was an attempt to screen impact of water stress and application of SA, trehalose or their interaction on the morphological and water relations variability, which in turn affect protein and nucleic acids (DNA and RNA) content in flag leaves of Triticum aestivum (L.). In this respect, we could investigate the ability of Triticum aestivum to grow under water stress and the characterized responses of the plants to water stress and application of SA, trehalose or their interaction in order to increase our understanding of the distribution of these cultivars in relation to water stress.
MATERIALS AND METHODS
Plant material and growth conditions
Pure strains of Triticum aestivum L . Gemmieza-7 (sensitive cultivar) and Sahel-1 (tolerant cultivar) were kindly supplied by the Agricultural Research Center, Ministry of Agriculture, Giza, Egypt.
For soaking experiment, a homogenous lot of Triticum aestivum L . (i.e. either sensitive or tolerant cultivar) grains were selected. The grains were separately surface sterilized by soaking in 0.01 M HgCl 2 solution for three minutes, then washed thoroughly with distilled water. The sterilized grains from each cultivar were divided into two sets (≈ 500 g per set for each cultivar). Grains of the 1st set were soaked in distilled water to serve as control, while those of the 2nd were soaked in salicylic acid (3 mM) each for about 12 hours.
After soaking, thoroughly washed grains were drilled on 21 November 2011/2012 in plastic pots (20 cm in diameter) filled with 5.5 kg soil (clay/sand 2/1, v/v), where fifteen grains was sown in each pot. The pots were then kept in a greenhouse at Botany Department, Faculty of Science, Mansoura University, Egypt. The plants were subjected to natural day/night conditions (minimum/maximum air temperature and relative humidity were 15/25°C and 35/45%; respectively) at mid-day during the experimental period. The plants in all sets were irrigated to field capacity by tap water.
After two weeks from sowing, thinning was started so that five uniform seedlings were left in each pot for the subsequent studies.
On the day 65 after planting (at the beginning of heading) the pots of the 1st set was allocated to four groups (20 pots per each group) as follows: control (cont.), water stress (WS), trehalose control, trehalose + water stress (trehalose + WS). The 2nd set group was allocated to four groups as follows: salicylic acid control (SA ) , salicylic acid + water stress (SA+WS), control trehalose + salicylic acid (SA + trehalose) and salicylic acid + trehalose + water stress (SA+ trehalose +WS). For trehalose (1.5 mM) treatment, the plants were sprayed by trehalose 48 hrs before starting the stress period and weekly during the stress period.
Water deficit was imposed by withholding water at the reproductive stage for 30 days within two periods: on the day 65 from planting (heading stage) and the day 80 from planting (anthesis stage). Each droughty pot received 500 ml water at the end of 1st stress period. At the end of stress periods, re-watering to the field capacity was carried out. The un-droughty (control) plants were irrigated to the field capacity during the stress period, and all plants were left to grow until grain maturation under normal irrigation with tap water. After thinning and at heading, the plants received 36 kg N ha -1 as urea and 25 kg P ha-1 as superphosphate.
To estimate the growth parameters during grain-filling (21 days post-anthesis) (i.e. 106 days after sowing) ten samples were taken from each treatment. Moreover, only three samples were taken from each treatment for the biochemical analyses.
Growth parameters:
Leaf area = Length X Breadth X 0.75 (Quarrie and Jones, 1979)
Specific leaf area = Leaf area / Dry mass (Beadle, 1993)
Water amount = Fresh Mass - Dry mass
Degree of succulence = Water amount / Leaf area (Delf, 1912)
Degree of sclerophylly = Dry mass / Leaf area (Witkoswski and Lamont, 1991)
Shoot or root distribution = Fresh Mass / Length (Arduini et al. , 1994)
Shoot or root density = Dry Mass / Length (Arduini et al. , 1994)
Determination of relative water content (RWC)
For measuring relative water content, the method of Weatherly (1950) and its modification by Weatherly and Barrs (1962) was adopted.
Determination of saturation water deficit (SWD)
Saturation water deficit was calculated according to Weatherly and Barrs (1962) from the following equation: SWD (%) = 100 - RWC (%).
Estimation of protein
The method of protein extraction was adopted by Scarponi and Perucci (1986). Protein content was determined spectrophotometrically according to the method adopted by Bradford (1976).
Estimation of DNA and RNA
Protein and nucleic acids (DNA and RNA) content were determined according to the method adopted by Sadasivam and Manickam (1996) as described by Devi (2000).
Statistical analysis
A test for significant differences between means at P ≤ 0.05 was performed using least significant difference (LSD) test (Snedecor and Cochran, 1976). The correlation coefficients were estimated according to SPSS programme.
RESULTS AND DISCUSSION
Abiotic pressures like water stress can impose limitations on crop productivity and also limit land available for farming, often in regions that can ill afford such constraints, thus highlighting a greater need for understanding how plants respond to adverse conditions with the hope of improving tolerance of plants to environmental stress (Joseph et al. , 2010). Furthermore, the quantity and quality of plant growth depend on cell division, cell enlargement and cell differentiation and all of these events are affected by water stress (Jaleel et al . 2007 a&b; Aldesuquy et al ., 2014).
The current results showed that water stress generally caused a noticeable reduction in almost all growth criteria of root, shoot and flag leaf of both wheat cultivars Gemmieza-7 (drought sensitive cultivar) and Sahel-1 (drought tolerant cultivar) during grain-filling. Extent of reduction was more obvious particularly in Gemmieza-7 (Figures 1- 6). These results were in agreement with those of many authors (Zaki et al., 2009; Khalid and Teixeira da Silva, 2010) who reported that water stress causes growth reduction through its osmotic effect, which is equivalent to a decrease in water activity through specific toxic effects of ions and by disturbing the uptake of essential nutrients. Hence, the inhibition of wheat growth characters under water stress would almost certainly be due to exposure to injurious levels of drought causing a decrease of turgor which would result in a decrease of growth and development of cells (Aldesuquy et al., 2012).
Water stress has a strong influence on the allocation of energy between assimilation and dissimilation. Assimilation was found to be reduced (photosynthesis slowed and root uptake decreased), while respiration was found to be increased (to synthesize organic solutes that neutralize the inorganic ions and restore damage caused by the stressor). These may explain why growth is slowed under water stress (Guo et al ., 2009, Aldesuquy et al ., 2012).
More interestingly, the morphology of roots affects the amount of salt taken into the plant (Maggio and Ramnarayan, 2001). Hence, plants must develop a vigorous root system that allows them to grow and overcome any stress conditions. However, water stress could lead to marked changes in the growth, morphology and physiology of roots that will in turn change water and ion uptake and as a result, the whole plant is then affected when its root is growing in unfavorable medium.
It is clear from this current study that root biomass (fresh and dry masses), root length, number of adventitious roots, root/shoot ratio as well as density and distribution were significantly and adversely influenced by water stress in both wheat cultivars where, magnitude of reduction was greater in Gemmieza-7 than Sahel-1 (Figure 1). These results were supported by Aldesuquy et al. (2012) who observed that water stress partial inhibit or total inhibit root development of wheat cultivars. Hence, water stress assumed to reduce root growth of water-stressed wheat plants by inhibiting root formation and branching, growth of the existing roots and inducing root decay.
Data in figure 1 showed that, Water stress significantly decreased root fresh and dry masses of both wheat cultivars. Earlier, Grewal (2010) stated that water stress had significant depressing effect on root biomass (fresh and dry masses). In this investigation, the deleterious effect of water stress on root biomass may be attributed to the inhibitory effect of Abscisic acid (ABA), induced by water stress, on cell division and/or cell expansion as stated by Hassanein (2000) or reduced water absorption due to osmotic effect, specific ion toxicity and nutritional imbalances as mentioned by Joseph et al . (2011).
The present work showed that, root length and the number of adventitious roots as well as root/shoot ratio calculated on length basis adversely affected by water-stress in both cultivars (Figures 1 and 2). These results were in harmony with those obtained by Aldesuquy et al . (2012) who observed massive increase in both root length and the number of adventitious roots in two wheat cultivars in response to water stress. Similar findings have been reported by Kaymakanova and Stoeva (2008). This could be evaluated as water stress reduces growth rate and tissue differentiation of the plant root (Shannon and Grieve, 1999).
Results in figure 2 indicated that, water-stress caused noticeable decreases in root density and root distribution of both cultivars. Since root density relates dry mass production to the unit root length and root distribution represents the fresh mass accumulated per the unit root length, the reduction in both density and distribution of wheat root may reflect the effect of water stress on decreasing root biomass (fresh and dry masses). In this respect, Chopart et al. (2008) stated that evaluation of root density and distribution could be considered as a key factor for water and nutrient uptake by a plant in soil.
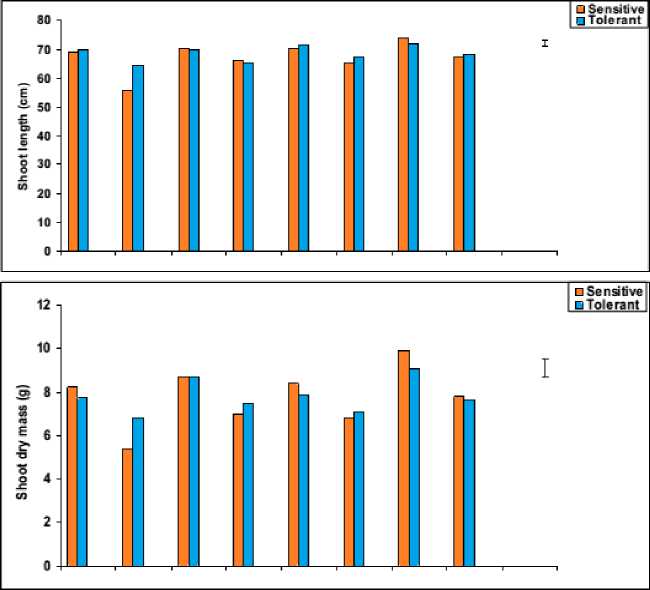
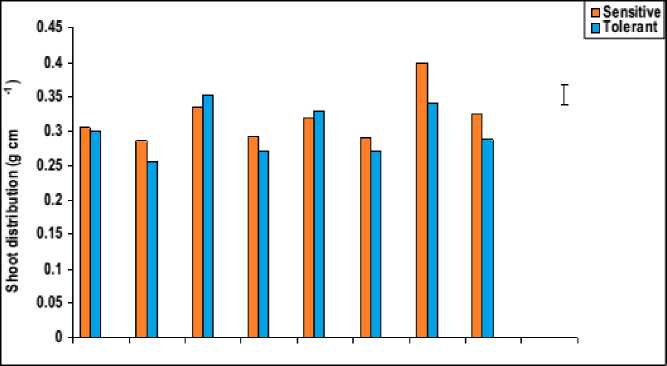
The data in figures 3 and 4 revealed that, waterstress induced a significant decrease in all growth parameters of the shoot i.e. shoot biomass (fresh and dry masses), shoot length and the number of tillers, as well as shoot density and shoot distribution of both wheat cultivars with more pronounced effect on Gemmieza-7 as compared to Sahel-1. These results are also in conformity with those obtained by Aldesuquy et al . (2012) who stated that water stress caused marked reduction in the shoot biomass, shoot length and plant height as well as the number of tillers in wheat plants. The inhibition of shoot growth of both wheat cultivars under drought stress could be explained on the basis that the absence of the sufficient amount of water in soil solution reduced the ability of plants to take up water ultimately causing the slower plant growth rate. Additionally, the initial reduction in shoot growth was probably due to hormonal signals generated by the roots (Munns, 2002) and/or inhibition of the efficiency of the translocation and assimilation of photosynthetic products (Xiong and Zhu, 2002).
In wheat plants, shoot biomass (fresh and dry masses) were adversely affected by water stress (Figure 3). These results were in harmony with those obtained by Seckin et al. (2010) who reported that shoot fresh and dry weights of two barley cultivars were decreased under water stressed conditions. This reduction in shoot dry matter yield of water-stressed wheat plants could be attributed to inadequate availability of nutrients present in growth medium and the decreased water entry rate into the plants and/or the decreased in photosynthetic output with suppressed supply of CO2. Moreover, Chaparzadeh et al. (2004) stated that the reduction in shoot dry mass may be a consequence of turgor limitation or cell wall hardening which may be due to altered wall structure induced by water stress.
The pattern of shoot growth revealed that water stress greatly affected shoot length, the development and viability of tillers per plant (Figures 3 and 4). These results were in good harmony with those obtained by Hasamuzzaman et al. (2009). This reduction in shoot length of water-stressed plants may be attributed to reduction in the meristematic activity as well as cells elongation and enlargement (Manivannan et al ., 2007). In this regard, the decrease in fresh and dry masses of the shoot system of water-stressed wheat plants was assumed to be due to the decrease in either the number of tillers and/or shoot length, which might be due to the disturbance in metabolic activities affected by the decrease in water absorption and/or disturbance in water balance. This conclusion came also in agreement with those of Sairam and Tyagi (2004).
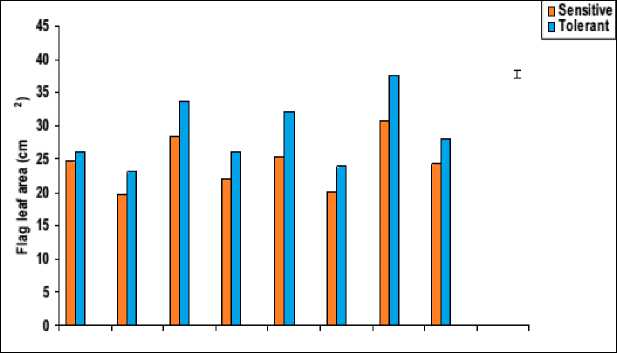
Flag leaf plays the key and the most important role in plant life as it transport assimilates to spike and developing grains. It is clear from the results that, all growth parameters of wheat flag leaf (i.e. biomass, area, specific area as well as the degree of succulence and sclerophylly) were negatively affected by water-stress in both cultivars with more conspicuous effect in Gemmieza-7. Overall, these results were in accordance with those obtained by Silva et al. (2010) with different plant species.
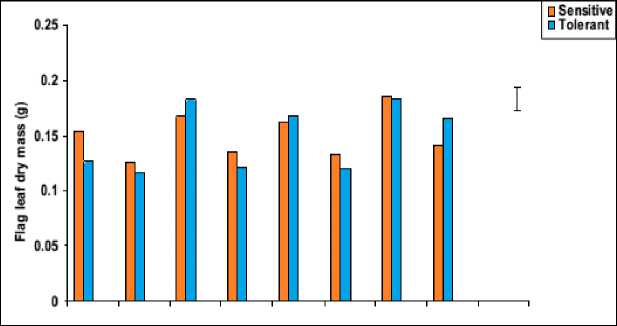
The flag leaf biomass (fresh and dry masses) of both cultivars was greatly inhibited by water stress (Figure 5). In wheat, genotypic differences in biomass production under water-stress were observed but all showed a reduction in this growth parameter. These results were in agreement with those obtained in other studies (Djanaguiraman et al., 2006; Siddiqui et al., 2008). The observed reduction in leaf biomass of water-stressed wheat plants might be due to induction of ionic stress, which causes premature abscission and senescence of adult leaves, causing a reduction in the available photosynthetic area. Additionally, this reduction also could be attributed to a combination of slower growth and development as a result of osmotic stress (Hatzig et al., 2009), as well as the inhibition in photosynthesis as a result of direct effects of water stress on the photosynthetic apparatus or indirect effects caused by the reduction in sink capacity (Moradi and Ismail, 2007).
Development of optimal leaf area is important to photosynthesis and dry matter yield. Water stress mostly reduced leaf growth and in turns reduced the leaf area and specific leaf area of water-stressed wheat plants (Figure 6). The observed reduction in leaf area and dry mass of water-stressed wheat plants could be attributed to the changes in plant water relations under water stress, which cause a reduction in meristem activity as well as cell elongation, thereby inhibiting leaf expansion after the loss of cell turgor pressure. These results were in a good agreement with those obtained by Shah (2007). Moreover, plant tried to cope with the water stress by reducing its leaf area in order to allow the conservation of energy, minimize the deleterious effects of loss of water and to complete their life cycle under stress conditions (i.e. avoidance and/or tolerance mechanisms).
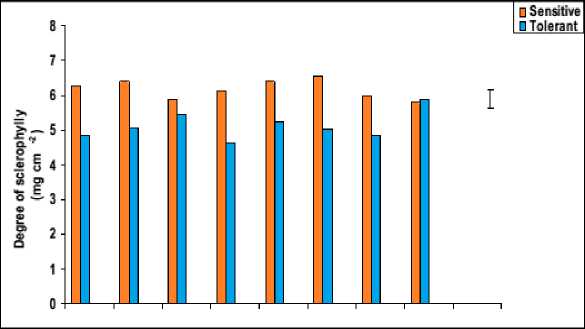
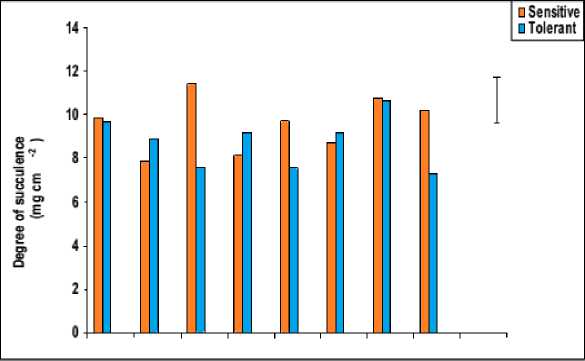
Perusal of data in figure 6 showed that waterstress induced a significant decline in the degree of leaf succulence with a concomitant increase in its degree of sclerophylly. In accordance with these results, leaf succulence was found to decrease in three varieties of water-stressed sunflower plants (Welch and Rieseberg, 2002). This may be explained on the basis that less absorbed water means less water content of the growing leaves, indicated by less relative water content and more saturation water deficit (Figure 7) as well as less succulence and more sclerophylly (Figure 6). From the obtained results we could conclude that, greater leaf succulence and less leaf sclerophylly could be recorded as a means of increasing drought tolerance since, the resistant cultivar showed less reduction in the degree of leaf succulence and more increment in the degree of leaf sclerophylly in comparing to sensitive cultivar under water stress.
Edwards et al . (2000) reported that the functional significance of sclerophylly remains controversial, with three main groups of hypotheses proposed to explain its adaptive significance. These centre on sclerophylly as (1) an adaptation to water deficit, (2) an adaptation to, or consequence of, low nutrients in the growing medium, and (3) enhancement of leaf longevity by leaf protection, thereby increasing leaf carbon gain.
It is clear from the current study that water stress significantly decreased almost all growth criteria of root, shoot and flag leaf of both wheat cultivars. Among the cultivars used in this study, Gemmieza-7 showed more susceptibility to waterstress since it showed a worse performance and produced less biomass under water-stress when compared with Sahel-1. Hence, this reduction in growth might be due to low osmotic potential and a decrease in wall extensibility. In this respect, morphologically, the most typical symptom of water deficit injury in plants is growth retardation due to inhibition of cell expansion.
In the present study, the applied SA and/or trehalose significantly enhanced almost all the studied growth characteristics of stressed or unstressed wheat plants in relation to their relative untreated plants (Figures 1 up to 6). Generally, the interaction of SA and trehalose appeared to be the most effective treatment in counteracting the negative effects of water stress on almost all growth criteria of wheat plants (Figures 1 up to 6), indicating the nullification of the negative effects induced by water stress. The repairing effect of SA and/or trehalose on the growth of water-stressed wheat plants may be due to the increased water uptake by root system since SA act as antitranspirant and trehalose hydrolysis to release two water molecules which are used by plants to regain water lost. These results can be related to earlier studies which reported that exogenous application of SA promotes growth and counteracts the stress induced growth inhibition due to abiotic stresses in a range of crop species (El-Tayeb, 2005; Arfan et al., 2007). Furthermore, salicylic acid-induced increase in growth of wheat under nonsaline or saline conditions can be attributed to an increase in photosynthesizing tissue, i.e., leaves (Zhou et al., 1999). The prevention of water loss from leaves of droughty wheat plants as a result of SA application might be the principal reason for the significant increase in shoot fresh weight. The observed increase in growth of droughty wheat plants resulting from SA (antitranspirant) may due to its effect on improvement of turgidly at a time when the growth of that particular plant part was more dependent on water status than in photosynthesis (Davenport et al., 1972). Moreover, trehalose is an important storage carbohydrate and stress protectant.
The positive effect of trehalose is probably due to the ability of trehalose to increase RWC (Figure 7) of wheat plants as well as translocation via transpiration stream. In addition, SA and/or trehalose application during water stress may reduce water loss rates and cause a concomitant increase in leaf water potential and carbon gain rates.
The inhibition of leaf area in response to water stress was alleviated when the grains were soaked in SA (Figure 5). This recovery may result from the role of SA in stimulating the rate of movement of nutrients and hormones from the plant root towards the developing leaves, and thus accelerating the rate of leaf expansion.
The potentiality of trehalose to mitigate the adverse effects of water stress on morphological features of wheat plants may be also due to the ability of trehalose to act as compatible solutes may help to maintain the relatively high water content necessary for growth and cellular function.
Determination of water relations is critical for any study of plant resistance to water stress (Netondo et al ., 2004). Additionally, the importance of the internal water balance in plant water relations is generally accepted because of the close relationship between the balance and turgidity to the rates of physiological processes that control the quality and quantity of growth (Aldesuquy et al ., 2009).
Leaf relative water content (RWC) has been emphasized as a better indicator of water status of a plant than water potential (Farhat et al., 2008). Thus, data in figure 7 showed that water-stress considerably lowered the RWC % with a concomitant increase in saturation water deficit (SWD %) of flag leaf of both wheat cultivars. Hence, Gemmieza-7 showed more reduction in RWC % and more increment in SWD% under water stress conditions in comparing to Sahel-1. These results were in agreement with those obtained by Aldesuquy and Ibrahim (2001) who demonstrated that water stress significantly reduced RWC % and increased SWD % of the stressed plants as compared with the control ones. The suppression of leaf RWC under water stress implies that there is a reduction in turgor and the plant suffers from restricted water availability to the cells (Munns, 2002). This decrease in turgor under water stress conceivably reduces expansive growth of cells (Netondo et al., 2004). Furthermore, the reduction in RWC under water stress to decreased water uptake due to water deficit.
Exogenous application of SA and/or trehalose appeared to mitigate the ill effect of water stress by maximizing RWC with minimizing SWD of wheat plants (Figure 7). These results are similar to those obtained by Aldesuquy et al. (1998). Hence, trehalose act as compatible solutes may help to maintain the relatively high water content necessary for growth and cellular functions. Additionally, trehalose hydrolysis to release two water molecules which are used by plants to regain water lost.
Water molecules are critical components of the reaction mechanism; they contribute to the stability of proteins, nucleic acids (DNA and RNA) and lipids. Stress conditions, as manifested by salinity, are a detrimental factor which adversely affects the cellular contents of plant cells of different species, such as proteins and nucleic acids (JungKlang, 2005). In the present investigation, water stress induced a noticeable decrease in total proteins and nucleic acids (DNA and RNA) content in stressed leaves of both wheat cultivars during grain filling. Note, for resistant cultivar in order to increase its ability to overcome water stress, it was able to keep out its protein and nucleic acids (DNA and RNA)
contents under water stress at higher levels than the sensitive one (Figure 8).
In this respect, a close relation between the changes in total protein and nucleic acids contents under water stress conditions was observed. Since the processes of DNA and RNA synthesis are related to protein synthesis, reductions in RNA synthesis ultimately reduce the enzymatic protein content (Siddiqui, 2006). In agreement with our results, Debouba et al . (2006) found that water stress decreased total leaf protein and nucleic acids (DNA and RNA) contents in tomato plants.
Likewise, water stress induced depletion of proteins and nucleic acids could be ascribed to the enhancement of ROS production (Aldesuquy et al ., 2013). ROS can affect many cellular functions by rapidly attacking all types of bio-molecules such as proteins and nucleic acids (Luna et al ., 1994), leading to irreparable metabolic dysfunction and cell death. Furthermore, ROS may spontaneously react with nucleophilic centers in the cell, thereby forming covalent bonds to protein and nucleic acids (Mates and Sanchez-Jimenez, 1999).
The protein content in plant cells is an important indicator of their physiological state but under water stress, total protein contents is usually decreased (Parida et al., 2002). The reduction in protein content of various plant tissues under water stress conditions may be due to the inhibition of transamination process, the increase of proteolysis as well as the decrease of protein synthesis (Baraka, 2008). The adverse effect of water stress on protein content could be also attributed to disturbance in nitrogen metabolism, inhibition of nitrate absorption or the decrease in the availability of amino acids and denaturation of the enzymes involved in amino acid and protein synthesis (Tawfik et al., 2006). Moreover, Mohammadkhani and Heidariturk (2008) found that, the decrease in total soluble proteins under water stress conditions was due to a severe decrease in photosynthesis and as a result materials for protein synthesis aren’t provided. Therefore, protein synthesis dramatically reduced or even stopped.
Nucleic acids metabolism is another cellular function known to be affected by exposure to water stress. Therefore, the observed reduction in nucleic acids (DNA and RNA) contents in flag leaves of water-stressed wheat plants (Figure 8) can be attributed to alteration in the activities of their synthetic and/or hydrolytic enzymes. Therefore, the exogenous application of SA and/or trehalose induced marked increase in total protein and nucleic acids content in flag leaf of stressed or unstressed wheat plants (Figure 8). The increase in DNA and RNA in wheat plants in response to SA could be attributed to the enhancement of their biosynthesis and/or inhibition of their degradation and preventing the hydrolysis of nucleic acids. Since the level of RNA and DNA may depend not only on the rate of synthesis but also on the rate of breakdown, it was thought that the effect of SA and/or trehalose on increasing the amount of RNA, DNA and protein may depend on the activity of ribonuclease, deoxy - ribonuclease and protease.
The mechanisms by which trehalose protects biological molecules can be divided into three categories: water replacement, glass transformation, and chemical stability. These three mechanisms are not mutually exclusive and all may contribute to the stabilizing effects of trehalose (Richards et al. 2002). Trehalose is one of the most chemically stable sugars and it essentially nonreducing. It is highly resistant to hydrolysis and in general chemically inert in its interactions with proteins. In addition, trehalose serves as energy or carbon reserve, protection of biomolecules from water stress (Elbein et al. 2003). Hence, desiccation tolerance achieved by trehalose could be caused by the stabilization of cellular structures and macromolecules by accumulating trehalose. Trehalose act as osmoprotectants which tend to be uncharged at neutral pH, and are highly soluble in water (Ballantyne and Camberlin, 1994). Hence, the accumulation of compatible solutes may help to maintain the relatively high water content necessary for growth and cellular functions. It might be considered preferable for these natural compounds to have the capacity to form hydrogenbonds with free water without any interaction with macromolecules, even at low water contents (Rudolph and Crowe, 1985).
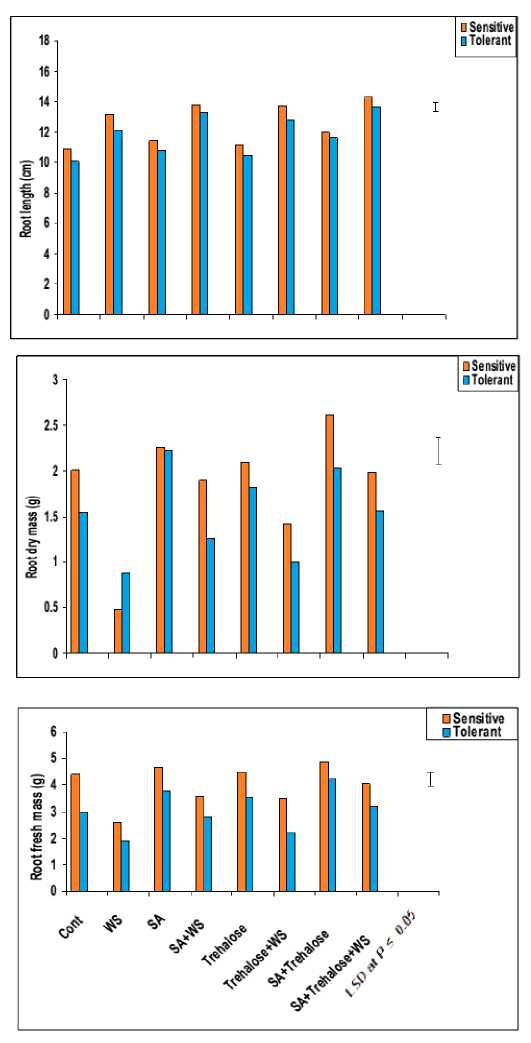
Figure 1: Effect of salicylic acid, trehalose and their interaction on root fresh mass (g), root dry mass (g) and root length (cm) of droughted wheat cultivars during grain-filling. Vertical bars represent LSD at P ≤ 0.05.
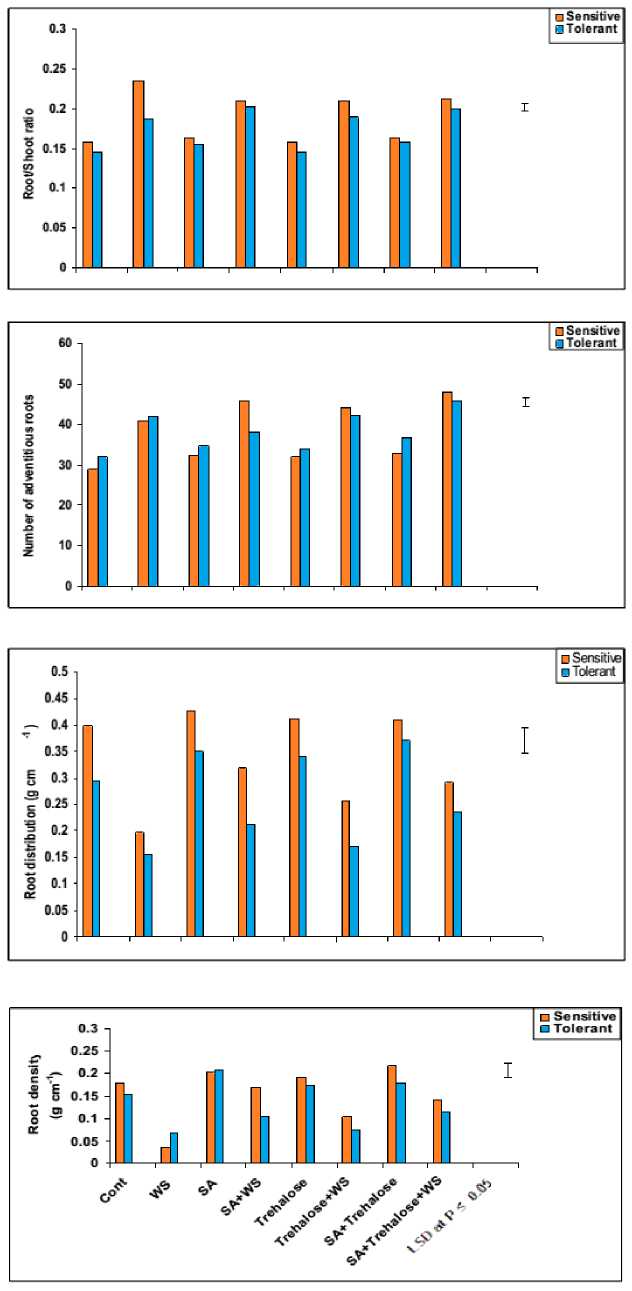
Figure 2: Effect of salicylic acid, trehalose and their interaction on root/shoot ratio, number of adventitious roots, root density (g cm-1) and root distribution (g cm-1) of droughted wheat cultivars during grain-filling. Vertical bars represent LSD at P ≤ 0.05.
□ Sensitive a Tolerant
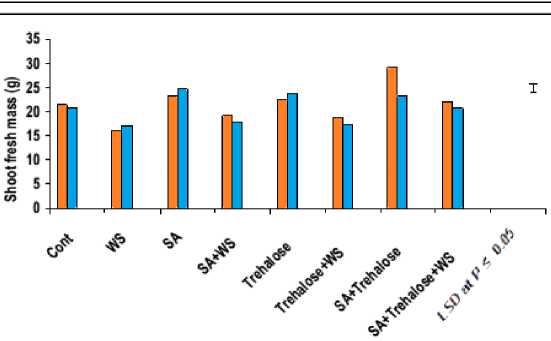
Figure 3: Effect of salicylic acid, trehalose and their interaction on shoot fresh mass (g), shoot dry mass (g)and shoot length (cm) of droughted wheat cultivars during grain-filling . Vertical bars represent LSD at P ≤ 0.05.
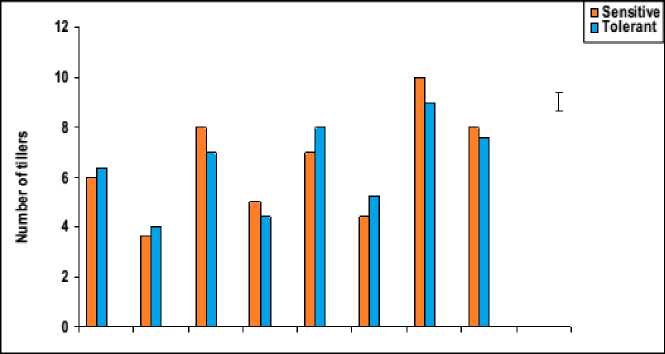
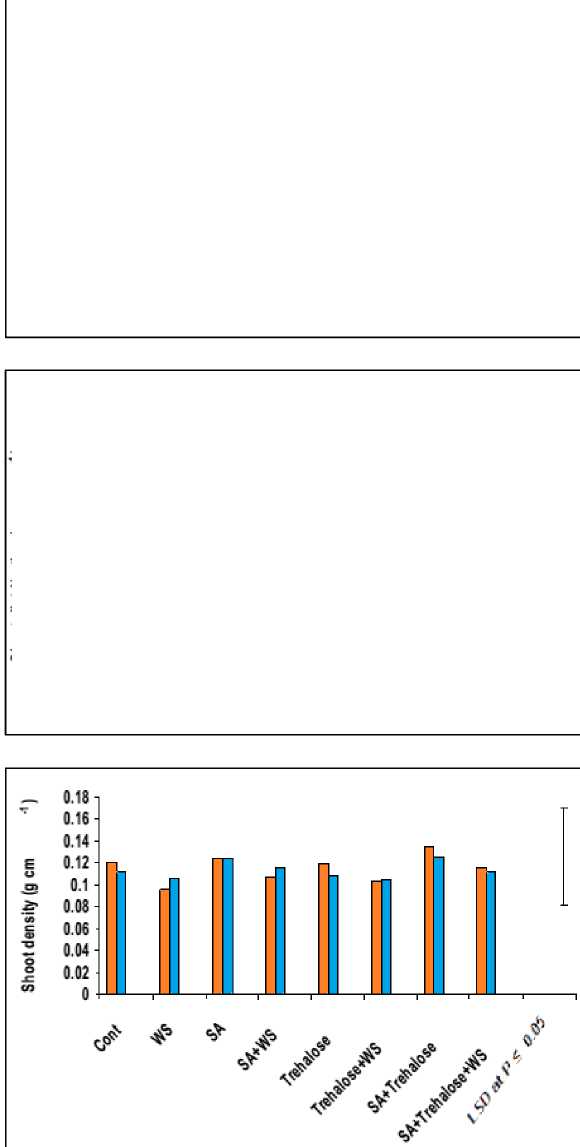
Figure 4: Effect of salicylic acid, trehalose and their interaction on shoot density (g cm-1), shoot distribution (g cm-1) and the number of tillers of droughted wheat cultivars during grain-filling. Vertical bars represent LSD at P ≤ 0.05.
□ Sensitive ■ Tolerant

□ Sensitive ■ Tolerant
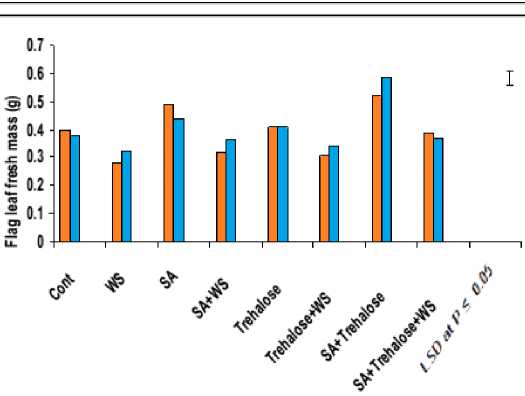
Figure 5: Effect of salicylic acid, trehalose and their interaction on flag leaf fresh mass (mg), dry mass (mg) and leaf area (cm2) of droughted wheat cultivars during grain-filling. Vertical bars represent LSD at P ≤ 0.05.

D Sensitive ■ Tolerant
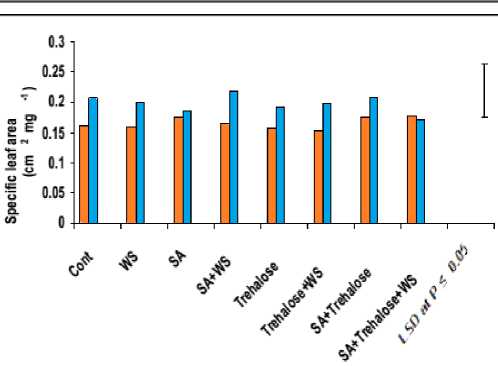
Figure 6: Effect of salicylic acid, trehalose and their interaction on flag leaf specific area (cm2 mg-1), degree of succulence (mg cm-2) and degree of sclerophylly (mg cm-2) of droughted wheat cultivars during
grain-filling .Vertical bars represent LSD at P ≤ 0.05.
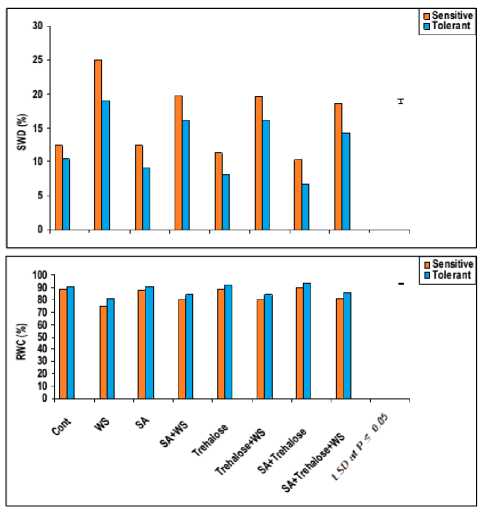
Figure 7: Effect of salicylic acid, trehalose and their interaction on RWC (%) and SWD (%) of flag leaf of droughted wheat cultivars during grain-filling. Vertical bars represent LSD at P ≤ 0.05.
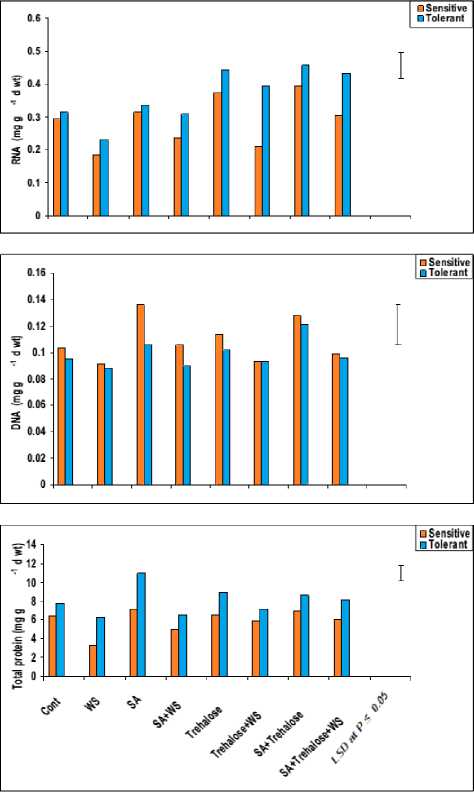
Figure 8: Effect of salicylic acid, trehalose and their interaction on total protein and nucleic acids (DNA & RNA) content (mg g-1 d wt) in flag leaf of droughted wheat cultivars during grain-filling. Vertical bars represent LSD at P ≤ 0.05
It was concluded from this work that the plant growth is significantly affected by water stress in both wheat cultivars (Gemmieza-7 and Sahel-1) in comparison to non-stressed plants. According to the obtained results, Gemmieza-7 would be classified as a species susceptible to drought, because its growth and physiological parameters, such as dry and fresh weight at the whole plant level, leaf area, specific leaf area, shoot to root ratio on the basis of length, some water relations (i.e. relative water content, saturation water deficit, degree of leaf succulence and degree of leaf sclerophylly) and leaf protein and nucleic acids (DNA and RNA) contents were severely affected by water stress in comparison to Sahel-1. Hence, SA and/or trehalose significantly stimulate almost all growth characteristics of stressed or unstressed wheat plants in relation to their relative untreated plants. Trehalose metabolism increases tolerance to water-deficit stress.
Finally, measuring the morphological parameters of both cultivars gave a good indication of the plant status under water-stress and applied SA and/or trehalose conditions.
Список литературы Comparative morpho-biochemical responses of wheat cultivars sensitive and tolerant to water stress
- Akıncı, Ş. and Lösel, D.M. (2012) Plant water-stress response mechanisms, water stress,” I. Md. and M. Rahman, Eds., Water Stress, Intech Europe, 15-42
- Aldesuquy, H.S. (2014): Glycine betaine and salicylic acid induced modification in water relations and productivity of drought wheat plants. Journal of Stress Physiology & Biochemistry 10(1): in press
- Aldesuquy, H. S. and Ibrahim, A. H. (2001) Interactive effect of seawater and growth bioregulators on water relations, abscisic acid concentration and yield of wheat plants. J. Agronomy & Crop Science, 187: 185-193
- Aldesuquy, H.S.; Mankarios, A.T. and Awad, H.A. (1998) Effect of some antitranspirants on growth, metabolism and productivity of saline-treated wheat plants. Induction of stomatal closure, inhibition of transpiration and improvement of leaf turgidity. Acta Botanica., 41: 1-10
- Aldesuquy, H.S.; Abo-Hamed, S.A.; Abbas, M.A. and Elhakem, A.H. (2009) Effect of glycine betaine and salicylic acid on growth and productivity of droughted wheat cultivars. I. Osmolytes in relation to osmotic adjustment and grain yield. Journal of Environmental Science, Mansoura University, 37: 13-33
- Aldesuquy, H.S.; Abo-Hamed, S.A.; Abbas, M.A. and Elhakem, A.H. (2012) Role of glycine betaine and salicylic acid in improving growth vigour and physiological aspects of droughted wheat cultivars. Journal of Stress Physiology & Biochemistry. 8: 150-171
- Aldesuquy H.S.; Baka, Z.A.; El-Shehaby, O.A. and Ghanem, H.E. (2013) Growth, Lipid peroxidation and antioxidant enzyme activities as a Selection Criterion for the salt tolerance of wheat cultivars irrigated by seawater. Phyton 53: 153-165
- Arduini, I.; Godbold, D.G. and Onnis, A. (1994) Cadmium and copper change root growth and morphology of Pinus pinea and Pinus pinaster seedlings. Physiologiae Plantarum, 92: 675-680
- Arfan, M., Atharb, H.R. and Ashraf, M. (2007) Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? Plant Physiology, 164: 685-694
- Ballantyne, J.S., Chamberlin, M.E. (1994) Regulation of cellular amino acid levels. In "Cellular and Molecular Physiology of Cell Volume Regulation" (K Strange ed), CRC Press, Boca Raton, pp.111-122
- Baraka, D.M. (2008) Osmotic adjustment of wheat grain germination to hyperosmotic saline by nicotine hermone. Research Journal of Agriculture and Biological sciences, 4: 824-831
- Beadle, C.L. (1993) Growth analysis. In: Photosynthesis and Production in a Changing Environment. A field and laboratory manual. (eds. Hall, D.C.; Scurlock, J.M.O.; Bolhar H.R.; Leegod, R.C. and Long, S.P.), Chapman and Hall, London, 36-46
- Chaparzadeh, N.; D'Amico, M.L.; Khavari-Nejad, R.A.; Izzo, R. and Navari-Izzo, F. (2004) Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiology and Biochmistry, 42: 695-701
- Chopart, J.L.; Le Mézo, L. and Mézino, M. (2008) Software application for processing root data from impact counts on soil profiles. User Guide, Technological document, 26-28
- Davenport, D. C.; Martin, P.E. and Hagan, R.M. (1972) Antitranspirants for conservation of leaf water potential of transplanted citrus trees. Hort. Sci., 7:511
- Debouba, M.; Gouia, H.; Suzuki, A. and Ghorbel M.H. (2006) NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato (Lycopersicon esculentum) seedlings. Plant Physiology, 163: 1247-1258
- Delf, E.M. (1912) Transpiration in succulent plants. Annals of Botany, 26: 409-440
- Devi, P. (2000) Principles and methods in plant molecular biology, In: biochemistry and genetics. Agrobios, India, 57-59
- Djanaguiraman, M.; Arun, A.S.; Shanker, J.; Devi, K.D. and Bangarusamy, U. (2006) Rice can acclimate to lethal level of salinity by pretreatment with sub-lethal level of salinity through osmotic adjustment. Plant Soil, 284: 363-373
- Edwards, C.; Read, J. and Sanson, G. (2000) Characterizing sclerophylly: Some mechanical properties of leaves from heath and forest. Oecologia, 123: 158-167
- Elbein, A.D., Pan, Y.T., Pastuszak, I. and Carroll, D. (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13: 17R-27R
- El-Tayeb, M. A. (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul., 45: 215-224
- Farhat, J.; Shahbaz, M. and Ashraf, M. (2008) Discriminating some prospective cultivars of maize (Zea mays L.) for drought tolerance using gas exchange characteristics and proline contents as physiological markers. Pakistan Journal of Botany, 40: 2329-2343
- Grewal, H.E. (2011) Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a Sodic vertosol with variable subsoil NaCl salinity. Agricultural Water Management, 97: 148-156
- Guo, L.Q.; Shi, D.C. and Wang, D.L. (2009) The key physiological response to alkali stress by the alkali-resistant halophyte Puccinellia tenuiflora is the accumulation of large quantities of organic acids and into the rhyzosphere. Journal of Agronomy and Crop Science, 196: 123-135
- Hasamuzzaman, M., Fujita, M., Islam, M.N., Ahamed, K.U. and Nahar, K. (2009) Performance of four irrigated rice varieties under different levels of salinity stress. International Journal of Integrative Biology, 6: 85-90
- Hassanein, A.A. (2000) Physiological responses induced by shock and gradual salinization in rice (Oryza sativa L.) seedlings and the possible roles played by glutathione treatment. Acta Botanica Hungarica, 42: 139-159
- Hatzig, S.; Kumar, A.; Neubert, A. and Schubert, S. (2009) PEP-carboxylase activity supports organic acid metabolism of maize (Zea mays) under salt stress. Institute of Plant Nutrition, Justus Liebig University, Germany
- Jaleel, A.C.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R. and Panneerselvam, R. (2007a): Water deficit stress mitigation by calcium chloride in Catharanthus roseus effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids and Surfaces, B: Biointerfaces, 60: 110-116
- Jaleel, A.C., Manivannan, P., Sankar, B., Kishorekumar, A., Gopi, R., Somasundaram, R. and Panneerselvam, R. (2007b): Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation. Colloids and Surfaces, B: Biointerfaces. 60: 201-206
- Jaleel, C.I., Gopi, R. and Panneerselvam, R. (2008a) Growth and Photosynthetic Pigments Responses of Two Varieties of Catharanthus roseus to Triadimefon Treatment,” Comptes Rendus Biologies, 331: 272-277
- Jaleel, A.C.; Sankar, B.; Sridharan, R. and Panneerselvam, R. (2008b) Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turkish Journal of Biology, 32: 79-83
- Joseph, B.; Jini, D. and Sujatha, S. (2010) Biological and physiological perspectives of specificity in abiotic salt stress response from various rice plants. Asian Journal of Agriculture, 2: 99-105
- Joseph, B.; Jini, D. and Sujatha, S. (2011) Development of salt stress-tolerant plants by gene manipulation of antioxidant enzymes. Asian Research Journal of Agriculture, 5: 17-27
- Jungklang, J. (2005) Physiological and biochemical mechanisms of salt tolerance in Sesbania rostrate (Brem. and Oberm.). Ph. D. Thesis, Tsukuba University, Tsukuba
- Kaymakanova, M. and Stoeva, N. (2008) Physiological reaction of bean plants (Phaseolus vulgaris L.) to salt stress. General and Applied Plant Physiology, 34, 177-188
- Khalid, K.A. and Teixeira da Silva, J.A. (2010) Yield, essential oil and pigment content of (Calendula officinalis L.) flower heads cultivated under salt stress conditions. Scientia Horticulturae, 126: 297-305
- Luna, C.M.; Gonzalez, C.A. and Trippi, U.S. (1994) Oxidative damage caused by an excess copper in oat leaves. Plant and Cell Physiology, 35: 11-15
- Mahajan, S. and Tuteja, N. (2005) Cold, salinity and drought stresses: an overview,” archives of biochemistry and biophysics, 444: 139-158
- Maggio, E.T and Ramnarayan, K. (2001) Recent development in computational proteomics. Trends Biotechnol., 19: 262-277
- Manivannan, P.; Jaleel, C.A.; Kishorekumar, A.; Sankar, B. Somasundaram, R.; Sridharan, R. and Panneerselvam, R. (2007) Changes in antioxidant metabolism of Vigna unguiculata L. (Walp.) by propiconazole under water deficit stress. Colloids and Surfaces, B: Biointerfaces, 57: 69-74
- Mates, J.M. and Sanchez-Jimenez, F. (1999) Antioxidant enzymes and their implications in pathophysiologic processes. Front Bioscience, 4: 339-345
- Mohammadkhani, N. and Heidariturk, R. (2008) Effects of drought stress on soluble proteins in two maize varieties. Journal of Biology, 32: 23-30
- Moradi, F. and Ismail, A.M. (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany, 99: 1161-1173
- Munns, R. (2002) Comparative physiology of salt and water stress. Plant and Cell Physiology, 25: 239-250
- Netondo, G.W.; Onyango, J.C. and Beck, E. (2004) Sorghum and salinity. I. Response of growth, water relations and ion accumulation to NaCl salinity. Crop Science, 44: 797-805
- Parida, A.K., Das, A.B. and Das, P. (2002) NaCl stress causes changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a true mangrove (Bruguiera parviflora) in hydroponic cultures. Journal of Plant Biology, 45: 28-36
- Quarrie, S.A. and Jones, H.G. (1979) Genotype variation in leaf water potential, stomatal conductance and abscisic acid concentration in spring wheat subjected to artificial drought stress. Annals of Botany, 44: 323-332
- Rudolph, A.S., Crowe, J.H. (1985) Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology; 22(4):367-377
- Richards, G. T., Gregg, M. D., Becker, R. H., and White, R. L. (2002) ”First: A first/2MASS Quasar with an Overabundance of C IV Absorption Systems”, ApJ, 567, L13
- Sadasivam, S. and Manickam, A. (1996) Biochemical methods. 2nd ed. New Age International Limited Publishers, New Delhi, India,159-160
- Sairam, R.K. and Tyagi, A. (2004) Physiology and molecular biology of salinity stress tolerance in plants. Current Science, 86: 407-421
- Scarponi, L. and Perucci, P. (1986) The effect of a number of S-triazines on the activity of maize delta aminolivulinate dehydratase. Agrochimica, 30: 36-44
- Seckin, B.; Turkan, I.; Sekmen, A.H. and Ozfidan, C. (2010): The role of antioxidant defense systems at differential salt tolerance of Hordeum marinum (sea barley grass) and Hordeum vulgare L. (cultivated barley). Environmental and Experimental Botany, 69: 76-85
- Shah, S.H. (2007) Effects of salt stress on mustard as affected by gibberellic acid application. General and Applied Plant Physiology, 33: 97-106
- Shannon, M.C. and Grieve, C.M. (1999) Tolerance of vegetable crops to salinity, Scientia Horticulturae, 78: 5-38
- Shao, H.B.; Chu, L.Y.; Abdul Jaleel, C. and Zhao, C.X. (2008) Water-deficit stress induced anatomical changes in higher plants. Journal of Biology, 331: 215-225
- Siddiqui, Z.S. (2006) Biochemical responses of dimorphic seeds of Arthrocnemum indicum (L. Willd) during germination, inhibition, and alleviation under saline and non-saline conditions. Turkish Journal of Biology, 30: 185-193
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N. and Khan, M.M.A. (2008) Cumulative effect of soil and foliar application of nitrogen, phosphorus, and sulfur on growth, physico-biochemical parameters, yield attributes, and fatty acid composition in oil of erucic acid-free rapeseed-mustard genotypes. Journal of Plant Nutrition, 31: 1284-1298
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Viégas, R.A. and Silveira, J.A.G. (2010) Comparative effects of salinity and water stress on photosynthesis, water relations and growth of Jatropha curcas plants. Journal of Arid Environments, 74: 1130-1137
- Snedecor, G.W. and Cochran, W.G. (1976) Statistical Methods. 6th ed. Oxoford IBH Publishing Co. New Delhi
- Tawfik, M.M.; Bahr, A.A. and Salem, A.K.M. (2006) Response of kaller grass (Leptochloa fusca L.) to biofertilizer inoculation under different levels of seawater irrigation. Research Journal of Applied Sciences, 2: 1203-1211
- Weatherly, P. E. (1950) Studied on the water relations of the cotton plants. Ι. The field measurement of water deficits in leaves. New Phytology, 49: 81-97
- Weatherly, P.E. and Barrs, C. (1962) A re-examination of relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Science, 15: 413-428
- Welch, M.E. and Rieseberg, L.H. (2002) Habitat divergence between a homoploid hybrid sunflower species, Helianthus paradoxus (Asteraceae), and its progenitors. American Journal of Botany, 89: 472-479
- Witkoswski, E.T.F. and Lamont, B.B. (1991) Leaf specific mass confounds leaf density and thickness. Oecologia, 84: 362-370
- Xiong, L. and Zhu, J.K. (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell and Environment, 25: 131-139
- Zaki, M.F., Abou-Hussein, S.D., Abou El-Magd, M.M. and El-Abagy, H.M.H. (2009) Evaluation of some sweet fennel cultivars under saline irrigation water. European Research Journal of Science, 30: 67-78
- Zhou, X. M.; Mac-Kenzie, A.F.; Madramootoo, C.A. and Smith. D.L. (1999) Effects of stem-injected plant growth regulator, with or without sucrose, on grain production, biomass and photosynthetic activity of field-grown corn plants. Journal of Agronomy and Crop Science, 183: 103-110


