Concentration of the total hemolymph protein of the Australian red-clawed crayfish (Cherax quadricarinatus, Von Martens 1868) grown in artificial conditions
Автор: Skafar D.N., Givlyud N.N., Antsupova A.M., Strelkova O.V., Shumeyko D.V.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.20, 2024 года.
Бесплатный доступ
Aim. The concentration of total protein in the hemolymph of the Australian red-clawed crayfish Cherax quadricarinatus, Von Martens 1868, contained in artificial conditions in closed water supply installations and an aquarium complex, was studied. Methodology. The object of the study was the Australian red-clawed crayfish, a total of 161 individuals were used in the experiment. Crayfish were kept in closed water supply installations. To study the protein concentration depending on the time of year, samples were taken and analyzed in the period 2021-2023: January - winter; April - spring; August - summer; September, October and November - autumn. To conduct an experiment to study the total protein content of cancer hemolymph, depending on the water temperature, three experimental groups of 15 cancers were formed in each. The experimental groups were seated in three aquariums, with a water temperature of 20, 25, 30 ° C. The duration of the crayfish exposure was 10 days. On the tenth day, hemolymph was collected. The total hemolymph protein was determined by the refractometric method.
Australian red claw crayfish, cherax quadricarinatus, hemolymph, total protein, ras
Короткий адрес: https://sciup.org/143182396
IDR: 143182396
Текст научной статьи Concentration of the total hemolymph protein of the Australian red-clawed crayfish (Cherax quadricarinatus, Von Martens 1868) grown in artificial conditions
Due to the increase in animal protein consumption, the amount of aquaculture supplied increases annually as fisheries are unable to fully meet the growing demand (FAO, 2020). Thus, in 2018, the catch of industrial fishing amounted to 96.4 million tons, and the volume produced by aquaculture was 82.1 million tons, which is 46% of the total production. Of the total volume produced by aquaculture in 2018, only 9.4 million tons are crustaceans. Although this number represents only 11.5% of total aquaculture production, it exceeds the catch of crustaceans from natural reservoirs. The main objects of crustacean aquaculture are: Natantia decapod shrimp, Japanese blue crab ( Portunus trituberculatus ), Akiami shrimp ( Acetes japonicus ), Antarctic krill ( Euphausia superba ), sea crabs nei, Brachyura, blue swimming crab ( Portunus pelagicus ), Argentine red shrimp ( Pleoticus muelleri ), southern rough shrimp ( Trachypenaeus curvirostris ) (FAO, 2020).
However, the Australian red-clawed crayfish ( Cherax quadricarinatus ) von Martens 1868 is not included in this list. Meanwhile, the Australian red-clawed crayfish is a promising warm-water aquaculture object with a rapid growth rate and valuable consumer qualities. Its development as an object of cultivation abroad began in the 90s of the last century (Jones, 1990; FAO, 2023). In Russia, work on developing technology for its cultivation began in the late 2000s (Lagutkina, Ponamarev, 2008). The prerequisites for this were the depressed state of natural populations of numerous marine species of crustaceans, a reduction in crayfish stocks and a ban on their fishing in some regions, not fully satisfied demand for gourmet products from live crustaceans on the Russian market, and the development of resort infrastructure (Kovacheva et al. , 2018), the underutilized potential of inland water bodies, as well as the availability of warm waters from thermal power plants and geothermal sources allowing almost continuous breeding of this tropical species. But at the same time, the cultivation technology requires refinement and optimization.
An urgent task is to study the physiology of this species, in particular the study of physiological and biochemical indicators of hemolymph, since in aquaculture conditions it is necessary to assess the physiological state of the body (Kovacheva, Aleksandrova, 2010), to monitor the health of the cultivated object in the conditions of fishery enterprises. One of the physiological and biochemical indicators is the concentration of total hemolymph protein.
Total hemolymph protein is the sum of all proteins circulating in the hemolymph and is the main component of blood. The total blood protein of crustaceans contains: hemocyanin, fibrinogen (coagulogen), protein hormones, antimicrobial proteins, etc. (Depledge & Bjerregaard, 1989; Fredrick & Ravichandran, 2012). The bulk of the total protein in crustacean hemolymph is hemocyanin, which can range from 60 to 90 percent or more (Depledge & Bjerregaard, 1989; Magnum, 1983). Hemolymph proteins have many functions: saturating the body with oxygen, removing carbon dioxide, maintaining pH and oncotic pressure of the plasma, transporting chemical compounds, storing amino acids, and immune defense (Gianazza et al. , 2021).
It is known that the total protein content may vary depending on various conditions. Thus, the concentration of total protein in the hemolymph of crustaceans can be subject to seasonal fluctuations (Sladkova and Kholodkevich, 2011), change depending on the diet (Lu et al., 2020 Shirina et al., 2023), and with a lack of food (Pascual et al., 2006 ; Sánchez-Paz et al., 2007) and under the influence of toxicants (Chen, Cheng, 1995; Vijayavel, Balasubramanian, 2006; Sladkova, Kholodkevich, 2011). Based on the total protein content, one can give a qualitative assessment of crustaceans used to form broodstock (Cherkashina et al., 1989) and assess the viability of individuals (Paterson et al., 2005). At the same time, there is a lack of information on the content of total protein in the hemolymph of Australian red-clawed crayfish cultivated under artificial conditions in closed water supply installations, which requires study. This issue requires detailed coverage since there is evidence that some physiological and biochemical parameters of hemolymph differ in organisms kept in natural and artificial conditions (Taylor et al., 2009). This is especially true for the Australian red-clawed crayfish, an important part of the breeding biotechnology of which is its maintenance in recirculating aquaculture system (RAS) (Borisov, Nikonova, 2018; Shumeyko et al., 2022).
The purpose of this work is to study the concentration of total protein in the hemolymph of the Australian red claw crayfish when kept under artificial conditions, to identify seasonal changes in protein concentration, and to determine it during a short stay in water at different temperatures.
MATERIALS AND METHODS
The work was carried out in the laboratory of advanced technologies in aquaculture on the basis of the business incubator of the Federal State Budgetary Educational Institution of Higher Education “Kuban State University”.
Animal keeping. . The crayfish were kept in three recirculation units. Individuals weighing 10 g or more were kept in two recirculation systems, including two pools with a volume of 2.5 m3 and an area of 3.14 m2 each at a water temperature of 20–28 °C and stocking densities of 6 to 20 individuals/m2. Crayfish weighing less than 10 g were kept in a RAS, which included five pools with a volume of 0.9 m3 each and an area of 0.18 m2 at a water temperature of 20–28 °C and stocking densities from 20 to 50 individuals/m2. Shelters made of PVC pipes were placed in the RAS. and polycarbonate to reduce intraspecific aggression. Feeding was carried out daily with Coppens Start Premium 1.5 mm compound feed (Netherlands) (protein – 54%, fats – 15%, ash – 10.4%, phosphorus – 1.59%), the daily norm was 3% of the crustacean biomass. The photoperiod was 12 hours. The oxygen concentration in the water was 5 mg/l, pH 7.9. A total of 161 individuals were used in the experiment, weighing from 1.7 to 118 g.
To study protein concentration depending on the time of year, samples were taken and analyzed in the period 2021–2023: January – winter; April - spring; August – summer; September, October and November -autumn.
To conduct an experiment to study the content of the total protein of the crayfish hemolymph depending on the water temperature, three experimental groups of 15 crayfish each were formed, the average weight of individuals was 3.05 ± 0.68 g. The experimental groups were seated in three aquariums with dimensions of 0.60 x 0.30 x 0.35 (L x W x H) each, with water temperature 20, 25, 30 oC. The deviation from the set temperatures during the experiment was ± 1 oC. The water was heated using Aquael Janusz Jankiewicz 100W heaters (Poland). The water was saturated with oxygen using a Hailea ACO-500 compressor (China). The oxygen level in the water was 5 g/l. The duration of holding the crayfish was 10 days. During the experiment, the main hydrochemical indicators (ammonia, nitrites, nitrates, pH) were within the limits of fish farming standards (Pyatikopova, et al., 2022). On the tenth day, hemolymph was collected.
Hemolymph collection. Hemolymph for analysis was collected intravitally using a 2 ml syringe with a 23G needle in compliance with the rules of asepsis and antisepsis by puncture of the ventral sinus of crayfish. This method allows blood to be collected intravitally without causing significant damage to the health of the crayfish (Alexandrova, Kovacheva, 2010).
Determination of total protein. Total protein was determined by the refractometric method on an IRF-22 refractometer (Kovacheva, Aleksandrova, 2010), protein concentration was expressed in g/l.
Statistical processing of data. Calculations of the data obtained were carried out using Microsoft Excel (Microsoft Corporation, USA) and Statistica 14 (TIBCO Software Inc.) programs. Results are presented as mean ± standard deviation (median values are used in graphs). To test the statistical significance of differences, the Mann-Whitney U test and the Kruskal-Wallis test were used. Differences were considered statistically significant at p<0.05. To detect correlations, the Spearman correlation coefficient was used; the correlation was considered statistically significant at p<0.05.
RESULTS AND DISCUSSION
Total protein content in the hemolymph of the Australian red claw crayfish.
The total protein concentration varied over a wide range from 10.8 to 108.8 g/L and averaged 50.5 ± 21.1 g/L (Fig. 1). The distribution of total protein concentration is normal; most often, individuals have a protein content of 40 to 80 g/l. The differences in protein concentration between males and females are not statistically significant (p = 0.96). A statistically significant weak positive correlation (r=0.24) was found between body weight and total protein content (p<0.01). No statistically significant differences in the protein content in the hemolymph were found in the five weight groups (p>0.05) (Fig. 2).
Our data on the concentration of total protein in the hemolymph of the Australian red-clawed crayfish differ greatly from other studies. Thus, in the work of Mauro et al. , crayfish with an average weight of 55 g were kept in aquariums after being transported from an aqua farm; the concentration of total blood protein in these individuals was 2.5 ± 0.8 g/l (Mauro et al. , 2022), which is approximately 20.2 times less than in our study. The closest to the values obtained in our work are the data of Lagutkina et al. , so in their study the concentration of total protein in the hemolymph of the Australian red claw crayfish was 40.8 ± 4.5 g/l when grown in a pond (Lagutkina et al. , 2021), however it is 0.8 times lower than in our study. When keeping crayfish in a pool setup, the protein concentration was 29.4 ± 4.2 g/l at the beginning of the experiment, and 35.7 ± 5.0 g/l at the end of the experiment (Lagutkina et al. , 2020). In the work of Shirina et al. , the amount of protein in the hemolymph of the studied individuals of C. quadricarinatus kept in aquariums, fed with three experimental foods, varied greatly depending on the composition of the feed - from 30.1 to 123.1 g/l (Shirina et al. , 2023 ). In almost all of the above studies, crustaceans were kept under different conditions, which can undoubtedly affect the protein content. It is also worth noting differences in sample preparation and methods for determining total protein concentration.
The above examples probably indicate that a wide range of concentrations of total hemolymph protein may be due to different conditions of keeping the crustacean and be considered as an adaptation mechanism to these conditions.
In addition, in crayfish, hemolymph was analyzed with abnormal signs: in the hemolymph there are many fragments of hemocytes, and bacteria are present. The total protein content in such hemolymph was 32.7 ± 21.9 g/l (n=10), which is 1.6 times less than in individuals without abnormalities (p<0.01). The hemolymph of crayfish that were in unfavorable conditions was also analyzed: during the winter in 2023, the heating radiators were turned off and the temperature in one RAS dropped to 10–15 °C, as a result of which the crayfish contained in it were subject to prolonged exposure to non-optimal temperatures. Individuals were affected by rust spot disease. The concentration of total protein in the hemolymph of these individuals was 34.9 ± 20.8 g/l (n=6). These values were 1.5 times less than those of individuals kept at optimal temperatures (p<0.01).
A decrease in total protein is observed in other types of crustaceans when infected with various pathogens. Thus, in white shrimp, the protein concentration decreases by 1.5 times when infected with the TSV virus (Song et al. , 2003). However, the protein concentration in individuals infected with a bacterial infection does not always decrease, and probably depends on the strain of the bacterium and the number of bacterial cells in the body (Joseph, Philip, 2020). The decrease in total protein concentration in crayfish with bacteria present in the hemolymph can be interpreted as an immune response. It is known that the main enzyme involved in the immune response to various pathogens is phenoloxidase (Söderhäll, Cerenius, 1992; Cerenius, Söderhäll, 2021). Hemocyanin also exhibits phenoloxidase activity under the influence of hemocyte components and during proteolytic cleavage, this is accompanied by a decrease in its concentration (Adachi et al. , 2003; Lee et al. , 2004). In our case, apparently in response to the penetration of the pathogen into the body, the phenoloxidase activity of hemocyanin is activated, accompanied by a decrease in its level and leading to a decrease in the concentration of total protein.
The hemolymph of two females was also analyzed 24 hours after giving birth to their offspring. The protein concentration was 49.8 ± 20.9 g/l, however, due to the small sample, it is premature to draw any conclusions. It is worth noting that the topic of studying the physiological and biochemical parameters of females with eggs, or with larvae hatched from the shell located on them, undoubtedly deserves attention, since such a condition should cause changes. It is known that females with eggs or crustaceans practically do not feed during the incubation of the eggs and until the young hatch from the female, which in theory should lead to a decrease in protein concentration (Pascual et al., 2006; Sánchez-Paz et al., 2007).
In addition, in November 2021, two females had a hemolymph color different from normal (instead of a bluish color with a grayish tint, it was cream-colored). The protein concentration in such hemolymph was 58.5 ± 8.5 g/l. Probably, this color is characteristic of mature females ready for reproduction (Alexandrova, Kovacheva, 2010).
Observations of protein concentrations at different times of the year.
Comparison using the Kruskal-Wallis test did not yield statistically significant differences between the four compared groups (p>0.5) (Fig. 3). Pairwise comparisons also did not show statistically significant differences (p>0.5). The protein concentration in winter was 50.3 ± 23.0 g/l, in spring the content was 53.6 ± 23.5 g/l, which is statistically not significantly more than in winter by 1.1 times. In summer, the protein concentration was 1.2 times less than in spring, amounting to 45.3 ± 16.1 g/l, and in autumn 50.3 ± 19.6 g/l.
Crustaceans exhibit seasonal fluctuations in blood protein concentrations. Thus, in Parastacus varicosus, the concentration of total protein decreases in the spring-summer period and increases in the autumnwinter period (Da Silva-Castiglioni et al., 2007). However, in a representative of the family Aeglidae -Aegla platensis, the protein concentration is reduced in the autumn-winter period, and increases in the springsummer period (Oliveira et al., 2007), similar changes are observed in Parastacus brasiliensis (Dutra et al., 2008), in the listed In the works, crustaceans were kept in natural conditions. There is information that in Pontastacus leptodactylus caught from its natural habitat and acclimatized to the conditions of the aquarium complex, seasonal changes in the concentration of hemolymph protein are observed (Sladkova, Kholodkevich, 2011). However, there is no information about seasonal fluctuations in the concentration of total hemolymph protein in crustaceans that spent their entire life cycle in artificial conditions (as in our case) - that is, in “domesticated” individuals. In the broad-clawed crayfish, molting occurs towards the end of summer, beginning of autumn. In this connection, the observed increase in the concentration of total hemolymph protein in the summer and its sharp decrease in the fall may be due to the cancer being in the premolting stage (Sladkova, Kholodkevich, 2011). While in the Australian red-clawed crayfish kept under artificial conditions, molting occurs at any time of the year (Borisov, Nikonova, 2018), such phenomena may not be observed. Thus, it is difficult to assert any seasonal fluctuations in the content of total hemolymph protein in Australian red claw crayfish kept under artificial conditions.
Total protein concentration in molted crayfish. In the process of collecting material on the concentration of hemolymph protein, we discovered molted individuals (n=8) from which hemolymph was also sampled and the protein concentration was determined. Significantly lower protein content was noted in individuals after molting. The protein concentration in molted individuals ranged from 6.3 to 52.5 g/l, the average value was 20.4 ± 15.3 g/l, which is statistically significant (p<0.005) 2.7 times lower compared to non-molted crayfish (Fig. 4).
A similar picture with a decrease in protein concentration is observed in other crustaceans; for example, in the crayfish Pontastacus leptodactylus, the protein content immediately after molting decreases by approximately 1.7 times (Sladkova and Kholodkevich, 2011). A similar situation is observed in other species. Apparently, a decrease in hemolymph protein during molting is characteristic of all representatives of the order Decapoda. The reason for the observed changes is primarily an increase in the volume of water in the hemolymph immediately after molting, and thereby a decrease in protein concentration. To facilitate the shedding of the old carapace and the stretching of the new soft carapace before hardening, crustaceans increase their water consumption, which dilutes the hemolymph to increase their body volume (Depledge and Bjerregaard, 1989). The second reason that reduces the concentration of total protein in the hemolymph after molting is the use of circulating hemolymph proteins in the construction of the carapace (Terwilliger, 1999).
Total protein concentration as a function of temperature.
The optimal temperature range for cultivating the Australian red claw crayfish is considered to be 25–29 °C, and at temperatures less than 20 °C and more than 30 °C, the growth rate and weight of individuals decrease (Meade et al. , 2002; Zhigin et al. , 2017).
This experiment examined changes in total protein concentrations to short-term exposure to temperatures above and below optimal. It was found that at the end of the experiment the survival rate in all three groups was 100%. In addition, crayfish that were in water with a temperature of 30 °C showed greater motor activity and a reaction to adding food to the aquarium. When crayfish were kept for a short time in water at different temperatures, no statistically significant changes in the total protein content were found (p>0.05). However, it was noted that in crayfish kept in water at a temperature of 30 °C there is a slightly higher concentration of protein in the hemolymph (48.2 ± 27.5 g/l) compared to crayfish kept in water at a temperature of 25 °C (41.8 ± 19.2 g/l ) and 20 °C (42.7 ± 17.1 g/l) (Fig. 5). This may be explained by the fact that at 30 °C the maximum rate of metabolic processes in the crustacean is observed.
The data obtained are comparable with the work of Bone et al. (Bone et al. , 2014), which also found no changes in the concentration of total protein when crayfish were kept in water at different temperatures for a long time, while in our study the protein content in the hemolymph was higher . However, in some crustacean species, changes in protein concentration occur after short-term exposure to water at different temperatures (Sánchez et al. , 2001; Matozzo et al. , 2011).
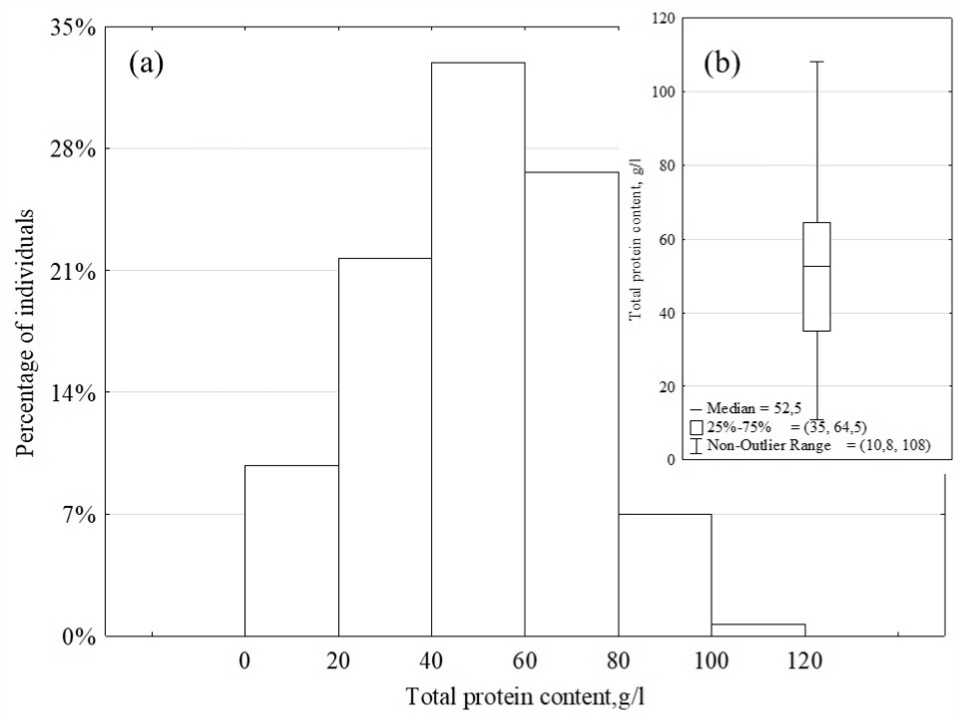
Figure 1. The total protein content of C. quadricarinatus : (a) – distribution of total protein concentration; (b) – the range
of total protein concentration.
Ъй
g
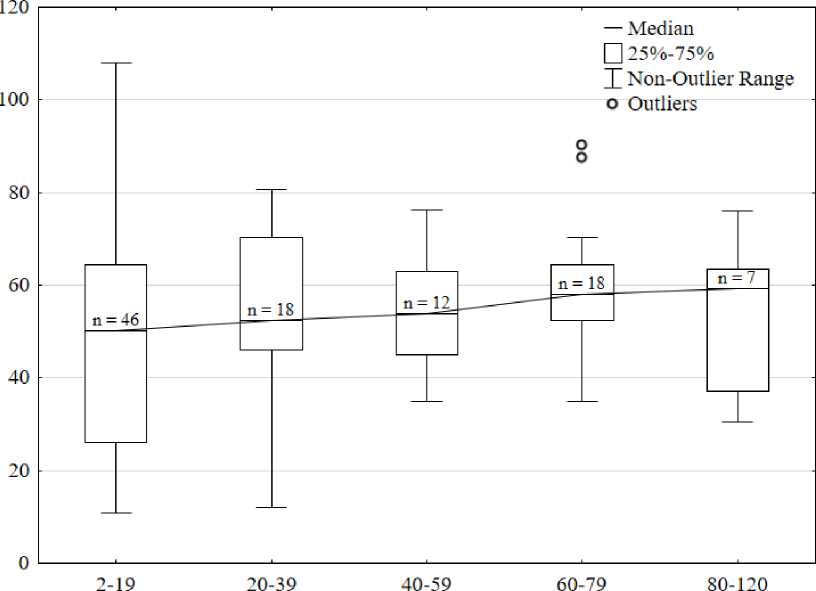
Weight groups, g
Figure 2. The concentration of total protein in weight groups of australian red-clawed crayfish.
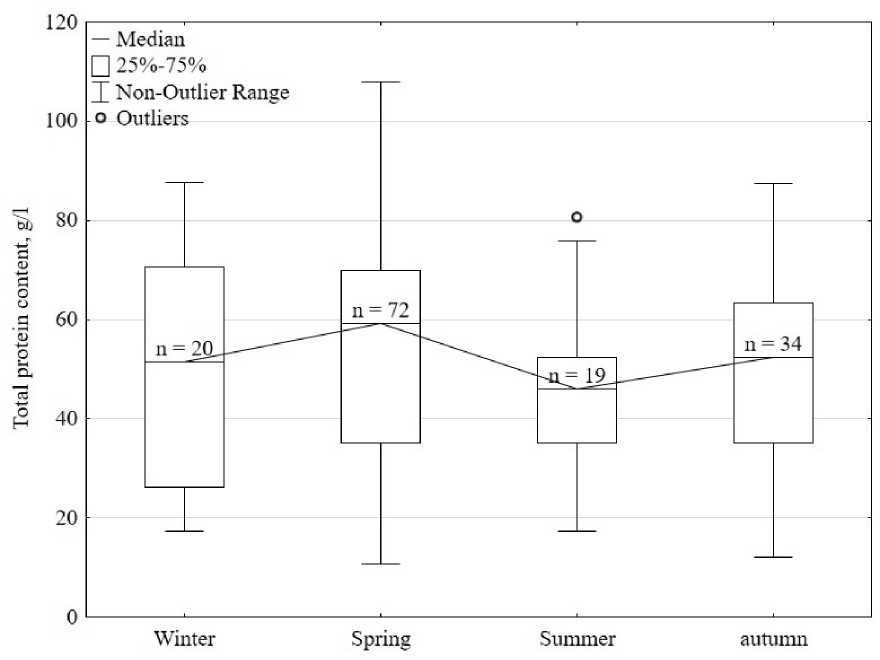
Figure 3. The concentration of total protein in the hemolymph of crayfish at different times of the year.
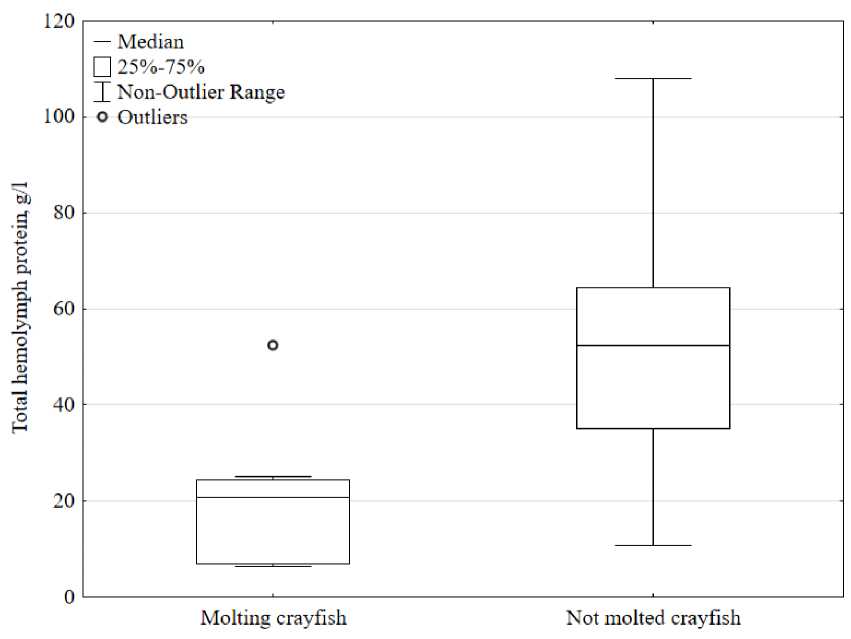
Figure 4. The concentration of total protein in molted and non-molted crayfish.
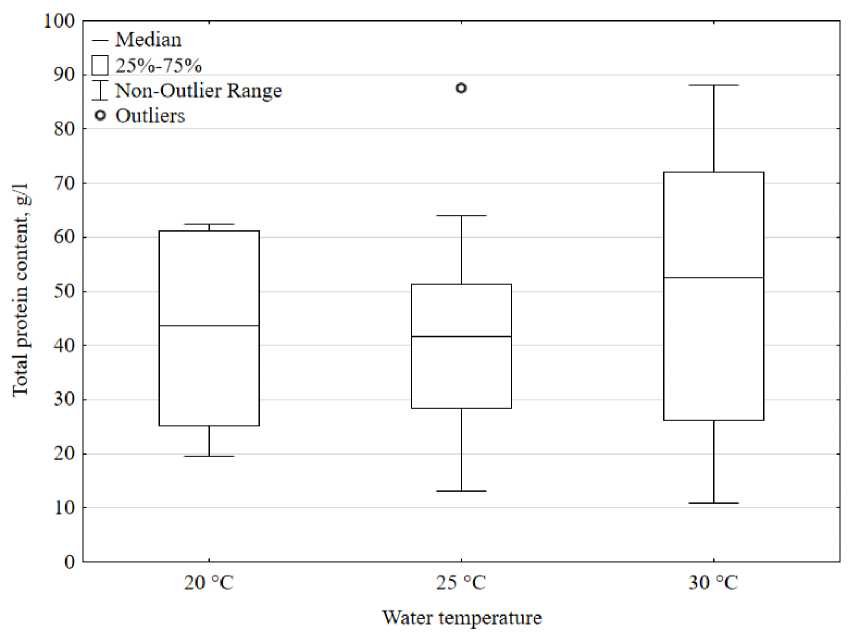
Figure 5. The concentration of total protein depends on different temperatures.
CONCLUSION
Thus, in the course of our studies, it was established that the concentration of total protein in the hemolymph of the Australian red claw crayfish can be in wide ranges, which may indicate the high adaptive potential of this organism. A decrease in the concentration of total protein may be due to the crustacean being in unfavorable conditions and being damaged by a bacterial agent. It was noted that changes in protein concentration at different times of the year in crustaceans under artificial conditions cannot be interpreted as seasonal fluctuations. It was found that after molting, the concentration of total protein in the hemolymph of Cherax quadricarinatus decreases by 2.7 times. It was also found that short-term exposure of crayfish in water at different temperatures does not lead to a change in the total protein content.
COMPLIANCE WITH ETHICAL STANDARDS
During the study, all applicable international principles for the use of laboratory animals were observed. Due to the work with invertebrates, the ethical standards specified in the World Declaration of Animal Rights have not been violated.
AUTHOR CONTRIBUTIONS
All the authors made a significant contribution to the design of the work, research and preparation of the materials of the article, read and approved the final version of the article before publication.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Concentration of the total hemolymph protein of the Australian red-clawed crayfish (Cherax quadricarinatus, Von Martens 1868) grown in artificial conditions
- Adachi K., et al. (2003) Hemocyte components in crustaceans convert hemocyanin into a phenoloxidase-like enzyme. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 134 №1, 135-141. https://doi.org/10.1016/s1096-4959(02)00220-8
- Alexandrova E.H., Kovatcheva N.P. (2010) In life determination of the physiological status of decapod crustaceans (Crustacea: Decapoda) by hematological characteristics. Progress in physiological science. 41 №2, 51-67. (In Russ)
- Bone J.W.P., et al. (2014) Using biochemical markers to assess the effects of imposed temperature stress on freshwater decapod crustaceans: Cherax quadricarinatus as a test case. Journal of Comparative Physiology B. 185 №3, 291-301. https://doi.org/10.1007/s00360-014-0883-3
- Borisov R.R., Nikonova I.N. (2018) Special aspects of decapod crustaceans growth in recirculating aquaculture systems as exemplified by Australian crayfish Cherax quadricarinatus (Decapoda: Parastacidae). Marine Biological Journal. 3 №3, 312. https://doi.org/10.21072/mbj.2018.03.3m
- Cerenius L., Söderhäll K. (2021) Immune properties of invertebrate phenoloxidases. Developmental & Comparative Immunology. 122, 104098 https://doi.org/10.1016/j.dci.2021.104098
- Chen J.C., & Cheng S.Y. (1995) Hemolymph oxygen content, oxyhemocyanin, protein levels and ammonia excretion in the shrimp Penaeus monodon exposed to ambient nitrite. Journal of Comparative Physiology B. 164 №7, 530-535. https://doi.org/10.1007/bf00261393
- Cherkashina N.Ya., et al. (1989) The quality of females and males of long-toed Kuban cattle Astacus leptodactylus cubanicus Bir. and Vin. Sat. scientific tr. GosNIORH. Issue. 300, 49-55. (In Russ)
- Da Silva-Castiglioni D., et al. (2007) Seasonal variations in the intermediate metabolism of Parastacus varicosus (Crustacea, Decapoda, Parastacidae). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 148 №1, 204213. https://doi.org/10.1016/j.cbpa.2007.04.005
- Depledge M.H., & Bjerregaard, P. (1989) Haemolymph protein composition and copper levels in decapod crustaceans. Helgoländer Meeresuntersuchungen. 43 №2, 207-223. https://doi.org/10.1007/bf02367900
- Dutra B.K., et al. (2008) Seasonal variations in the intermediate metabolism of the crayfish Parastacus brasiliensis (Crustacea, Decapoda, Parastacidae) in the natural environment and experimental to a change in the total protein content. COMPLIANCE WITH ETHICAL culture. Iheringia. Série Zoologia. 98 №3, 355-361. https://doi.org/10.1590/s0073-47212008000300010
- FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. https://doi.org/10.4060/ca9229en
- FAO (2023) Cherax quadricarinatus. Cultured Aquatic Species Information Programme. Text by Jones, C.M. Fisheries and Aquaculture Division [online]. Rome. Updated 2011-10-10 [Cited Thursday, May 18th 2023].
- Fredrick W.S., & Ravichandran S. (2012) Hemolymph proteins in marine crustaceans. Asian Pacific Journal of Tropical Biomedicine. 2 №6, 496-502. https://doi.org/10.1016/s2221-1691(12)60084-7
- Gianazza E., et al. (2021) Hemolymph proteins: An overview across marine arthropods and molluscs. Journal of Proteomics. 245
- https://doi.org/10.1016/jjprot.2021.104294 Jones C.M. (1990) The Biology and Aquaculture Potential of the Tropical Freshwater Crayfish Cherax quadricarinatus. Queensland Department of Primary Industries Information Series QI90028, p. 109.
- Joseph A., & Philip R. (2020) Immunocompetence of Penaeus monodon under acute salinity stress and pathogenicity of Vibrio harveyi with respect to ambient salinity. Fish & Shellfish Immunology. 106, 555-562. https://doi.org/10.1016/j.fsi.2020.07.067
- Kovacheva N.P., et al. (2018) Crustaceans aquaculture: current status and development trends. Fisheries. №2, 78-82. (In Russ)
- Kovatcheva N.P., Aleksandrova E.N. (2010) Hematological parameters as an indicators of physiological status of the decapods: red king crab Paralithodes camttshaticus and freshwater crayfish genus Astacus and Pontastacus. VNIRO Publishing, Moscow P. 92. (In Russ)
- Lagutkina L.Yu., et al. (2020) Providing factual support for efficient techniques of breeding tropical freshwater species. Vestnik of ASTU. Series: Fishing industry. №2, 94-105. (In Russ) https://doi.org/10.24143/2073-5529-2020-2-94-105
- Lagutkina L.Yu., et al. (2021) Hematologicaland biochemical indicators of Australian red-claw crayfish hemolymph. Bulletin of the ASTU. Series: Fishing Industry. №2, 134-143. (In Russ). https://doi.org/10.24143/2073-5529-2021-2-134-143
- Lagutkina L.Yu., Ponomarev S.V. (2008) New object of aquaculture - australian redclaw crayfish (Cherax quadricarinatus). Vestnik AGTU. №6, 220-224. (In Russ)
- Lee S.Y., et al. (2004) Processing of crayfish hemocyanin subunits into phenoloxidase. Biochemical and Biophysical Research Communications. 322 №2, 490-496. https://doi.org/10.1016/j.bbrc.2004.07.145
- Lu X., et al. (2020) Effects of dietary protein levels on growth, muscle composition, digestive enzymes activities, hemolymph biochemical indices and ovary development of pre-adult red swamp crayfish (Procambarus clarkii). Aquaculture Reports. 18 https://doi.org/10.1016/j.aqrep.2020.100542
- Magnum C.P. (1983) Oxygen transport in the blood. Biology of Crustacea 5, 382-428.
- Matozzo V., et al. (2011) Effects of temperature on cellular and biochemical parameters in the crab Carcinus aestuarii (Crustacea, Decapoda). Marine Environmental Research. 71 №5, 351-356. https://doi.org/10.1016/j.marenvres.2011.04.001
- Mauro M., et al. (2022) Haemolymphatic Parameters in Two Aquaculture Crustacean Species Cherax destructor (Clark, 1836) and Cherax quadricarinatus (Von Martens, 1868). Animals. 12 №5, 543. https://doi.org/10.3390/ani12050543
- Meade M.E., et al. (2002) Effects of Temperature and Salinity on Weight Gain, Oxygen Consumption Rate, and Growth Efficiency in Juvenile Red-Claw Crayfish Cherax quadricarinatus. Journal of the World Aquaculture Society. 33 №2, 188-198. https://doi.org/10.1111/j.1749-7345.2002.tb00494.x
- Oliveira G.T., et al. (2007) Seasonal variations in the intermediate metabolism of Aegla platensis (Crustacea, Aeglidae). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 147 №3, 600-606. https://doi.org/10.1016/j.cbpa.2006.08.025
- Pascual C., et al. (2006) Biochemical, physiological, and immunological changes during starvation in juveniles of Litopenaeus vannamei. Aquaculture. 251(2-4): 416-429. https://doi.org/10.1016/j.aquaculture.2005.06.001
- Paterson B.D., et al. (2005) Prediction survival of western rock lobster Panuluris cygnus, using discriminant analysis of hemolymph parameters taken immediately following simulated handling treatments. New Zealand Journ. of Marine and Freshwater Research. 39 №5, 1129-1143. https://doi.org/10.1080/00288330.2005.9517380
- Pyatikopova O.V., et al. (2022) Hydrochemical conditions of cultivation of the Australian red-clawed crayfish (Cherax quadricarinatus) in the Astrakhan region. Aquatic bioresources and habitat. 5 №3, 32-47. (In Russ.) https://doi.org/10.47921/2619-1024_2022_5_3_32
- Sánchez A., et al. (2001) Hemolymph metabolic variables and immune response in Litopenaeus setiferus adult males: the effect of acclimation. Aquaculture. 198 №1-2, 13-28. https://doi.org/10.1016/s0044-8486(00)00576-7
- Sánchez-Paz A., et al. (2007) Effect of short-term starvation on hepatopancreas and plasma energy reserves of the Pacific white shrimp (Litopenaeus vannamei). Journal of Experimental Marine Biology and Ecology. 340 №2, 184-193. https://doi.org/10.1016/jjembe.2006.09.006
- Shirina Y.M., et al. (2023) Prospects for using alfalfa flour as part of feed for Australian red-claw crayfish (Cherax quadricarinatus) Vestnik of ASTU Series: Fishing industry. №1, 72-81. (In Russ) https://doi.org/10.24143/2073-5529-2023-1-72-81
- Shumeyko D.V., et al. (2022) Rearing of Cherax quadricarinatus (von martens, 1868) juveniles using feed for sturgeons. Sel'skokhozyaistvennaya biologiya [Agricultural Biology]. 57 №4, 803-816. (InRuss). https://doi.org/10.15389/agrobiology.2022A803ms
- Sladkova S.V., Kholodkevich S.V. (2011) Total protein in crawfish hemolymph as a parameter of functional state of animals and biomarker of quality of habitat. Journal of Evolutionary Biochemistry and Physiology. 47, 160-167.
- Söderhäll K., Cerenius L. (1992) Crustacean immunity. Annual Review of Fish Diseases. №2, 3-23. https://doi.org/10.1016/0959-8030(92)90053-z
- Song Y-L., et al. (2003) Haemolymph parameters of Pacific white shrimp (Litopenaeus vannamei) infected with Taura syndrome virus. Fish & Shellfish Immunology. 14 №4, 317-331. https://doi.org/10.1006/fsim.2002.0440
- Taylor S., et al. (2009) Flow cytometric characterization of freshwater crayfish hemocytes for the examination of physiological status in wild and captive animals. Journal of Aquatic Animal Health. 21 №3, 195-203. https://doi.org/10.1577/h09-003.1
- Terwilliger N.B. (1999) Hemolymph Proteins and Molting in Crustaceans and Insects. American Zoologist. 39 №3, 589-599. https://doi.org/10.1093/icb/39.3.589
- Vijayavel K., & Balasubramanian M.P. (2006) Fluctuations of biochemical constituents and marker enzymes as a consequence of naphthalene toxicity in the edible estuarine crab Scylla serrata. Ecotoxicology and Environmental Safety. 63 №1, 141-147. https://doi.org/10.1016/j.ecoenv.2005.02.004
- Zhigin A.V., et al. (2017) Determination of the optimal temperature and consumption of oxygen in rearing young australian red clawed crayfish. Prirodoobustrojstvo. №3, 121-128. (In Russ)


