CXCR4 expression in different subsets of CTCS and single (detached) breast cancer cells
Автор: Savelieva Olga E., Tashireva Liubov A., Buldakov Mikhail A., Mukhamedzhanov Rustam H., Kaigorodova Evgenia V., Denisov Evgeny V., Zavyalova Marina V., Perelmuter Vladimir M.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Клинические исследования
Статья в выпуске: 4 т.17, 2018 года.
Бесплатный доступ
The aim of this study was to assess CxCR4 expression in different subsets of CTCs and single (detached) breast cancer cells. materials and methods. Thirty five patients with invasive breast carcinoma of no special type (IC NST) (T1-4N0-2M0), between 29 and 69 years of age were included in this study. Different subsets of CTCs with CxCR4 expression were evaluated by flow cytometry. A confocal microscopy was used to assess CxCR4 expression in different subsets of single (detached) cancer cells in breast tissue. results. The CxCR4 was expressed in CTCs without stem-like and EMT phenotype, in CTCs with EMT but not stem markers and in stem-like CTCs without EMT features. In all blood samples, the CxCR4 expression in CTCs with stem-like and EMT phenotype was absent. In breast tumor the CxCR4 was expressed in the non stem-like single (detached) breast cancer cells with EMT features, in the single (detached) breast cancer cells with stem and EMT features. In all tumor samples the stem-like or non stem-like single (detached) breast cancer cells without EMT features were absent. Conclusions. Different subsets of the CTCs exhibited CxCR4. The CxCR4 expression did not depend on the presence or absence of stem or/and EMT features in tumor cells. We showed that some subsets of single (detached) breast cancer cells in the primary tumor were characterized by the ability to express CxCR4 and may be a source of the respective CTC subsets.
Cxcr4, circulating tumor cells, single (detached) cancer cells, epithelial-mesenchymal transition, breast cancer, cancer stem-like cells
Короткий адрес: https://sciup.org/140254203
IDR: 140254203 | УДК: 618.19-006.6-091.8 | DOI: 10.21294/1814-4861-2018-17-4-75-80
Текст научной статьи CXCR4 expression in different subsets of CTCS and single (detached) breast cancer cells
Circulating tumor cells (CTCs) mediate tumor dissemination and play the key role in the metastatic cascade [1, 2]. The prognostic value of CTCs in breast cancer has been previously shown. Thus, presence of CTCs in the peripheral blood is associated with the poor prognosis in breast cancer [3, 4]. However, little is known about exact mechanism of migration and trafficking of CTCs in the peripheral blood circulation [2]. Metastasis of cancer cells to distant locations needs intravasation from the primary tumor sites into blood vessels, survival in the circulation, migration to secondary organs, adhesion, and proliferation of cancer cells in targeting organs and tissues [5]. Chemokines might affect the selection of target organs for metastases formation. For example, the CXCR4/ CXCL12 axis makes breast cancer cells move out of the circulation and traffic into organs with high amounts of chemokines, and thus forming metastases [6, 7]. In our previous study, we showed that CTCs were represented by heterogeneous population. Some cancer cells had stem-like or/and EMT (epithelialmesenchymal transition) phenotype, other cancer cells did not have EMT and stemness features [8]. We suggested that CTCs did not always precede metastasis, because they could not have the necessary features, including their CXCR4 expression. So, in present study we investigated the CXCR4 expression in different subsets of CTCs and single (detached) breast cancer cells in primary tumor.
Material and Methods
Patients (n=35) with invasive breast carcinoma of no special type (IC NST) (T1-4N0-2M0) within the range of 29 - 69 years of age (mean age: 49.06 ± 9.78) were treated at the Cancer Research Institute, Tomsk NRMC (Tomsk, Russia) between 2011 and 2017 included (Table 1). IC NST was defined according to the World Health Organization’s recommendations [9]. The venous blood samples were collected in EDTA-treated tubes before surgical intervention and were used for flow cytometry. The formalin-fixed, paraffin-embedded (FFPE) primary tumor samples were used for immunofluorescence analysis. This study was approved by the institutional review board, all patients signed an informed consent for voluntary participation.
Flow Cytometry
Confocal microscopy
Results
As CXCR4 promotes breast cancer metastasis to distant organs where its ligand, SDF-1, is generated in large quantity [10, 11], we examined the expression of these molecules in different subsets of CTCs in the blood of breast cancer patients (Figure 1). The
CXCR4 expression in CTCs without stem-like and EMT phenotype (CK7+CD45-CD44-CD24+/-N-cadherin-CXCR4+) was present in 31.8% (7/22) patients (0.00(0.00-0.95) cell/ml). In 68.2% (15/22) patients, CTCs with CXCR4 exhibited EMT but not stem markers (CK7+CD45-CD44-CD24+/-N-cadherin+CXCR4+) (1.96(0.13-3.59) cell/ml). The stem-like CTCs expressed CXCR4 in 27.3% (6/22) patients. All this cells (0.00(0.00-0.13) cell/ml) didn’t have EMT features (CK7+CD45-CD44+CD24-N-cadherin-CXCR4+). In all patient blood samples (22/22) the CXCR4 expression in CTCs with stem-like and EMT phenotype (CK7+CD45-CD44+CD24-N-cadherin+CXCR4+) was absent (Table 2).
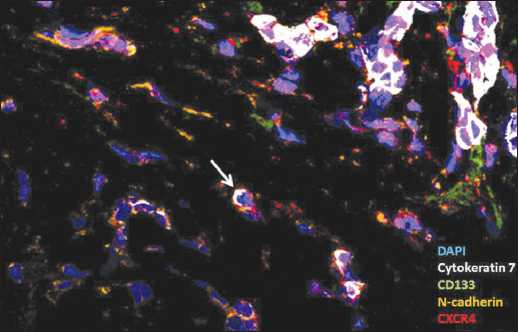
Figure 2. The single (detached) breast cancer cells with CXCR4 expression. The white cursor points to the non stem single (detached) breast cancer cell with EMT features and CXCR4 expression
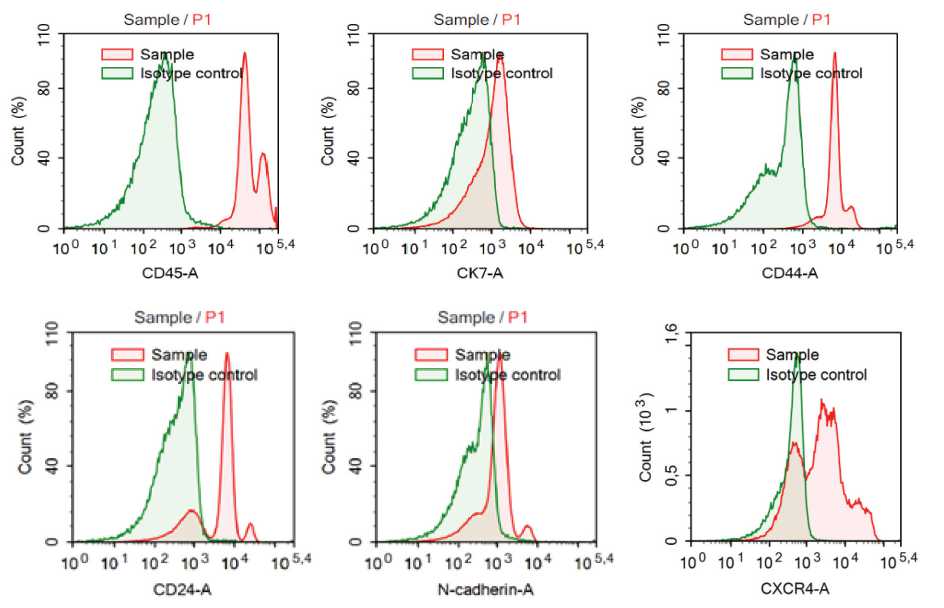
Figure 1. Flow cytometry analysis of the circulating tumor cells with CXCR4 expression
The non stem-like single (detached) breast cancer cells with EMT features (CK7+CD133-N-cadherin+CXCR4+) expressed CXCR4 in 44.0% (11/25) tumor samples (Figure 2). Our results suggested that only 0.00(0.00-8.00)% of single (detached) CK7+CD133-N-cadherin+ cells exhibited CXCR4 in primary tumor. The single (detached)
Список литературы CXCR4 expression in different subsets of CTCS and single (detached) breast cancer cells
- Fidler I.J. The pathogenesis of cancer metastasis: the ‘seed and soil'hypothesis revisited. Nat Rev Cancer. 2003; 3(6): 453-8. DOI: 10.1038/nrc1098
- Mego M., Cholujova D., Minarik G., Sedlackova T., Gronesova P., Karaba M., Benca J., Cingelova S., Cierna Z., Manasova D., Pindak D., Sufliarsky J., Cristofanilli M., Reuben J.M., Mardiak J. CXCR4-SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer. 2016 Feb 19; 16: 127. DOI: 10.1186/s12885-016-2143-2
- Lucci A., Hall C.S., Lodhi A.K., Bhattacharyya A., Anderson A.E., Xiao L., Bedrosian I., Kuerer H.M., Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012; 13(7): 688-95. DOI: 10.1016/S1470-2045(12)70209-7
- Zhang L., Riethdorf S., Wu G., Wang T., Yang K., Peng G., Liu J., Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012 Oct 15; 18(20): 5701-10. DOI: 10.1158/1078-0432.CCR-12-1587
- Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002; 2: 563-72. DOI: 10.1038/nrc865


