Determination of the role of ethanol extract of Parmelia perlata on glucose adsorption & diffusion-an in vitro study
Автор: Hari Haran G., Manjula B., Jothi G.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.19, 2023 года.
Бесплатный доступ
Diabetes mellitus is a group of chronic metabolic disease characterized by elevated level of glucose in blood. It is expected that by the year 2030, the number of diabetes will rise up to 439 million, and greater than 90% have type 2 diabetes. In vitro antidiabetic potential of ethanol extract of Parmelia perlata Ach. (EEPP) was determined by evaluating the inhibitory enzymes of α- amylase, sucrase and α- glucosidase. Glucose adsorption, diffusion, glucose uptake by yeast cell and non-enzymatic glycation parameters were also studied. Cytotoxic assay and glucose uptake assay were carried out in 3T3L1 cell line. Ethanolic extract of Parmelia perlata Ach. showed dose dependent effect on inhibition of sucrase, α- amylase and α- glucosidase. Results for glucose bounding capacity, glucose diffusion, glucose uptake by yeast cell and non- enzymatic glycation were also promising. In vitro studies in 3T3L1 preadipocyte cell line showed effective non cytotoxicity and enhanced glucose uptake. From the data of the experiment it was evident that EEPP might be a potent natural drug source for treating diabetes mellitus.
Α-amylase, α-glucosidase, glucose adsorption, glucose diffusion, parmelia perlata
Короткий адрес: https://sciup.org/143180314
IDR: 143180314
Текст научной статьи Determination of the role of ethanol extract of Parmelia perlata on glucose adsorption & diffusion-an in vitro study
Diabetes mellitus is a group of metabolic disorder with hyperglycemia, occurring due to defective insulin secretion, resistant to insulin action or combination of both. It causes metabolic disturbances of carbohydrate, protein, fat (Les et al ., 2020) and causes long-term damage, failure and dysfunction of various vital organs (Tran et al ., 2020). The data of International Diabetes Federation (IDF) reported 425 million people with diabetes in 2017, and the rate expected to raise over 700 million cases by 2045 (Saeedi et al., 2019).
Allopathic medicines like sulfonylureas, thiazolidinediones, biguanides and meglitinides have been used to cure diabetes mellitus with their diverse mechanisms. But on prolonged usage they cause many side effects like hypoglycemia, drug resistance toxicity and weight gain (Shobana et al ., 2018). Instead of modern medicines, to reduce the problems and consequences of diseases, complementary and alternative medicine take a part of role in treatment (Yakubu et al ., 2020). Traditional system of medicine is fully based on plants and its active metabolites. Worldwide 75-80 % of the population in developing as well as developed countries relies on herbs for curing various ailments, because of its lesser side effects, compatibility and acceptability. Literatures revealed that, more than 400 plant species have hypoglycemic effect (Patel et al ., 2012). Plants are used as an effective source for validating new antidiabetic drugs, and have still been active because of its phytoconstituents like phenols, alkaloids, terpenoids, carotenoids, glycosides, flavonoids, etc., that can be used as optional medication for diseases like diabetes mellitus (Jothi & Brindha, 2014).
Parmelia perlata Ach. is a lichen belonging to the family Parmeliaceae , and is widely distributed all over the India especially in hills station, rocky area and old trunk of trees. Lichens are recognized as a source of pharmacologically active bioequivalent enzyme, fatty acids and polysaccharides (Huneck & Yoshimura, 1996). Parmelia perlata was selected for this study on the basis of its unique biological constituents. It contains abundant metabolites such as Phenolic acid, atranorin, protolichesterinic acid, lecanoric acid and salazinic acid
(Momoh & Adikwu, 2008). It is widely distributed in Kerala, Bengal and Himachal Pradesh in India. In India Materia medica reported that Parmelia species are essential for curing number of ailments (Halama & Haluwin, 2004). It is known as Kalpasi in Tamil, Stone flower in English, Shilapushpha, Saileya in Sanskrit and Chadeela, PatharKa Pool in Hindi. The lichens have been used as a folk medicine to treat diseases like diabetes mellitus, aphrodisiac and anti-inflammatory activity (Pratibha & Mahesh, 2016). It is also used to cure amenorrhea, renal calculi, sores, dysentery, fever, diarrhea, cough, (Lakshmi et al ., 2013), seminal weakness, head ache, itching and skin problems (Hussain et al ., 2014). It acts as a kappa and pitta suppressant. The phytochemical constituents of the different Parmeliaceae family plants revealed the existence of some important secondary metabolites such as flavonoid, saponin, tannin, glycosides and alkaloids which is responsible for its hypoglycemic activity. Hence in vitro analysis of Parmelia perlata was used as a major screening tool to evaluate the therapeutic potential as well as to determine its antidiabetic mechanism of action (Roy & Geetha, 2013).
MATERIALS AND METHODS
Chemicals and reagents
Collection and Authentication
Parmelia perlata Ach. were collected from the local market of Trichy, Tamil Nadu, South India. The Lichen was recognized with the help of Compendium of Indian medicinal plants, (volume 3) and authenticated by Dr. S. John Britto. A specimen (Number: BM001) was deposited in the Rapinat Herbarium, Centre for Molecular Systematics, St. Joseph’s College (Autonomous), Tiruchirapalli, India.
Preparation of extract
Ethanol extract was prepared by soaking 250 g of dried material in ethanol for 48 hours. The solution was filtered and evaporated to dryness. The residue was dissolved in isotonic saline and used for the study (Rao et al ., 2003).
Maintenance of Cell
Animal Mouse cell line 3T3L1 was purchased from National center for Cell Science, Pune. They are squamous in nature and credent for the diffusion of substances across the tissue. They grow adherently as a monolayer in vitro . Minimum Essential Medium was used for cell growth in culture flask, supplemented with 3 % L-Glutamine, 10% Fetal bovine serum, Streptomycin (100 μg/ml), Amphotericin B and 7.5 % sodium bicarbonate in a humidified atmosphere of 5 % CO2 at 37°C, Pencillin (100 IU/ml). For further studies, the cells were included passaging for appropriate number of flask (Babu et al. , 2015).
In vitro antidiabetic evaluation assays
In vitro antidiabetic evaluation was performed based on the assays of α-amylase enzyme inhibition (Moushino et al ., 2013), α-glucosidase enzyme inhibition (McCue et al ., 2005), determination of glucose adsorption capacity (Jijith & Jayakumari, 2017), Sucrase inhibitory activity (Folin & Wu, 1919), glucose diffusion (Sathiyavelu et al ., 2013), determination of glucose uptake in yeast cells (Bhutkar & Bhise, 2013), Non enzymatic glycosylation of hemoglobin (Acharya & Manning, 1980) and in vitro cytotoxic assay of MTT assay (Mosmann, 1983) and Glucose uptake assays (Deutschlander et al ., 2009) were evaluated.
Statistical analysis
All the experiments were done in triplicate, and the results were expressed as mean ± standard deviation (SD).
RESULTS AND DISCUSSION
α-Amylase inhibitory effect of ethanol extract of Parmelia perlata was investigated. The EEPP (50-200 µg/ml) exhibited potent α-Amylase inhibitory effect in a dose dependent manner ranging from 25 % at lower dose and 85.9 % at higher dose (Table 1). IC50 value was 106.63 µg/ml (Fig.1). Experimental data’s of EEPP revealed its inhibitory potential on α-Glucosidase and Sucrase in dose (100 to 500 µg/ml) dependent manner (Table 2). The percentage of inhibition varies from 81.4 to 94.5 % for α-Glucosidase and 20 to 77.1 % for Sucrase enzyme inhibitory effect (Fig.2). The IC50 value of α-Glucosidase is 57.5 µg/ml, and for Sucrase is 251.53 µg/ml.
The EEPP showed increased glycosylation inhibition effect in a concentration dependent manner as given in Table-3 . Formation of Glucose-haemoglobin complex get reduced and free haemoglobin level increased, this represent the inhibitory effect of ethanol extract of test drug (Fig. 3) .
α Amylase is an important enzyme involved in digestion that, hydrolyze the polysaccharides to simple sugars like glucose and maltose (Chaudhari et al ., 2013) . Alpha amylase enzyme acts by delaying the digestion and absorption of carbohydrates, that slows down the rise of post prandial blood glucose value. (Odeyemi & Afolayan, 2018). In this study the inhibitory activities are reliable because of the presence of polyphenolic compounds against alpha amylase enzyme (Proenca et al ., 2019).
Inhibition of alpha glucosidase activity is one of the key approaches in controlling diabetes mellitus by delaying the digestions of polysaccharides and disaccharides. Thereby controls the rise of postprandial hyperglycemia, and its consequent onset of diabetes (Loodu et al ., 2019). The inhibitors of alpha glucosidase retard the digestion of carbohydrate and slow down the absorption. Alpha amylase and alpha glucosidase enzymes are inhibited by polyphenols through nonspecific binding mechanism. The EEPP has high polyphenolic acids and have more effective enzyme inhibition activity (Wang et al ., 2013). Polyphenols has rich amount of antioxidant, and is well known for its hypoglycaemic properties in managing type 2 diabetes (Lin et al ., 2016).
Sucrase is a key enzyme that digests starch and enhances absorption of glucose in the intestine. The inhibition of this enzyme can slow down the passage of carbohydrate into the bloodstream thereby significantly decrease the postprandial, level of glucose. The inhibitor of Carbohydrate digesting enzymes (alpha amylase, alpha glucosidase and sucrase) slows down the digestion of carbohydrate by reducing the rate of glucose absorption. It therefore mitigating the postprandial plasma glucose increase and play a substantial role in regulating postprandial blood glucose level (Kalidoss et al., 2017). EEPP revealed good inhibitory activity against inhibitory enzymes. The inhibitory effect of α-amylase and α-glucosidase enzymes was expounded by synergistic actions of some important secondary metabolites alkaloid, phenol, quinone, saponin and coumarin present in this plant (Sales et al., 2012).
Formation of reactive oxygen species increases blood glucose concentration in blood and increases its adhering capacity to the hemoglobin molecule. The Hb of Red blood corpuscles bound to glucose and forms abduct AIc (Absorbance inhibitory concentration). This Glycosylated hemoglobin is stable and last for 60 to 120 days. The plant extract inhibits the glycosylation of hemoglobin at various glucose concentrations over the period of 72 hours, thus the EEPP extract reduces the formation of glucose hemoglobin complex and increases free Hb (Sindhu et al ., 2013).
Effect of EEPP on Glucose Diffusion, Adsorption and Glucose Uptake in Yeast Cell
The glucose diffusion retardation index (GDRI) was calculated on the basis of its retardation of glucose diffusion. The GDRI of the EEPP (1%) showed significantly higher inhibition (57.4 %) at the end of 60 min treatment (Table 4) than the control, and diminished over time but diffusion of glucose was retarded compared to the normal control (Fig. 4) . The glucose adsorption capacity of EEPP was presented in Table 5 . There was an appreciable increase (p<0.05) in the glucose adsorption capacity at 50 mM (3.64%) and 100 mM (8.54%) of glucose solution (Fig. 5) . EEPP exhibited significantly higher glucose uptake efficacy at different glucose concentration, showing maximum increase in 5mM/ml glucose concentration (Table 6) in yeast cells (Fig. 6) .
The GDRI, is determined based on the retardation and diffusion index for glucose, and is an important in vitro assay to assess the delay in the absorption of blood glucose in the intestinal tract. Glucose molecule gets retarded within the network of fibers present in the plant during the diffusion process. A high GDRI value represents the glucose retardation index of glucose by the test sample (Jenkins et al ., 1978) . The GDRI of the EEPP was 57.4% at 60 min and gradually reduced to 17.9 % at 120 min. Inhibition of α-amylase, by the test drug might be a cause for retardation of glucose diffusion. EEPP showed a strong hindrance towards the flow of glucose across the dialysis membrane. It was evident from the results that the extract possesses inhibitory effect on glucose diffusion over time than the control due to its fiber content and phytoconstituents present in it (Gallagher et al ., 2003) .
Effective absorption was exhibited by EEPP at lowest (5mM) and highest (10mM) of glucose. The adsorption capacities of the sample were found to be related to the molar glucose concentration in the medium, resulting in increased glucose bound with the plant extract (Jain et al ., 2007). It was, therefore, presumed that EEPP might help to withhold the glucose in the intestinal cavity, even at low glucose concentration levels. The EEPP exhibited good retardation potential of glucose adsorption and glucose diffusion, thus attributing its ability to reduce the glucose available to diffuse into the bloodstream, which might be due to the fiber content of the plant (Singh et al ., 2007). Similar observations were reported for number of antidiabetic plants (Chau et al ., 2004). The result also revealed that the EEPP under study could conjugate with glucose even at lower concentration (5mM/L) and has beneficial role to lesser the amount of glucose accessible for transport across the intestinal lumen. Thus blunting the postprandial hyperglycemia and notified for its antidiabetic activity. The plant extract showed significantly higher glucose adsorption at 100 mM glucose concentration. The results depicted that the plant could still adsorb a small amount of glucose even at low glucose concentration.
The mechanism of glucose transport across the yeast cell membrane has been gaining importance as an
In-vitro screening method for hypoglycemic effect of test drug. In the present study it was observed that, EEPP increases glucose uptake by yeast cells dose dependently and the rate of glucose transport across the yeast cell membrane was due to of external glucose concentration as well as sample concentration. Glucose transport in yeast cell is extremely complex and is transported by a facilitated diffusion. (Pitchaipilli et al. , 2016)
MTT (2-(4,4-dimethyl-2-tetrazoyl)-2,5-diphenyl-2,4 tetrazolium salt) gets reduced by active metabolic cells and convert yellow tetrazolium to formazon by the mitochondrial succinate tetrazolium reductase system. The amount of formazon produced is directly equated with the number of viable cells (Asokan et al ., 2014). EEPP has potent maximum cytotoxicity in 24 hours; has increased cytotoxicity of 3T3-L1 cell lines which resulted due to altered mitochondrial assembly.
Hence, it can be assured that the ethanolic extract of Parmelia perlata Ach. is safe, nontoxic and useful in glucose uptake. The results confirmed that EEPP augment the glucose uptake in vitro conditions. It also reduces the gastrointestional glucose absorption through the inhibition of carbohydrate digesting enzymes viz alpha amylase, alpha glucosidase and sucrase. Thereby reduces the post prandial blood sugar level after carbohydrate diet. This is attributed by the effect of phytoconstitutents in the Parmelia perlata Ach. on the glucose receptors on the cell membrane.
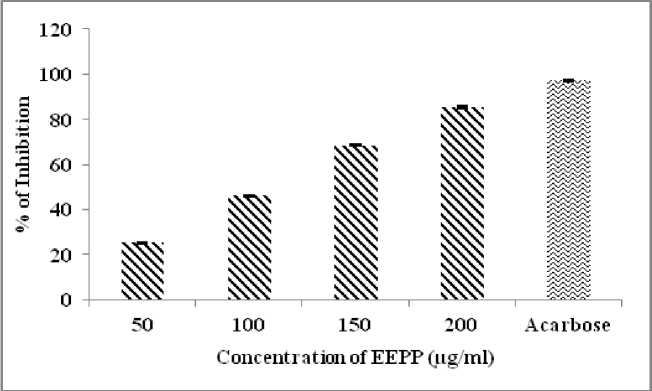
Figure 1. α-Amylase inhibitory effects of EEPP
Table 1. α-Amylase inhibitory effects of EEPP
|
S.No. |
Concentration of EEPP (μg/ml) |
Percentage of Inhibition |
|
1. |
50 |
25.2 ± 0.30 |
|
2. |
100 |
46.2 ± 0.34 |
|
3. |
150 |
68.6 ± 0.37 |
|
4. |
200 |
85.4 ± 0.40 |
|
5. |
Acarbose (500) |
97.2 ± 0.20 |
|
IC 50 = 106.6 µg/ml |
||
Each value was shown in mean±standard deviation (n=3)
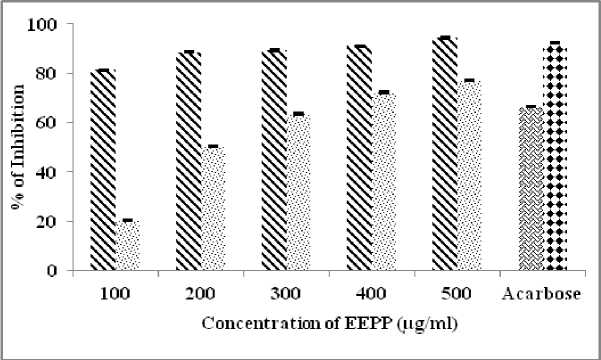
Figure 2. α-Glucosidase & Sucrase inhibitory effects of EEPP
Table 2. α-Glucosidase & Sucrase inhibitory effects of EEPP
|
S.No. |
Concentration of EEPP (μg/ml) |
α-Glucosidase % Inhibition |
Sucrase % Inhibition |
|
1. |
100 |
81.4 ± 0.20 |
20.1 ±0.20 |
|
2. |
200 |
88.6 ± 0.23 |
50.1 ± 0.20 |
|
3. |
300 |
89.5 ± 042 |
63.4 ± 0.32 |
|
4. |
400 |
91.0 ± 0.32 |
72.3 ± 0.29 |
|
5. |
500 |
94.5 ± 0.32 |
77.1 ± 0.37 |
|
6. |
Acarbose (500) |
66.3 ± 0.24 |
92.2 ± 0.17 |
|
IC 50 |
57.5 µg/ml |
251.5 µg/ml |
|
Each value was shown in mean±standard deviation (n=3)
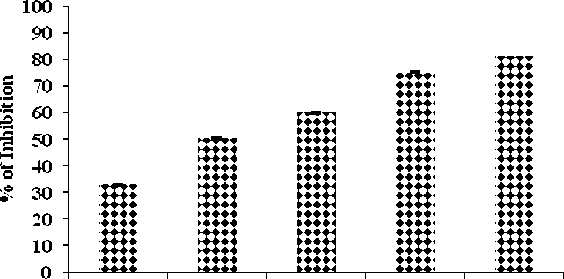
20 40 60 80 100
Concentr atinn of EEPP (ngini)
Figure 3. Non-enzymatic Glycosylation of Haemoglobin assay
Table 3. Non-enzymatic Glycosylation of Haemoglobin assay
|
S.NO |
Concentration of EEPP (μg/ml) |
% of Inhibition of plant sample |
|
1. |
20 |
32.7 ± 0.14 |
|
2. |
40 |
50.3 ± 0.57 |
|
3. |
60 |
60.1 ± 0.20 |
|
4. |
80 |
75.1 ± 0.21 |
|
5. |
100 |
81.0 ± 0.17 |
|
IC 50 |
43.5 µg/ml |
|
Each value was shown in mean±standard deviation (n=3)
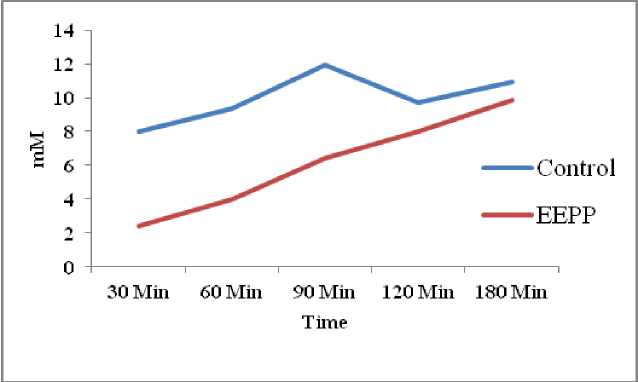
Figure 4. Effect of EEPP on Glucose Diffusion
Table 4. Effect of EEPP on Glucose Diffusion
|
Sample |
Glucose content in the dialysis (mM) |
||||
|
30 min |
60 min |
90 min |
120 min |
180 min |
|
|
Control |
7.97 ± 2.84 |
9.36 ± 2.36 |
11.96 ± 0.89 |
9.71 ± 0.72 |
10.92 ± 0.81 |
|
EEPP |
2.42 ± 0.13 |
3.98 ± 0.91 |
6.41 ± 0.54 |
7.97 ± 0.61 |
9.88 ± 0.42 |
|
GDRI (%) |
69.6 |
57.4 |
46.4 |
17.9 |
9.6 |
Each value was shown in mean±standard deviation (n=3)
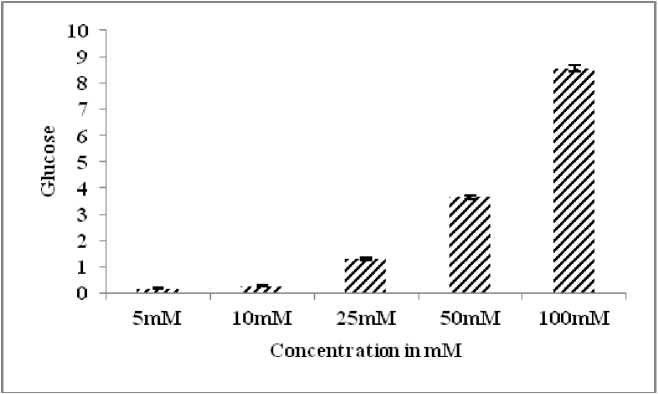
Figure 5. Effect of EEPP on Glucose Adsorption Capacity
Table 5. Effect of EEPP on Glucose Adsorption Capacity
|
Sample |
Glucose concentration |
||||
|
5mM |
10 mM |
25 mM |
50 mM |
100 mM |
|
|
1 % Plant Extract |
0.168 ± 0.02 |
0.284 ± 0.01 |
1.304 ± 0.04 |
3.644 ± 0.08 |
8.544 ± 0.11 |
Each value was shown in mean±standard deviation (n=3)
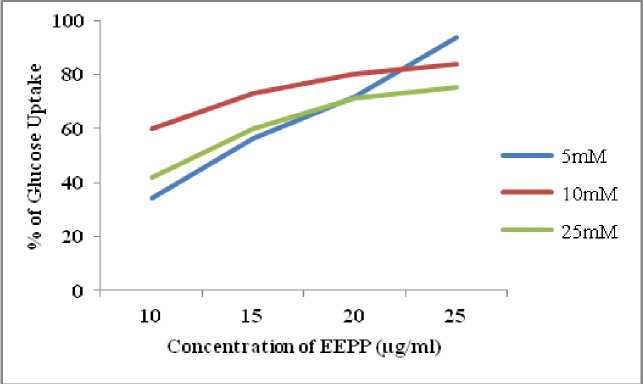
Figure 6. Effect of EEPP on Glucose Uptake in Yeast Cell
Table 6. Effect of EEPP on Glucose Uptake in Yeast Cell
|
EEPP (mg/ml) |
Glucose in |
||
|
5mM |
10mM |
25mM |
|
|
% of increase in glucose uptake |
% of increase in glucose uptake |
% of increase in glucose uptake |
|
|
10 |
34.3 ± 2.04 |
60 ± 3.41 |
42 ± 3.06 |
|
15 |
56.2 ± 3.46 |
73 ± 3.78 |
60 ± 3.45 |
|
20 |
71.8 ± 3.52 |
80 ± 4.62 |
71 ± 2.66 |
|
25 |
93.7 ± 3.61 |
84 ± 4.04 |
75 ± 3.91 |
Each value was shown in mean±standard deviation (n=3)
Table 7. Cytotoxic Effect of EEPP on 3T3L1 cell line- MTT assay
|
S.No. |
Concentration of EEPP (μg/ml) |
% of Dead Cell (24 Hrs) |
|
1. |
125 |
0.4% |
|
2. |
250 |
0.9% |
|
3. |
500 |
1.3% |
|
4. |
1000 |
2.7% |
|
5. |
2000 |
8.1% |
Each value was shown in mean±standard deviation (n=3)
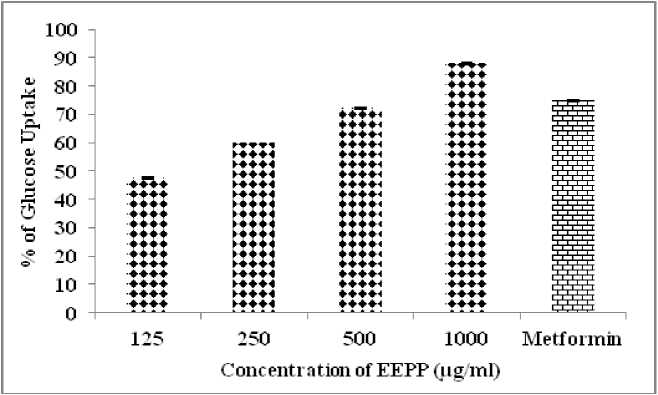
Fig. 7 Glucose Uptake in 3T3 Cell lines
Table 8. Effect of EEPP In vitro Glucose Uptake Study in 3T3L1 Cell lines
|
Ethanol extract of Parmeliaperlata |
Concentration (μg/ml) |
% Glucose uptake over control |
|
125 |
47.6 ± 0.24 |
|
|
250 |
60.1 ± 0.17 |
|
|
500 |
72.2 ± 0.24 |
|
|
1000 |
88.0 ± 0.14 |
|
|
Standard drug (Metformin) |
500 |
74.8 ± 0.17 |
Each value was shown in mean±standard deviation (n=3)
CONCLUSION
In this study, the antidiabetic activity of the ethanol extract of Parmelia perlata Ach. was carried out for its inhibitory effect on alpha amylase, alpha glucosidase and sucrase by in vitro methods. The obtained result showed that the plant extract has significant inhibitory effect on carbohydrates hydrolyzing enzymes to control the blood glucose at postprandial level, because of the presence of polyphenolic compounds exerting their bioactive inhibitory effect at different concentration of EEPP. In phytotherapy based research, isolation of the plant secondary metabolite especially phenolic acid plays an important role in management of type 2 diabetes. In addition to this, Glucose diffusion assay and Glucose adsorption assay envisaged the physical obstacle in the glucose utilization due to its efficacy in trapping of glucose within the network of fiber and phytoconstituents present in it. EEPP increased glucose uptake in yeast cells dose dependently. EEPP was active in reducing non enzymatic glycation of haemoglobin. The effect was found to be dose dependent. In vitro cytotoxic MTT assay were also carried out, plant extract disturbed the mitochondrial assembly and increases cytotoxicity in 3T3L1 Cell line. Parmelia perlata has pronounced activity against 3T3L1 cell line by GLUT4 translocation. Thus the result showed that the ethanol extract of Parmelia perlata had significant inhibitory activity in glucose hydrolyzing enzymes through these in vitro studies.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.


