Developing nanostructured composite of titanium dioxide and poly(triazine imide) for selective photooxidation
Автор: Golovin M.S., Mironova A.T., Zakharchenkova V.P., Sozykin S.A., Bolshakov O.I.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 4 т.16, 2024 года.
Бесплатный доступ
Pristine semiconductors are rarely suggested as efficient heterogenous photocatalysts, as they exhibit low efficiency, due to high recombination rate and narrow adsorption spectrum. Instead, various composites are proposed where intimate contact between two phases creates heterojunction, that improves photoactivity. In this report, we disclose a stepwise development of a grain-based hierarchical structure of photoactive composite based on anatase and poly(triazine imide) (PTI). This nanocomposite was efficient in benzyl alcohol (BA) oxidation and provided targeted benzaldehyde (BAl) with 94 % selectivity at 100 % conversion.
Poly(triazine imide), titanium dioxide, photocatalysis
Короткий адрес: https://sciup.org/147246061
IDR: 147246061 | УДК: 544.478-03 | DOI: 10.14529/chem240418
Текст научной статьи Developing nanostructured composite of titanium dioxide and poly(triazine imide) for selective photooxidation
and are harmful to the nature as it consumes hazardous reagents. For this reason, it is necessary to search for new, greener ways to synthesize these substances [1]. Benzaldehyde is an indispensable component in the production of a wide range of medicines, perfumes, dyes, and food flavors. The industrial production of this aldehyde is based on the selective catalytic oxidation of toluene. The reaction is quite simple, but its disadvantages are harsh reaction conditions and consumption of additional reagents [2–4]. Photoactivated chemical processes seem as a reasonable response to a growing demand for environmentally benign chemical technologies [5]. The advantages of photocatalysis over classical synthesis are mild conditions: room temperature, atmospheric pressure, reagentlessness.
Poly(triazine imide) (PTI) is a highly crystalline polymorph of carbon nitride. It is an affordable, inexpensive semiconductor, which is resistant to photocorrosion [6]. Its structure contains basic Lewis and Brønsted centers, which increase its activity in various catalytic processes [7]. The photocatalytic use of PTI is mainly limited to laboratory due to the disadvantage of rapid charge recombination. The optimal way to increase the photoactivity of carbon nitride is to produce composite photocatalysts with another semiconductor. This way, higher efficiency is achieved by improved charge separation, preventing charge recombination [8] and widened adsorption spectrum [9].
Titanium dioxide is one of the most promising materials for the formation of photoactive composites with PTI with type 2 heterojunction [10]. Optimal promising method of composite formation is hydrothermal treatment. It allows to ensure intimate contact of 2 phases [11, 12]. In this work, a series of TiO2@PTI composites were prepared and showed excellent results in photo oxidation of BA.
Experimental part
Equipment. JEOL JSM 7001F, Rigaku Ultima IV diffractometer, Netzsch 449 F1, Shimadzu IRAf-finity-1S FTIR sp., Shimadzu UV-2700 UV-vis sp., HPLC Shimadzu Prominence LC-20.
Synthesis of TiO2@PTI composite. Ti-peroxo complex was prepared to be the precursor of the TiO 2 phase. For this, 5 mL of aqueous ammonia (NH 3aq , 30%) was added to the 15 mL solution containing 0.4 g of titanium oxysulfate (TiOSO 4 ). The resulting colloidal titanium dioxide (TiO 2 ) was then centrifuged, washed with deionized water 8 times and redissolved with 4 mL of Hydrogen peroxide (H2O2, 30%). The pH of the solution was adjusted to (3, 5, 7 or 9) with aqueous ammonia (NH 3aq , 30%), and the volume was filled up to 20 mL with distilled water. Then, this solution was put into a 40 mL autoclave with the PTFE lining containing a different amount of PTI powder (50, 100, 200 or 400 mg). The autoclave was heated at a specified temperature and treatment time. After cooling, the obtained solid was washed 3 times and dried at 100 °C.
Photocatalytic reaction . The photocatalytic performance of was studied in the oxidation of benzyl alcohol (BA) to benzaldehyde (BAl) in acetonitrile on air. Benzyl alcohol solution in acetonitrile (30 ml, 2 mM) together with 50 mg of photocatalyst were placed into a water jacketed quartz reactor. The quartz reactor was irradiated with thirty 1 Wt UV-diodes with a sharp luminosity peak at 395 nm, a light power of 600 W/m2 and constant stirring on a magnetic stirrer; the irradiation time was 5 hours, on air at 20 °C. Samples were filtered through a PTFE syringe filter prior to HPLC analysis. An HPLC calibration curve was established using BA and BAl with concentrations: 2, 1.5, 1, 0.5, 0.2, and 0.1 mM.
Results and discussion
Photocatalytic studies. It search of the optimal composite photocatalyst, we adapted hydrothermal synthesis employing Ti-peroxo complex as a precursor of TiO2 mixed with prefabricated PTI. Various parameters of hydrothermal synthesis was considered for screening, which were all optimized step by step. Efficiency criterion was the yield of the benzaldehyde in a standard single-stage reaction of photo-catalytic oxidation of BA involving synthesized composites as a photocatalysts. Also, before each experiment, the photocatalyst and reaction mixture were stirred for half an hour in the dark to establish adsorption equilibrium. No significant change was detected in the concentration of reagents in the mixture. Table 1 shows the results of photocatalytic tests of samples with different optimization parameters. To begin with, a previously developed method of composite formation was used, in which the Ti-peroxo complex equilibrated at pH 9 was hydrothermally treated with 200 mg of PTI for 3 days [13]. Firstly, the effect of hydrothermal treatment time on the photocatalytic properties of the samples was studied. Sample after 3 days of treatment showed the best result. Next, we studied the effect of the starting Ti-peroxo complex pH on the photocatalyst functional properties. We did not try a higher pH of the media, as it dissolves the PTI [14]. The next optimization parameter was the temperature of the hydrothermal synthesis. A set of obtained samples showed that the lower the temperature provided the most efficient photocatalyst. Photocatalytic tests were also carried out with a batch of samples which were synthesized with various amounts of PTI at fixed amount of Ti-peroxo complex. An increase in the starting PTI content in the starting mixtures leads to an improvement in photocatalytic characteristics.
Table 1
Optimization of hydrothermal synthesis of TiO 2 @PTI composite
|
Entry |
Condition parameters |
BA conversion, % |
Selectitvity to BAl, % |
BAl yield, % |
Other products, % |
Pseudo 1st order cons., h–1 |
AQE, % |
|
Effect of hydrothermal treatment time |
|||||||
|
1 |
1 day |
49.8 |
99.6 |
49.6 |
0.2 |
0.138 |
0.170 |
|
2 |
3 days |
56.4 |
99.9 |
56.4 |
0 |
0.166 |
0.197 |
|
3 |
5 days |
48.4 |
93.8 |
45.4 |
3 |
0.132 |
0.150 |
|
Effect of pH of Ti-peroxo complex |
|||||||
|
4 |
pH3 |
33.3 |
99.5 |
33.1 |
0.2 |
0.081 |
0.124 |
|
5 |
pH5 |
30.4 |
98.9 |
30.1 |
0.3 |
0.072 |
0.112 |
|
6 |
pH7 |
39.0 |
99.0 |
38.6 |
0.2 |
0.042 |
0.070 |
|
7 |
pH9 |
56.4 |
99.9 |
56.4 |
0 |
0.166 |
0.197 |
|
Effect of synthesis temperature |
|||||||
|
8 |
100 °C |
56.4 |
99.9 |
56.4 |
0 |
0.166 |
0.197 |
|
9 |
140 °C |
9.0 |
19.8 |
1.8 |
7.2 |
0.019 |
0.007 |
|
10 |
180 °C |
23.6 |
49.1 |
11.6 |
12 |
0.054 |
0.040 |
|
Effect of the amount of added PTI during synthesis |
|||||||
|
11 |
50 mg |
32.8 |
87.3 |
28.7 |
4.1 |
0.080 |
0.112 |
|
12 |
100 mg |
29.6 |
95.5 |
28.3 |
1.3 |
0.070 |
0.103 |
|
13 |
200 mg |
56.4 |
99.9 |
56.4 |
0 |
0.166 |
0.197 |
|
14 |
400 mg |
71.9 |
99.5 |
71.5 |
0.4 |
0.253 |
0.267 |
|
Comparison of the composite with pristine phase samples |
|||||||
|
15 |
TiO 2 |
38.1 |
99.9 |
38.1 |
0 |
0.096 |
0.145 |
|
16 |
PTI |
76.8 |
87.3 |
67.0 |
9.8 |
0.270 |
0.251 |
Physicochemical characteristics of TiO 2 @PTI composite. Figures 1a,b depict microphotographs where photocatalyst is represented by nanostructured titanium dioxide grains that envelop the surface of poly(triazine imide) aggregates. Nanograins are 200–300 nm in length and 60–80 nm in width.

Fig. 1. SEM images of composite TiO 2 @PTI sample (a) x2000 (a) x50000
X-ray diffraction (Fig. 2a) was used to characterize the TiO2@PTI sample. The composite’s pXRD profile exhibits reflexes of two phases. Anatase phase (JCPDS Card no. 21-1272) is manifested by reflexes at 25.2°, 37.7°, 47.9°, 53.8°, 54.9° 2Ɵ degrees [15,16]. Poly(triazine imide) phase is represented by a series of peaks at 12.0°, 20.9° and 32.1° 2Ɵ degrees [17], respectively. TiO 2 @PTI shows a significant change in the reflex positions, such that the most intense one at 26.5° is shifted by 0.9° toward larger angles compared to pristine phase. It shows partial incorporation of titanium dioxide particles into the PTI matrix, changing of distance between layers and the formation of a composite [18].
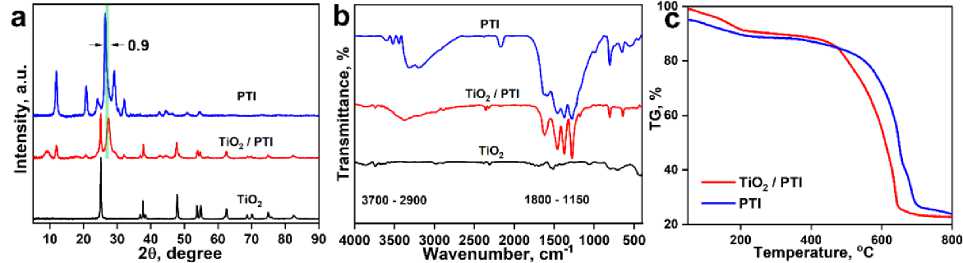
Fig. 2 (a) XRD patterns; (b) FTIR spectra of samples; (c) Comparison TG curves of samples
IR spectra of the samples are shown in Fig. 2b. Spectra of TiO 2 demonstrate broad bands near 3700–3400 cm–1, 1800–1450 cm–1, related to the vibrations of OH groups and 400–900 cm–1, corresponding to vibrations of the Ti–O–Ti lattice [19]. The composite’s absorption bands related to TiO 2 are not visible because of their low intensity compared to the vibrations of the organic fragments of PTI. Absorption bands of poly(triazine imide) and composites in the “fingerprint region” up to 1800 cm–1 exhibit same profile. The peak at 808 cm–1 is the “breathing mode” of triazine units, bands at 1800–1150 cm–1 correspond to the stretching vibrations of s-triazine units [20]. The differences in the spectra of TiO 2 @PTI and pristine PTI is the absence of a peak at 2170 cm–1 related to vibrations of cyano groups, as well as a narrowed set of bands at 1800–1150 cm–1. Their hydrolysis of cyano fragment at alkaline conditions could be one of the explanation.
Thermal analysis of the TiO 2 @PTI are shown in Fig. 2c. Three main stages can be distinguished on the TG curves. The first stage (up to 200 °C) is physisorbed water desorption. The second stage is a partial oxidation of organic substances (up to 600 °C). Third stage is combustion of PTI (higher 600 °C). The significant difference in the combustion temperature of the PTI phase resembles the low-temperature shift in the decomposition of the g-C 3 N 4 phase in its composites [21]. With this thermal behavior of the synthesized sample, we assume the composite is formed by close contact between phases.
UV spectroscopy studies of the samples (Fig. 3 a,b) revealed the several adsorption maxima composites in the composite material, which indicates the presence of several band gaps [22, 23]. The optical band gap was calculated from the linear approximation of the largest straight segment of the spectrum after the Kubelka-Munk transformation [24]. The calculated main band gap of the composite was found to be 2.11 eV, which was a significant drop, compared to pristine PTI (2.85 eV) and TiO2 (3.25 eV).
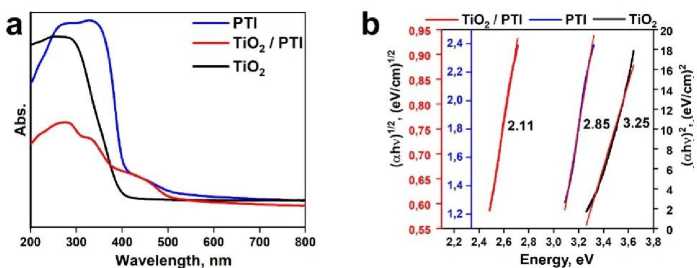
Fig. 3 (a) UV-vis spectra of PTI, TiO 2 @PTI, TiO 2 ; (b) Optical band gap of samples
Practical applicability of the optimal TiO 2 @PTI photocatalyst was examined at prolonged irradiation. The complete conversion was achieved after 10 hours of irradiation, providing with a good selectivity of 94% Fig. 4a. The reaction is described by a pseudo-first-order kinetic model, as shown in Fig. 4b, in agreement with previous reports about heterogeneous photocatalysts. Figure 4c shows a comparison of chromatograms of commercial BA and the reaction mixture after photocatalysis (at 9.8 min) using a TiO2@PTI composite. The chromatogram of the reaction mixture shows benzoic acid (8.1 min), but the peak area is insignificant.
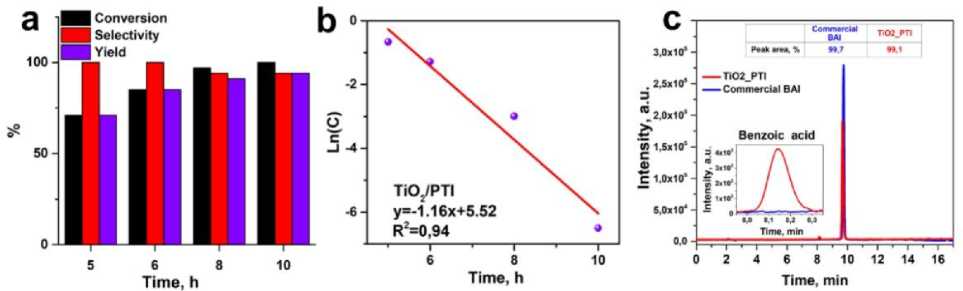
Fig. 4. (a) Kinetics of BA photooxidation using TiO 2 @PTI; (b) linear fit of the quasi-first-order kinetic model;
(c) HPLC chromatograms of commercial BAl and reaction mixture using TiO 2 @PTI sample
Scavenger test (Table 2) revealed that p-Benzoquinone diminished selectivity. It speaks for active O 2 • radical involvement in the selective oxidation. Application of methanol alcohol used to “trap” OH• radicals showed a decrease in conversion by 2 times, showing their major role in the reaction [25]. Based on obtained data, we hypothesize that the reaction follows a complex mechanism: exposure of the TiO 2 @PTI material with light causes the formation of a pair of photoseparated charges (an electron, a “hole”). An electron, interacting with O2, forms a superoxide radical. “Hole” reacts with BA, forms a BA radical. Further, the resulting superoxide radical and BA radical react to form BAl [25]. Reaction with sun light showed 24,1% conversion.
Table 2
Influence of scavengers and solar irradiation on photocatalytic oxidation of benzyl alcohol
|
Entry |
Scavenger |
BA conver., % |
Selectitvity to BAl, % |
BAl yield, % |
Other products, % |
Pseudo 1st order cons., h–1 |
AQE, % |
|
TiO 2 @PTI (pure) |
71.9 |
99.5 |
71.5 |
0.4 |
0.253 |
0.267 |
|
|
1 |
p-Benzoquinone |
61.9 |
80.9 |
50.0 |
11.9 |
0.193 |
0.186 |
|
2 |
MeOH |
32.0 |
99.9 |
32.0 |
0 |
0.077 |
0.120 |
Conclusions
For the first time, a composite nanomaterial based on titanium dioxide and poly(triazine imide) was used for the photocatalytic synthesis of BA. Photocatalytic properties were optimized by screening hydrothermal conditions, providing material with improved selectivity. Major factor influencing photoactivity of the resulting composite was the starting ratio of components and the temperature of the synthesis. The photocatalyst comprised discrete 200–300 nm long and 60–80 nm wide grains. Strong interaction between phases was confirmed with pXRD, thermal analysis and UV-spectroscopy, creating a heterojunction for widened adsorption spectrum. The photocatalyst showed the highest value of conversion (100%) and selectivity (94%).
Список литературы Developing nanostructured composite of titanium dioxide and poly(triazine imide) for selective photooxidation
- Belousov A.S., Suleimanov E.V. // G. Ch. The R. S. of Ch., 2021. V. 23, No 17. P. 6172. DOI: 10.1039/D1GC01690C.
- Fazli H., Akkol Ç., Osmanogullari S. et al. // J. Organomet. Chem. Elsevier, 2023. V. 983. P. 122553. DOI: 10.1016/j.jorganchem.2022.122553.
- Song H., Liu Z., Wang Y. et al. // Green Energy Environ., 2019. V. 4, No 3. P. 278. DOI: 10.1016/J.GEE.2018.09.001.
- Bao X., Lv X., Wang Z. et al. // Int. J. Hyd. En. P., 2021. V. 46, No 76. P. 37782. DOI: 10.1016/j.ijhydene.2021.09.052.
- Pomilla F., Garcia-Lopez E, Marci G. et al. // M. T. Sustain., 2021. V. 13. P. 100071. DOI: 10.1016/j.mtsust.2021.100071.
- Morozov R., Golovin M., Uchaev D. et al. // JCSS., 2021. V. 133, No 133. P. 1. DOI: 10.1007/S12039-021-01999.
- Thomas A., Fischer A., Goettmann F. et al. // J. Mater. Chem. The Royal S. of Chem., 2008. V. 18, No. 41. P. 4893-4908. DOI: 10.1039/B800274F.
- Tay Q., Wang X., Zhao X. et al. // Journal of Catalysis, 2016. V. 342. P. 55. DOI: 10.1016/j.jcat.2016.07.007.
- Marschall R. // Adv. F. Mat. John Wiley & Sons, Ltd, 2014. V. 24, No 17. P. 2421. DOI: 10.1002/ADFM.201303214.
- Baca M., Kukulka W., Cendrowski K. et al. // C.SusChem.2019. V. 12, No 3. P. 612. DOI: 10.1002/cssc.201801642.
- Yan H., Yang H. // J. Alloys Compd. Elsevier, 2011. V. 509, No 4. P. L26. DOI: 10.1016/j.jallcom.2010.09.201.
- Zarate R., Fuentes S., Wiff J. et al. // J. Phys. Ch. Solids. 2007. V. 68, No 4. P. 628. DOI: 10.1016/j.jpcs.2007.02.011.
- El-Akaad S., Morozov R., Golovin M. et al. // Talanta. 2022. V. 238. P. 123025. DOI: 10.1016/j.talanta.2021.123025.
- Horvath-Bordon E, Kroke E, Svoboda I. et al. // DT. The RS. Ch., 2004. No 22. P. 3900. DOI: 10.1039/B412517G.
- Theivasanthi T., Alagar M. // Chemical Physics, 2013. arXiv:1307.1091 https://arxiv.org/abs/ 1307.1091v1.
- Wei X, Zhu G, Fang J. et al. // Int. J. P. J. Wiley & Sons, Ltd, 2013. V. 2013, No 1. P. 726872. DOI: 10.1155/2013/726872.
- Ham Y., Maeda K., Cha D.// Chem. - An Asian J. J. W. & Sons, Ltd, 2013. V. 8, No 1. P. 218. DOI: 10.1002/ASIA.201200781.
- Yan X., Ning G., Zhao P. // MDPI., 2019. V. 9, No 1. P. 55. DOI:10.3390/CATAL9010055.
- Zhang B., Wang Q., Zhuang J. et al. // J. Photochem. Photobiol. A Chem. 2018. V. 362. P. 1. DOI: https://DOI.org/10.1016/jjphotochem.2018.05.009.
- Zhu Z, Pan H., Gong J. et al. // Appl. Catal. B Env,. Elsevier, 2018. V. 232. P. 19. DOI: 10.1016/j.apcatb.2018.03.035.
- Tseng I., Sung Y., Chang P. et al. // Polymers (Basel). 2019. V. 11, No 1. P. 1. DOI: 10.3390/polym11010146.
- Li J., Liu Y., Li H. et al. // JPP. A Chem., 2016. V. 317. P. 151. DOI: 10.1016/J.JPHOTOCHEM.2015.11.008.
- Wang C., Zhu W., Xu Y. et al. // Ceram. Int. 2014. V. 40, No 8. P. 11627. DOI: 10.1016/J.CERAMINT.2014.03.156
- Valencia S., Marin J.M., Restrepo G. // TOM. Sc. J., 2010. V. 4, No 2. P. 9. DOI: 10.2174/1874088X01004020009.


