Development and Validation of a Simple HPLC PDA Method for the Simultaneous Analysis of 13- Docosenamide, Squalene and n-Tetracosanol-1 from the Leaf Extracts of Wagatea spicata
Автор: Girish Nandini, Vaidya Vikas
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.16, 2020 года.
Бесплатный доступ
Wagatea spicata (Dalzell) Wight [Moullava spicata (Dalzell) Nicolson] exhibits a diverse concentration of biologically active constituents such as Lupeol, Bergenin, Stigmasterol, Friedelin, n-Hexadecanoic acid, Palmitic acid, Gamma sitosterol,13docosenamide, squalene and n-Tetracosanol-1. Present work focuses on development and validation of a precise, accurate and reproducible HPLC method for simultaneous quantification of three pharmacologically active phytochemical markers 13docosenamide, squalene and n-Tetracosanol-1 from the aerial parts of Wagatea spicata.
HPLC, 13-docosenamide, squalene, n-Tetracosanol-1, simultaneous, method development, method validation
Короткий адрес: https://sciup.org/143173839
IDR: 143173839
Текст научной статьи Development and Validation of a Simple HPLC PDA Method for the Simultaneous Analysis of 13- Docosenamide, Squalene and n-Tetracosanol-1 from the Leaf Extracts of Wagatea spicata
Traditional knowledge of India is gaining increasing acceptance all over the world for its therapeutic efficacy and safety (Chaudhary and Singh, 2011). However, only a small percentage of the entire treasure has been explored and there is a lot more to be put forth. To meet this objective, it is essential that the ethnomedicinal plants of Indian origin are scientifically studied for their known medicinal benefits and documented. Development of a formulation from such plants will also require extensive standardization for establishing the quality and/or efficacy of individual plant components as well as raw material or as a final product component. (Nandini et al. , 2017)
With the above objective in mind, the isolation of the compounds 13docosenamide, squalene and n-Tetracosanol-1 has been previously performed using HPTLC and the structural elucidation was performed with the help of FTIR, NMR and GCMS techniques (Surange and Deokule, 1986; Nandini and Vikas, 2019). Further, it was felt worthwhile to develop simultaneous methods for the quantitative analysis of the isolated components.
Thus, the present work explains the development of a simple, sensitive and accurate high-performance liquid chromatographic method for the simultaneous determination of 13docosenamide, squalene and n-Tetracosanol-1 from the leaf extracts of the plant Wagatea spicata .
MATERIALS AND METHODS
Chemicals and materials
HPLC grade Methanol, Ethanol was procured from E. Merck, Mumbai, India. Reference standards of 13 Docosenamide (Purity>95%), Squalene (Purity>95%) and n-Tetracosanol-1 also called as Lignoceryl alcohol (Purity>95%) were purchased from Sigma-Aldrich (Aldrich Division; Steinheim, Germany).
Plant Material
Wagatea spicata fresh plant was collected from the field area of Kankeshwar, Alibaug, District- Raigad, and Maharashtra, India in the month of November 2014; and the herbarium specimens were identified and authenticated by Botanical Survey of India, Pune.
Sample preparation
1 g of fine leaf powder in 10ml of ethanol was subjected to accelerated maceration by ultrasonication for 30 minutes, followed by overnight steady state extraction. After filtration using syringe filter (pore size 0.2 microns), the clear extract was used for HPLC analysis.
Preparation of standard solution(s)
Individual stock solutions of n-Tetracosanol-1, 13 docosenamide and Squalene (1000 µg/ mL) were prepared in ethanol. 1000 µg/mL stock solution of standard mixture was also prepared in ethanol.
RESULTS AND DISCUSSION
Optimization of the Chromatography
Initial trial experiments were conducted to select a suitable mobile phase for accurate analysis of the standards and HPLC grade acetonitrile containing formic acid (0.1%) in isocratic mode was finalized as the best for the separation of the three analytes 13 docosenamide, squalene and n-tetracosanol-1.
The separation of analytes of interest was also evaluated at different flow rates (0.6-1mL/min). Finally the flow rate was optimized to 1 mL/min to avoid the interference of solvent peaks. Considering the complexity of herbal samples, the total run time for the method was determined to be 35 mins.
The wavelength of 202 nm was selected for the detection of analytes eluting out from the column as phytoconstituents under study demonstrated the maximum absorption at the specified wavelength.
The optimized chromatographic conditions chromatograms and spectra of individual standards standard mixture and sample extract as obtained under the same chromatographic conditions are depicted in Table 1 and Figures 1-7 respectively.
Method Validation
The developed method was validated as per tripartite ICH guidelines (ICH Harmonised Tripartite Guideline, 2005).
Selectivity: As shown in figures 7A and 7B, there was no interference observed from diluent blank (ethanol) and mobile phase overlapping with the retention times of 13 docosenamide, squalene and n-tetracosanol-1. Hence the method is selective.
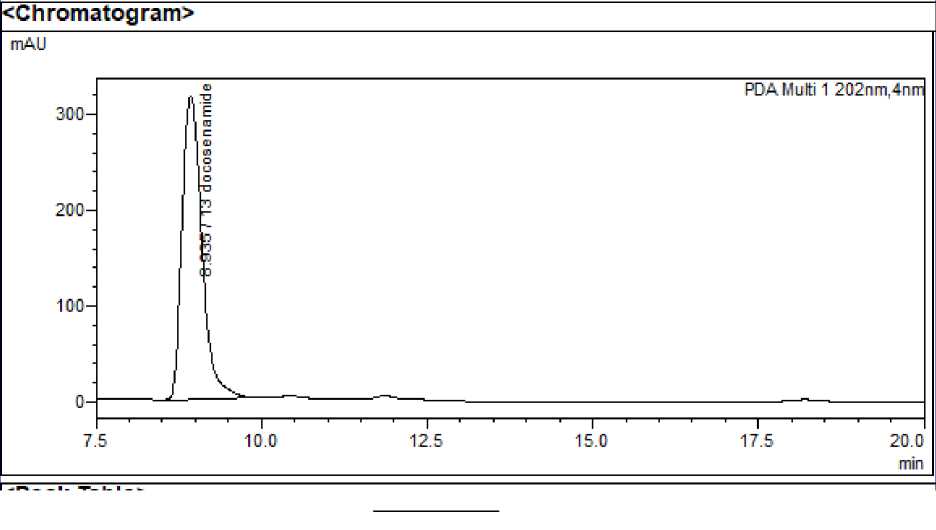
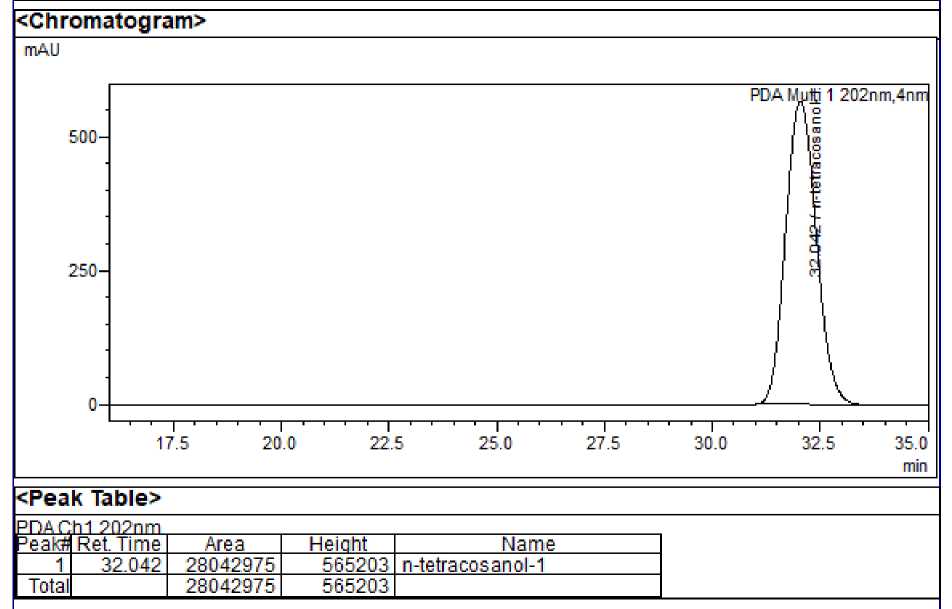
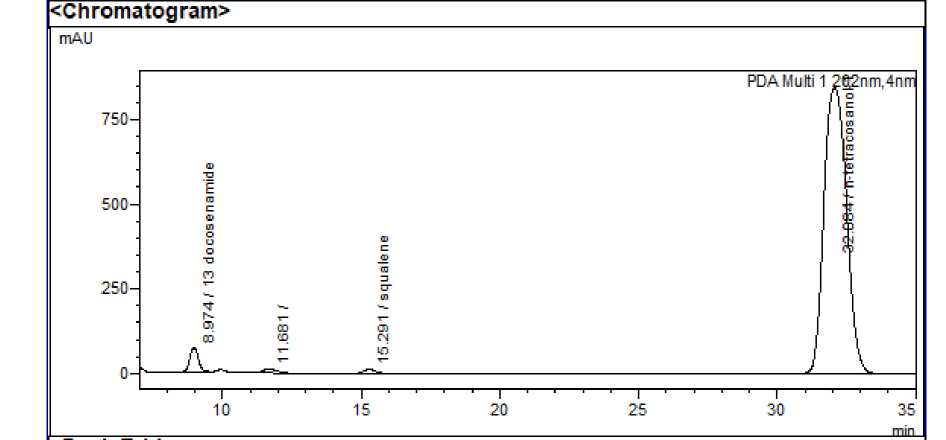
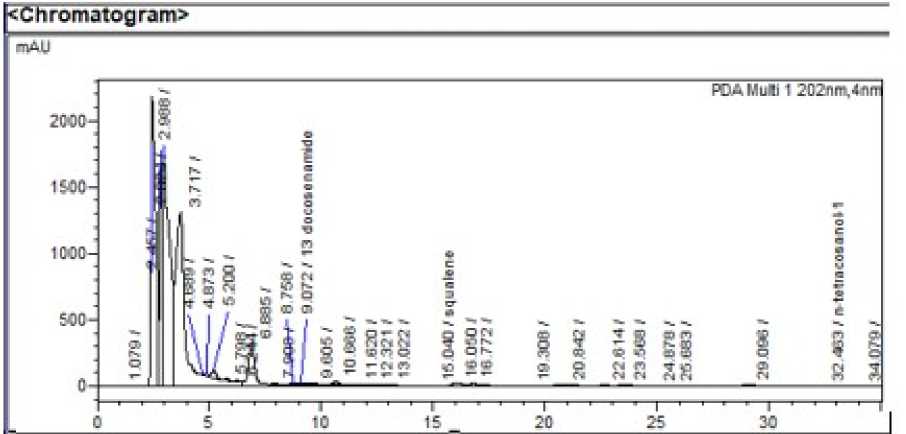
System suitability : The RSD values for area and retention time of 13 docosenamide, squalene and n-tetracosanol-1 and were found to be <2% indicating that the system was suitable to carry out further analysis. Squalene, 13 docosenamide and n-Tetracosanol-1 were detected at 15.298, 8.963 and 32.054 minutes respectively.
Linearity : The method was found to be linear from 50-300 μg/mL for 13 docosenamide, 100-300 μg/mL for squalene, 5-300 μg/ml for n-Tetracosanol-1. The correlation coefficient was found to be ≥0.99 for all the three components.
Sensitivity: Sensitivity of the method was affirmed in terms of LOD and LOQ for 13 docosenamide squalene and n-Tetracosanol-1.
The value of limit of detection was found to be 50 µg/mL for squalene and 13 docosenamide and while it was 5 µg/mL for n-tetracosanol-1, whereas the limit of quantification was found to be 100 µg/mL for squalene 50 µg/mL for 13 docosenamide and 5 µg/mL for n-Tetracosanol-1 (Griffiths, 2003; Vuppugalla et al ., 2003 Lu et al. , 2004; Wichitnithad et al , 2009; Le et al. , 2019).
Precision : In the repeatability study, intra-day and inter-day precision of the HPLC method were investigated using replicate injection ( n =3) of quality control samples of all the three standards. The developed method was found to be precise with RSD<2%.
Robustness: As inferred from the %RSD values of robustness testing results, it can be said that the proposed method was not influenced by slight change in the wavelength of analysis. Minor change in flow rate and mobile phase composition affected the separation of the three analytes. However, the simplicity of the method increases the robustness by minimizing mobile phase alterations.
Stability: Stability studies showed that the components were found stable in the mixture for at least 24.0 h at room temperature and 48 hours at 2-8°C of storage condition. The individual stock solutions however, were stable for up to 72 hours under both the conditions.
Assay: The percentage of n-tetracosanol-1, 13 docosenamide and Squalene in the leaf extracts of Wagatea spicata extract was found to be 0.009% 2.54% and 1.07% respectively. The method is sensitive and selective for the aforementioned phytoconstituents in presence of other phytochemicals present in the extract.
Recovery: The recovery values for all the three components were within acceptable limits (80.0 to 120.0%). This indicated that the method was reliable and accurate.
Thus, the proposed HPLC method was found to be suitable for qualitative and simultaneous quantitative analysis of 13 docosenamide, squalene and n-Tetracosanol-1 in the ethanolic extract of Wagatea spicata . The summary of validation is charted in Table 2.
Table 1: Optimised Chromatographic Conditions.
|
Parameter |
Description |
|
Instrument |
HPLC- Prominence-i, LC-2030C 3D Plus Liquid chromatograph |
|
Pump |
LC 2030 pump |
|
Injector |
LC 2030, Autosampler |
|
Injection volume |
50ul |
|
Column oven |
LC 2030 oven,40oC |
|
Column |
Shimadzu, C18 250mm X 4.6 mm, 5µm |
|
Mobile Phase |
HPLC grade acetonitrile containing formic acid (0.1%) |
|
Flow Rate |
1mL/min |
|
Detector |
LC 2030/2040 PDA |
|
Detection Wavelength |
202nm |
|
3eakS |
Ret. Time |
Area |
Heiqht |
Name |
|
1 |
3.693 |
7896636 |
270958 |
|
|
2 |
8.935 |
6632864 |
316005 |
13 docosenamide |
A
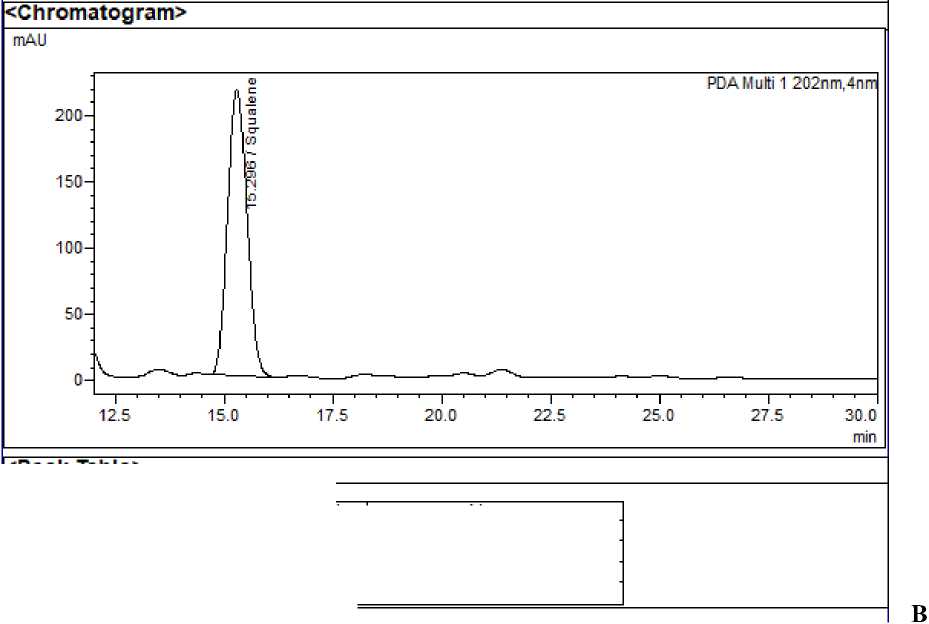
Figure 1: A - HPLC chromatogram of standard 13 docosenamide; B - HPLC chromatogram of standard squalene;
<Реак Table>
РПАСМ ?П?пт
|
=>63^ |
Ret. Time |
Area |
Heiaht |
Name |
|
1 |
2.837 |
2954943 |
372324 |
|
|
2 |
3.004 |
6410179 |
346734 |
|
|
3 |
3.696 |
4516941 |
179659 |
|
|
4 |
15.296 |
6679980 |
215758 |
Squalene |
A
' ^ПАПМ 909nm
|
эеакй |
Ret. Time |
Area |
Heiaht |
Name |
|
1 |
8.974 |
1684717 |
72330 |
13 docosenamide |
|
2 |
11.681 |
438963 |
11816 |
|
|
3 |
15.291 |
394912 |
12731 |
saualene |
|
4 |
32.084 |
47666422 |
844539 |
n-tetracosanol-1 |
|
Total |
50185014 |
941416 |
Figure 2: A - HPLC chromatogram of standard n-tetracosanol-1 ; B - HPLC chromatogram of standard mixture
B
|
рпд гы |
Fedc^ble Я№™ |
|||
|
РУ- |
Ret. Г'"? |
Am |
НчеЬл |
__ Name __ |
|
1 |
1.079 |
3333 |
352 |
|
|
2 |
2.457 |
31-047203 |
21305 56 |
|
|
3 |
2.324 |
1154515" |
17-525- |
|
|
4 |
2.933 3.717 |
39405051 423043"9 |
____________ 1 "25593 13-04431 |
— |
|
5 |
4.539 |
35499 |
10424 |
|
|
4.373 |
140736 |
14524 |
||
|
в |
5 200 |
359633 |
53472 |
|
|
g |
5.-93 |
139010 |
13590 |
|
|
10 |
5.244 |
85337 |
7453 |
|
|
11 |
5.335 |
3303191 |
317631 |
|
|
12 |
7908 |
55153 |
5545 |
|
|
13 |
3.753 |
114570 |
7643 |
|
|
14 |
9 072 |
2-9125 |
12353 |
13 Дocc«ramida |
|
._______Li. |
9.605 |
-10607 |
17034 |
|
|
1 16 |
10.666 |
4353 36 |
22043 |
|
|
17 |
11620 |
215341 |
5937 |
|
|
IB |
12.321 |
46645 |
1423 |
|
|
19 |
13.022 |
63122 |
2236 |
|
|
20 |
15.040 |
6917 |
333 |
mraffw__________________ |
|
21 |
16.050 |
632275 |
24801 |
|
|
22 |
15.772 |
615166 |
14355 |
|
|
23 |
19 30В |
27150 |
"45 |
|
|
24 |
20.342 |
49-251 |
10234 |
|
|
25 |
_____________ 22.51- |
128474 |
4120 |
|
|
26 |
_____________ 23.553 |
298015 |
341 |
|
|
27 |
24.373 |
54335 |
1532 |
|
|
23 |
25.633 |
54404 |
1441 |
|
|
29 |
29.096 |
______________ 295313. |
5953 |
|
|
30 |
32.453 |
47626 |
1039 |
ibtetracosmol-l |
|
31 |
34.079 |
171375 |
3305 |
|
|
Totil |
139096971 |
755-263 |
||
Figure 3: HPLC chromatogram of leaf extract of Wagatea spicata
A
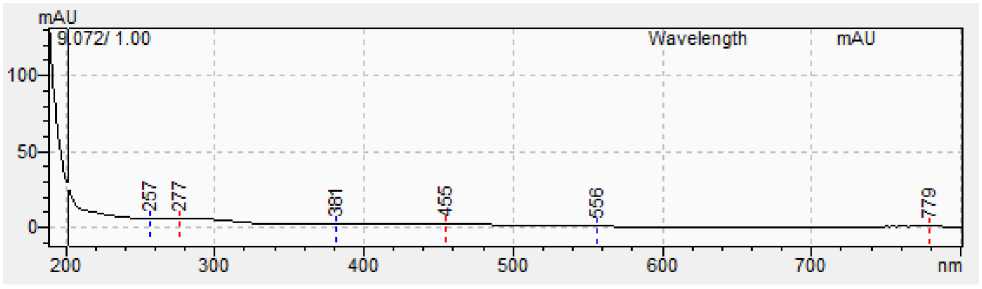
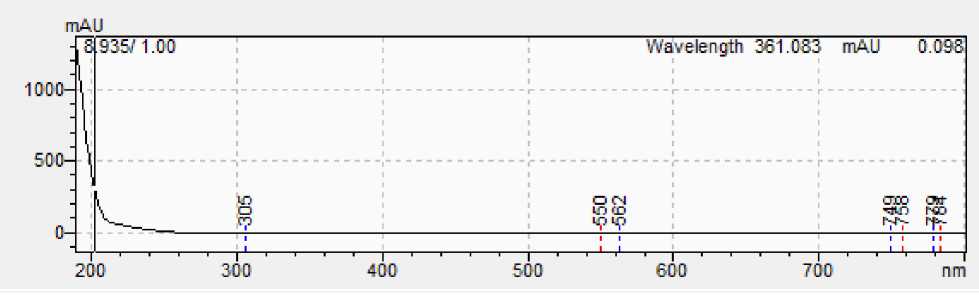
Figure 4: A - Spectrum of 13 docosenamide standard; B - Spectrum of 13 docosenamide from sample.
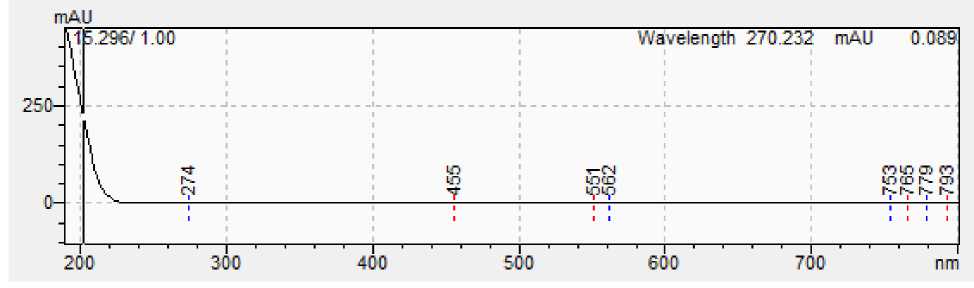
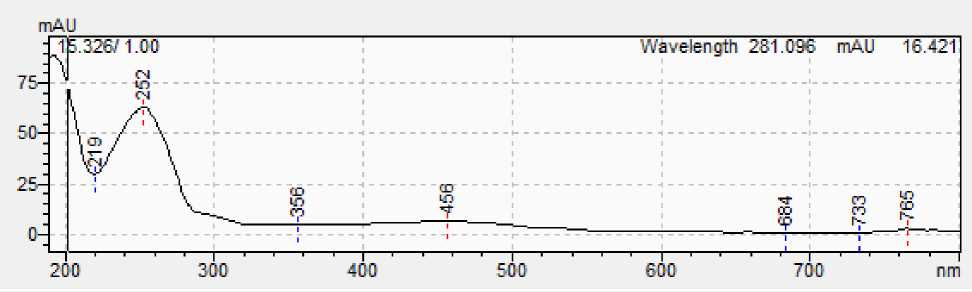
Figure 5: A - Spectrum of squalene standard; B - Spectrum of squalene from sample.
B
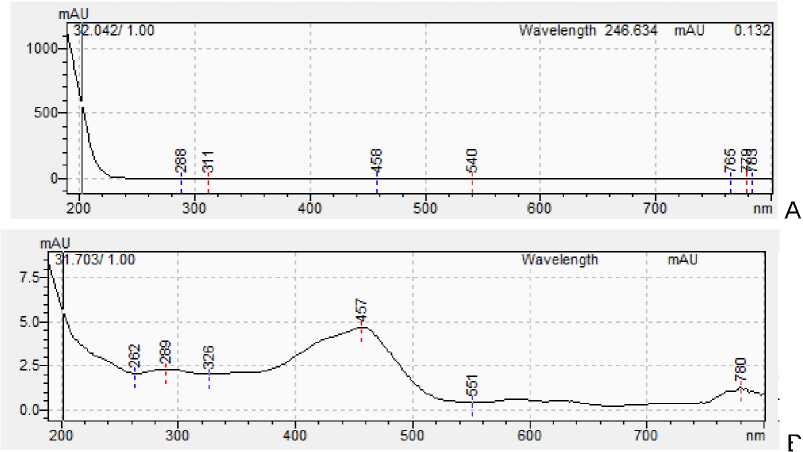
Figure 6: A - Spectrum of n-tetracosanol-1 standard; B - Spectrum of n-tetracosanol-1 from sample.
1243806 223511 184211 155756 110875
Peak#
Ret. Time
B
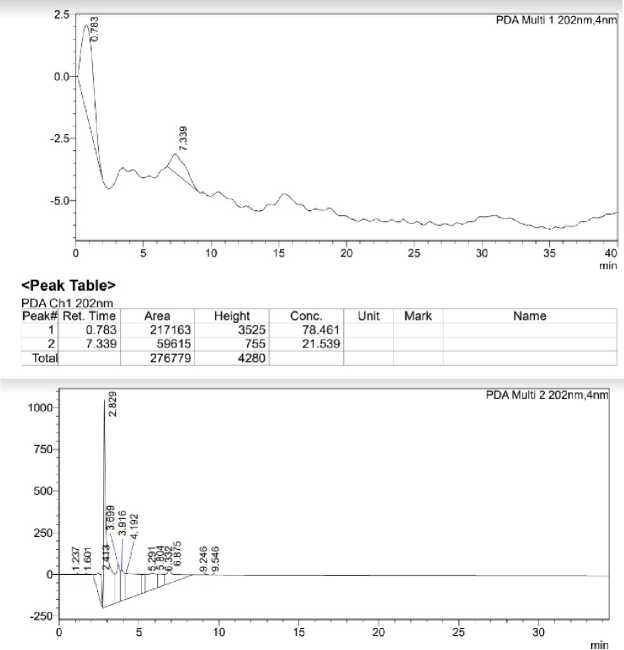
Figure 7: A - HPLC chromatogram of mobile phase; B - HPLC chromatogram of ethanol blank .
PDA Ch2 202nm
1.601
2.413
2.829
3.699
3.916
4.192
5.291
5.804
6.332
6.875
9.246
9.546
12 "
Total
Table 2: Summary of Validation
|
Parameter |
13 docosenamide |
Squalene |
n-Tetracosanol-1 |
|
System Suitability(%RSD) |
Area = 0.696 Rt = 0.52 |
Area=1.476 Rt = 0.589 |
Area = 0.393 Rt = 0.585 |
|
Specificity and Robustness |
Specific and Robust |
Specific and Robust |
Specific and Robust |
|
Precision(%RSD) Intraday,200(μg/ml) |
0.779 |
0.255 |
1.558 |
|
Precision(%RSD) Interday, 200(μg/ml) |
0.094 |
0.709 |
0.914 |
|
LOD |
50 μg/ml |
50 μg/ml |
5 μg/ml |
|
LOQ |
50 μg/ml |
100 μg/ml |
5 μg/ml |
|
Linearity |
50-300 μg/ml |
100-300 μg/ml |
5-300 μg/ml |
|
Assay |
2.54% |
1.075% |
0.009% |
|
Recovery |
100.894% |
97.550% |
100.787% |
CONCLUSION
The current work provides a simple, precise, accurate and reproducible method for the qualitative and quantitative analysis of 13 docosenamide, squalene and n-Tetracosanol-1 from Wagatea spicata . The developed method can be used as a tool to asses phytochemical variation caused due to diverse geographical, climatic genotypic factors. It can also be used as a quality control method for the plant and formulations containing Wagatea spicata .
ACKNOWLEDGEMENTS
The authors acknowledge the support received from Rashtriya Uchchatar Shiksha Abhiyan (RUSA) for the HPLC facility at the Department of Bioanalytical Sciences, Ramnarain Ruia Autonomous College.
Список литературы Development and Validation of a Simple HPLC PDA Method for the Simultaneous Analysis of 13- Docosenamide, Squalene and n-Tetracosanol-1 from the Leaf Extracts of Wagatea spicata
- Chaudhary, A., & Singh, N. (2011). Contribution of world health organization in the global acceptance of Ayurveda. Journal of Ayurveda and integrative medicine, 2(4), 179–186.
- Griffiths W.J. (2003) Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids. Mass Spectrom Rev. 22 (2), 81-152.
- ICH Harmonised Tripartite Guideline. (2005) Validation of Analytical Procedures: Text and Methodology Q2 (R1).
- Le, T., Phung, T. H., & Le, D. C. (2019). Development and Validation of an HPLC Method for Simultaneous Assay of Potassium Guaiacolsulfonate and Sodium Benzoate in Pediatric Oral Powder. Journal of analytical methods in chemistry, 2019, 6143061.
- Lu, HT., Jiang, Y. & Chen, F. (2004) Determination of Squalene Using High-Performance Liquid Chromatography with Diode Array Detection Chromatographia 59, 367.
- Nandini G & Vaidya V. (2019) Isolation of active constituents from Wagatea spicata using preparative HPTLC and structural elucidation using FTIR and NMR and GCMS techniques Journal of Pharmacognosy and Phytochemistry, 8(4), 805-810.
- Nandini, G., Palekar, S., Vaidya, V. & Shinde, M., (2017) Phytochemical profiling of wagatea spicata using gc-ms to reveal the pharmacological significance, International Journal of Current Research, 9(12), 62197-62204
- Surange S.R. & Deokule S. S., (1986) Pharmacognistic studies on Wagatea spicata Dalzell, Ancient Science of Life, VI (4), 238 – 243.
- Vuppugalla R, Agarwal V, Khan MA. (2003) A simple HPLC method for the simultaneous analysis of insulin and ovomucoid. Pharmazie. 58(11), 793-795.
- Wichitnithad W., Jongaroonngamsang N., Pummangura S., Rojsitthisak P. (2009) A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts, Phytochemical Analysis, 20(4), 314-319


