Development of Beta zeolite template free synthesis method
Автор: Monzharenko Margarita, Mikhailov Stepan, Brovko Roman, Doluda Valentin
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Химические науки
Статья в выпуске: 9 т.8, 2022 года.
Бесплатный доступ
The development of new methods for the synthesis of zeolites and the improvement of existing methods for the synthesis of zeolites in order to reduce their cost and improve the quality of the final product is an important task of modern chemistry and chemical technology. Existing methods for the synthesis of zeolites, including hydrothermal synthesis methods, are extremely time-consuming and expensive due to the widespread use of structure-forming agents. At the same time, traditional methods for solving the above problems by increasing the rate of hydrothermal synthesis by increasing the temperature are not optimal, due to the possibility of the formation of additional inorganic phases at high temperatures. Zeolite BETA is one of the most commonly used aluminosilicates both as a catalyst and as a material for creating inorganic membranes for various purposes, while the synthesis of the above zeolite is extremely long and takes from 5 to 10 days. The presented article presents the results of the development of a method for obtaining BETA zeolite by a hydrothermal method under the influence of ultrasound. A study of the influence of temperature and the ratio of silicon to aluminum in the reaction medium on the yield of zeolite is also given. It has been established that an increase in the temperature of hydrothermal synthesis from 80° C to 120 °C contributes to an increase in the yield of zeolite up to 97%. An increase in the molar ratio of silicon to aluminum, on the contrary, leads to a decrease in the zeolite yield to 15%. It is shown that the synthesis under conditions of ultrasonic treatment with a power of 6 mW/cm2 contributes to an increase in the yield of the target product by 2-4 times, and an increase in the rate of formation of BETA zeolite by 2-8 times.
Zeolites, synthesis, acidity, chemosorption
Короткий адрес: https://sciup.org/14125323
IDR: 14125323 | УДК: 544.47 | DOI: 10.33619/2414-2948/82/04
Текст научной статьи Development of Beta zeolite template free synthesis method
Бюллетень науки и практики / Bulletin of Science and Practice
High interest of scientific community and industry to zeolites is determined by their unique sorption, separation and catalytic properties [1–5]. Huge micropore surface and variety of possible pore diameters allow to apply zeolites as membrane materials for gas and ions separation. Among of different zeolites BETA zeolite attract considerable attention because of high internal surface area and volume. Zeolite BETA (Figure 1) has the structural formula |Na+ 7 | [Al 7 Si 57 O 128 ] and has tetragonal symmetry with unit cell parameters a = 12.6610 Å, b=12.6610 Å, c=24.4060 Å [6–8].
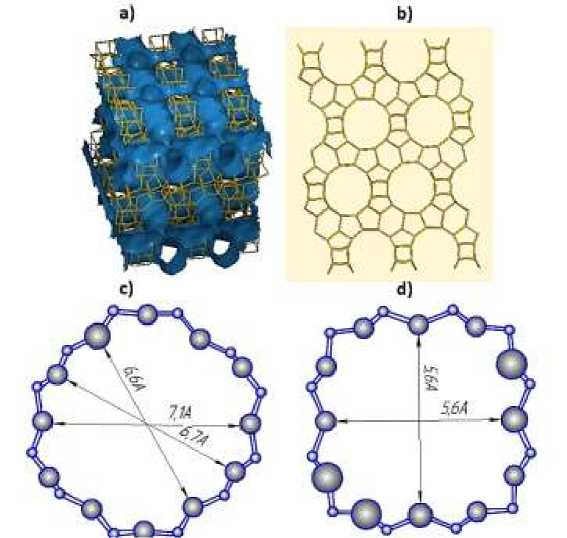
Figure 1. a) Channel system of BETA zeolite, b) pore structure of BETA zeolite c) pore dimensions along (100) axis, d) pore dimensions along (001) axis
The pores are represented by an elliptical ring consist of 12 silicon atoms, the pore sizes are 5.6–7.1 Å in the longitudinal and transverse directions, the available pore volume is 20.52%, while the calculated surface area is 1220 m2/g. The pore size allows molecules with a hydraulic radius of 5.95 Å to diffuse through them. However, pores can be modified and used for small molecules separation [9–12]. The main problem of BETA zeolite application is high price of their synthesis due to application of structure directing agents and longtime of BETA zeolite synthesis. One possible way to solve this problem is to develop seeds induced template free synthetic method of BETA zeolite synthesis [8, 13, 14].
Materials and methods
BETA zeolite synthesis was provided using chemical grade sodium hydroxide, sodium aluminate, silica gel and seeds of BETA zeolites produced by HKC with purity not less than 99%. Distillate water was purified using DE-25 aqua distillation system. For BETA zeolite synthesis silica gel was milled in laboratory milling machine and classified thought laboratory sieve to obtained 10 μm silica gel particles.
For BETA zeolite synthesis solution of sodium hydroxide was obtained at ambient temperature, then milled silica gel was added to sodium hydroxide solution and mixed for one hour at 70 C for gel formation. After cooling of reaction gel to ambient temperature solution of sodium aluminate and one gram of BETA zeolite seeds were to formed gel. Reaction was carried out at 80, 100, and 1200C in autoclave reactor. Quantity if used reagent are shown in (Table). After finishing of hydrothermal synthesis reaction media was centrifugated and zeolite was separated from reaction solution. Then zeolite was washed with distillate water and dried at 1400C. Synthesized samples have theoretical Si/Al rate from 15 to 1500 (Table). BETA zeolite silica to aluminum ratio was determined gravimetrically by addition hydrogen fluoride acid to BETA zeolite followed by silica fluoride formation and its evaporation. Yield of synthesized zeolite was made by dividing of dried solid weight on theoretical weight of BETA zeolite sample.
Results and discussions
Increasing of reaction media Si/Al mole fraction from 15 to 1500 results in decrease of solid zeolite yield (Table), that can be explained by low reaction rate of silica phase formation on zeolite surface.
Table
REACTION CONDITIONS FOR AND NA-ZSM-5 SYNTHESIS
|
Sample |
NaOH, mol fraction |
NaAlO 2 , mol fraction |
SiO 2 , mol fraction |
H 2 O, mol fraction |
Zeolite yield, % |
|
1 |
2 |
2 |
30 |
360 |
74.1 |
|
2 |
2 |
1 |
30 |
360 |
68.3 |
|
3 |
2 |
0.5 |
30 |
360 |
62.1 |
|
4 |
2 |
0.25 |
30 |
360 |
40.7 |
|
5 |
2 |
0.125 |
30 |
360 |
30.4 |
|
6 |
2 |
0.06 |
30 |
360 |
23.1 |
|
7 |
2 |
0.02 |
30 |
360 |
15.6 |
Temperature of hydrothermal synthesis is 100 0C, time of hydrothermal synthesis 120h
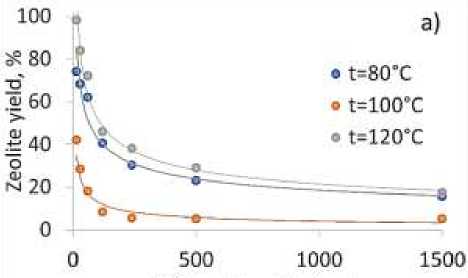
Increasing of hydrothermal reaction temperature from 80 C to 120 C. results in appropriate increase of BETA zeolite yield (Figure 2a), that can be explained by increasing of nucleation reaction rate and increasing of mass transfer under high temperature. Si/Al ratio in synthesized
BETA zeolite strongly depends on Si/Al ratio in reaction gel (Figure 2b). Increasing of Si/Al ratio in reaction gel from15 to 1500 results in increase of Si/Al ratio in BETA zeolite from 7 to 427. Difference between Si/Al ratio in reaction gel and BETA zeolite can be explained by reactions equilibrium during hydrothermal synthesis. Increase in reaction temperature (Figure 2b) results in appropriate increase of reactions rate there for Si/Al ratio in zeolite become closer to Si/Al rate in reaction gel. From the other side increase of reaction temperature higher than 1200C can result in formation of different phase of zeolite. For increasing of nucleation rate other methods should be applied. One of the possible solutions of this problem is application of ultrasonic irradiation during nucleation process. For this purpose, heating of reaction media was provided in ultrasonic laboratory bath under 800C and ultrasonic power density 0.6×10-2W/cm2.
Si/AI molar ratio in gel
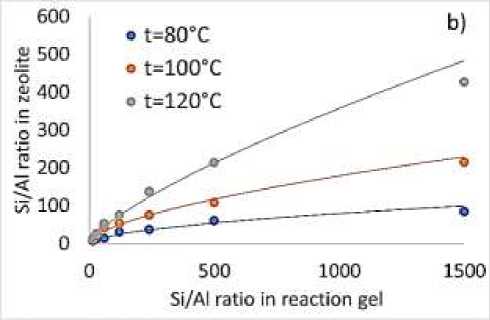
Figure 2. a) Yield for BETA zeolite for different Si/Al ratio under various reaction temperatures, b) obtained Si/Al ratio in BETA zeolite on Si/Al ratio in reaction gel for different temperatures
Application of ultrasound during BETA zeolite particles nucleation results in appropriate increase of BETA zeolite yield (Figure 3), that can be subscribed to increase in nucleation rate.
• with out sonication о with sonication
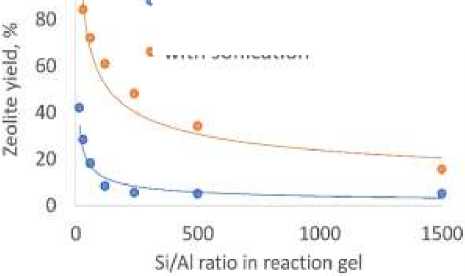
Figure 3. Sonication influence over BETA zeolite yield at 800C, hydrothermal treatment for 120 h
Calculated nucleation rate increase increased in 2–4 times compare to conventional method.
Conclusions
New and reliable template free method providing zeolite yield up to 97% for BETA zeolite synthesis using ultrasound sonication was discovered and tested. Developed ultrasound assisted method showed to increase nucleation rate in 2-4 times compare to conventional method. Study of reaction temperature influence on BETA zeolite yield showed appropriate increase of BETA zeolite yield from 15-20% to 85-97%. Increase of Si/Al ratio in reaction gel from15 to 1500 results in increase of Si/Al ratio in BETA zeolite from 7 to 427. Difference between Si/Al ratio in reaction gel and BETA zeolite can be explained by reactions equilibrium during hydrothermal synthesis.
The reported study was jointly funded by Russian Foundation for Basic Research (RFBR), Sirius University of Science and Technology, JSC Russian Railways and Educational Fund “Talent and success”, project number 20-38-51001.
Список литературы Development of Beta zeolite template free synthesis method
- Perez-Pariente, J., Martens, J. A., & Jacobs, P. A. (1988). Factors affecting the synthesis efficiency of zeolite BETA from aluminosilicate gels containing alkali and tetraethylammonium ions. Zeolites, 5(1), 46-53. https://doi.org/10.1016/S0144-2449(88)80029-0
- Benslama, R., Fraissard, J., Albizane, A., Fajula, F., & Figueras, F. (1988). An example of the technique of studying adsorbed xenon by 129Xe nmr: approximate determination of the internal void space of zeolite beta. Zeolites, 5(3), 196-198. https://doi.org/10.1016/S0144-2449(88)80307-5
- Ono, Y. (1990). Chapter II. 6 Recent Advances and Future Developments in Zeolite Catalysis. Studies in Surface Science and Catalysis, 54, 185-210. https://doi.org/10.1016/S0167-2991(08)60040-3
- Roland, E. (1989). Hindustrial production of zeolites. In Studies in Surface Science and Catalysis (Vol. 46, pp. 645-659). Elsevier. https://doi.org/10.1016/S0167-2991(08)61019-8
- Pérez-Pariente, J., Sanz, J., Fornés, V., & Corma, A. (1990). 29Si and 27Al MAS NMR study of zeolite P with different Si/Al Ratios. Journal of Catalysis, 124(1), 217-223. https://doi .org/10.1016/0021 -9517(90)90116-2
- Barrer, R. M. (1985). Synthesis of zeolites. In Studies in surface science and catalysis (Vol. 24, pp. 1-26). Elsevier. https://doi.org/10.1016/S0167-2991(08)65264-7
- Tomita, J., Elangovan, S. P., Itabashi, K., Chokkalingam, A., Fujinuma, H., Hao, Z., ... & Okubo, T. (2022). OSDA-free synthesis of zeolite beta: Broadening the methodology for a successful use of the product as a seed. Advanced Powder Technology, 33(9), 103741. https://doi.org/10.1016Zj.apt.2022.103741
- Chen, T., Gu, C., Ouyang, Y., Zhuang, L., Yao, Z., Zou, K., ... & Shu, X. (2022). Synthesis of high hydrothermal stability Beta zeolite with crosslinked starch and catalytic performance in catalytic cracking reaction. Fuel, 318, 123696. https://doi.org/10.10167j.fuel.2022.123696
- Cui, T. L., He, J. Y., Hu, M., Liu, C. S., & Du, M. (2020). Secondary template-free synthesis of hierarchical beta zeolite nanocrystals with tunable porosity and size. Microporous and MesoporousMaterials, 309, 110448. https://doi.org/10.1016/j.micromeso.2020.110448
- Yue, Y., Guo, X., Liu, T., Liu, H., Wang, T., Yuan, P., ... & Bao, X. (2020). Template free synthesis of hierarchical porous zeolite Beta with natural kaolin clay as alumina source. Microporous and Mesoporous Materials, 293, 109772. https://doi.org/10.1016/j.micromeso.2019.109772
- Zaykovskaya, A. O., Kumar, N., Kholkina, E. A., Li-Zhulanov, N. S., Maki-Arvela, P., Aho, A., ... & Murzin, D. Y. (2020). Synthesis and physico-chemical characterization of Beta zeolite catalysts: Evaluation of catalytic properties in Prins cyclization of (-)-isopulegol. Microporous and Mesoporous Materials, 302, 110236. https://doi.org/10.1016/j.micromeso.2020.110236
- Tang, L., Haw, K. G., Zhang, Y., Fang, Q., Qiu, S., & Valtchev, V. (2019). Fast and efficient synthesis of SSZ-13 by interzeolite conversion of Zeolite Beta and Zeolite L. Microporous and Mesoporous Materials, 280, 306-314. https://doi.org/10.1016/j.micromeso.2019.02.021
- Nakamura, T., Kamiya, Y., & Otomo, R. (2022). A rapid synthesis of Hf-Beta zeolite as highly active catalyst for Meerwein-Ponndorf-Verley reduction by controlling water content of precursor gel. Microporous and Mesoporous Materials, 333, 111743. https://doi.org/10.1016/j.micromeso.2022.111743
- Luo, D., Wang, Q., Fan, D., Yang, M., Fan, B., Cao, K., ... & Liu, Z. (2022). Hydrothermal synthesis of Siliceous Beta Zeolite by an inorganic cation-driven strategy and its crystallization mechanism. Microporous and Mesoporous Materials, 329, 111557. https://doi.org/10.1016/j.micromeso.2021.111557


