Differential responses of growth, metals accumulation, oxidative stress and osmo-compatible solutes in the halophyte Tamarix gallica under arsenic and aluminum stress alone or combined with salt
Автор: Sghaier Dhouha Belhadj
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.21, 2025 года.
Бесплатный доступ
Several studies have been investigated to determine the ability of plants to reduce heavy metals in polluted soils. Conducted within the strong halophyte Tamarix gallica , known for thriving under harsh environmental conditions, the major objective was to study the tolerance and accumulation capabilities of Tamarix gallica under Arsenic, Aluminium, and salt stress. Plants were exposed to different concentrations of Al and As alone (200, 500 and 800 µM), or added to NaCl (200 mM). The results evinced that both metals impaired the mature and expanation of T. gallica . Furthermore, the accumulation of the metals was dependent on the concentrations of the metals in the medium. Interestingly, when salt diminished As uptake, a rise in Al accumulation was noted. Besides, T. gallica demonstrated that APX was the predominant enzyme to scavenge excess ROS. Nonetheless, SOD and GPOX were responsive to the metal, demonstrating different behaviour in metal stress alone or when added with salt. Proline accumulated in a dose-dependent manner under Al and As stress, instead of lower or unchanged levels of glycine betaine. The application of NaCl led to a decrease in proline content but an increase in glycine betaine levels. Under combined stress, there was an augmentation in proline levels, while glycine betaine levels remained unchanged. This underscores T. gallica 's capacity to thrive under elevated concentrations of As/Al.
Heavy metals, salt addition, combined stress, enzymes activities, osmotic solutes
Короткий адрес: https://sciup.org/143184741
IDR: 143184741
Текст научной статьи Differential responses of growth, metals accumulation, oxidative stress and osmo-compatible solutes in the halophyte Tamarix gallica under arsenic and aluminum stress alone or combined with salt
Throughout their life cycle, plants confront various environmental stressors (Bhat et al., 2019). In recent decades, there has been a concerning escalation in environmental contamination by heavy metals (HM), recognized for their toxicity and persistence (Jamla et al., 2021). Furthermore, these metals are acknowledged as substantial obstacles to achieving optimal agricultural production on a global scale (Alsahli et al., 2021). Notably, Arsenic and Aluminium are among the heavy metals that merit particular attention in this context.
Arsenic, a toxic element, is commonly present in the environment and organisms (Ackova, 2018). Its introduction into terrestrial and aquatic environments is attributed to both natural processes and human activities (Jamla et al., 2021). To understand the effects of different As species, researchers have elucidated diverse physiological and biochemical parameters in different plant species (Dave et al., 2013). In fact, the phytotoxicity of arsenic to plants can vary based on concentration, soil properties and phosphorus content (Jamla et al., 2021). The influx of Arsenate into plants leads to damage to root membranes (Ackova, 2018). Elevated levels of arsenic can significantly impact the growth and development of halophytes (Dave et al., 2013).
Contrarily, Aluminium can play a beneficial role in promoting plant growth when present in small amounts, although not all plants require it (Rahman and Upadhyaya, 2021). The excessive use of ammonia fertilizers can lead to a decrease in soil pH, resulting in an increase in the concentration of several ions in the soil solution, like aluminum (Kar et al., 2021). The Al effect on plants can be identified as death of apical tissues, curly leaf, getting roots thickener and brown. Al may also produce callose and lignin deposits on root tips, causing imbalances in the metal homeostasis and signal transduction path (Shetty et al., 2021). Moreover, these heavy metals drop the antioxidant pools, and thus rise in ROS production (Hossain et al., 2012). Consequently, the latter gives rise to trouble in the various physiological activities of plants and results in reduced growth and yield (Kumari et al., 2019). Therefore, the plant susceptibility to heavy metals is contingent upon an intricate network of physiological and molecular mechanisms, which involve metal absorption and storage via linking to extracellular exudates and cell walls through various substances. Ions are complexed into cells, and a general biochemical stress defence feedback such as the stimulation of antioxidative enzymes. This response, along with alterations in plant metabolism, enables the proper functioning of metabolic pathways and the swift restoration of damaged cell structures (Branco-Neves et al., 2017).
Additionally, plants are exposed to another stress factor that can affect ecosystems, namely salinity, which is the main environmental element that deteriorates soils and reduces crop productivity. In Tunisia, for example, saline areas (salt marshes) are undergoing transformations due to heavy metal pollution, resulting from industrialization and waste disposal in these zones over the past few years. Therefore, HM phytoremediation of salt-altered soils is a challenge because the appropriate plant species must be able to cope with both saline and polluting stresses (Zhou et al., 2019). The utilization of halophyte species for the remediation of heavy metal-polluted soils holds considerable significance due to their innate ability to thrive in excessively salty soils. This approach is particularly valuable for its economic feasibility (kumari et al., 2019). Halophytes plants are grown in harsh environmental conditions, namely lack of water, unsteady temperature, high salinity, and contaminated soil. The Tamarix genus, in particular, flourishes in a wide range of natural territory marked by soils with varying levels of salinity (Terronos et al., 2016). However, limited data are available regarding the specific effects of metals on the growth and development of T.gallica (Moreno-Jiménez et al., 2009; Sghaier et al., 2019a; 2020; Bencherif et al., 2020).
In our previous studies, Sghaier et al. (2015; 2016; 2019b and 2023) focused not only on determining the effect of As and Al in some physiological parameters, but also on determining the subcellular localization of these metals inside plants. Unravelling the tolerance mechanisms adopted by plants to counteract oxidative stress caused by accumulated metal ions is pivotal for enhancing their resilience to heavy metals. This understanding is fundamental to bolstering their efficacy in the phytoremediation process. Taking all this into consideration, the present data are directed to investigate (1) the extent of Al and As leads to oxidative stress in T.gallica (2) antioxidant responses to each metal, and (3) the impact of As and Al under salt stress conditions. These objectives are followed by determining MDA content and examining the variation in proline and glycinebetaine content.
MATERIALS AND METHODS
-
2.1. Experimental plant material and growth condition
T. gallica was propagated by stem cuttings (5 cm) obtained from mother plants cultivated in the natural habitat of these species (sabkha of Ariana in Tunisia). This halophyte was identified at the Biotechnology Center of Borj-Cédria, and a voucher specimen were deposited at the Herbarium of the laboratory ((Laboratoire des Ecoprocédés et de Valorisation des Plantes Aromatiques et Médicinales). Cuttings were grown in plastic pots containing a mixture of perlite and gravel substrate (2:1; v/v). They were then irrigated with non-saline tap water for a period of six weeks (For more details see Sghaier et al., 2015; 2019). Next, young-rooted cuttings were supplied by Hewitt nutritive solution (Hewitt, 1966) either supplemented or not with NaCl (200 mM), three times a week for 90 days as described in Sghaier et al. (2015; 2019). Finally, plants were divided into groups of six plants: a) Control plants (0 µM Al or As ; 0 mM NaCl), b) Al or As 200 µM; c) Al or As 500 µM; d) Al or As 800 µM; e) NaCl 200 mM; f) Al or As 200 µM + NaCl 200 mM; g) Al or As 500 µM + NaCl 200 mM; h) Al or As 800 µM + NaCl 200 mM. The experiments were realized in a greenhouse under semicontrolled conditions with a natural photoperiod, mean temperature (night-day) of 20-30°C, and relative humidity between 60 and 90%.
-
2.2. Metal concentration
Fresh plant material was blended in HNO 3 : H 2 SO 4 : HClO 4 (10:1:0.5; v/v/v) in Teflon vessels for 2 h 30 min at 110°C (see details in Sghaier et al., 2015; 2019), and then filtered and diluted with distilled water to 50 ml.The blanks, used to set the atomic absorption spectrometer
-
2.3. Oxidative stress biomarkers
Leaves and roots were collected and immediately frozen in liquid N 2 and then stored at -80 °C. All enzymatic analyses were carried out at 4°C. For the extraction procedure, 500 mg of fresh leaves were added to12 ml of sodium phosphate buffer (50 mM, pH 7.6) with 0.1 mM Na-EDTA. The mixture underwent centrifugation at 8923 rpm for 20 min, at 4°C, and the supernatant was undertaken for enzymes assays.
-
2.4. MDA content
MDA content was determined according to Heath and Packer (1968) using a mixture of 20% trichloroacetic acid (TCA) and 0.5% thiobarbituric acid (TBA). The homogenate was extracted at 95°C for 30 min,followed by centrifugation at 3000 g for 5 min at 4°C. The absorbance of the supernatant was read at 532 and 600 nm in a Shimadzu UV-1603 spectrophotometer.
-
2.5. Osmo-compatible solutes
Free proline was extracted from blended samples in aqueous 3 % sulphosalicylic acid. It underwent centrifugation at 10,000 rpm for 15 min, and the proline in the supernatant was determined using ninhydrin according to Bates et al. (1973).
-
2.6. Statistical analysis
to zero, were treated in the same way as described above.
Ascorbate peroxidase (APX) was assayed as described by Tiryakioglu et al. (2006) by recording the drop in absorbance upon the oxidation of ascorbate at 290 nm. Guaiacol peroxidase (GPOX) was carried out following the method of Bergmeyer et al. (1974) by recording a rise in absorbance upon the formation of guaiacol oxidation products at 470 nm (ε= ¼ 26.6 mM-1 cm-1).
Superoxide dismutase (SOD) activity was assayed according to Marklund and Marklund (1974) by noticing the decline of pyrogallol at 325 nm. Proteins were set according to Bradford (1976).
A unidirectional analysis of variance (ANOVA) was used and when the hypotheses of the parametric tests were not verified, the Kruskal-Wallis test was used to compare the oxidative stress and the antioxidant responses vis-à-vis aluminum or arsenic on plants in the presence and absence of salt. Depending on the type of test (parametric or non-parametric), Bonferroni test or pairwise multiple comparisons were realized when significant differences were found (α = 0.05 significance level). The statistical analysis was determined via Statistica software Statistica 12 (Statasoft). All the samples were analyzed in three replicates (n=3) and all reported values refer to mean ± standard error.
RESULTS
Biomass production
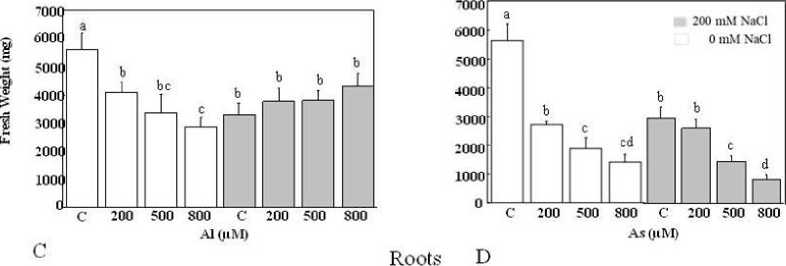
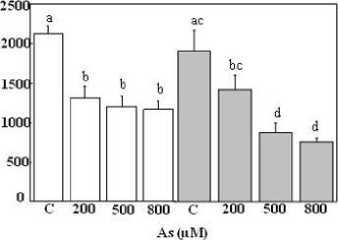
Figure 1 presents the alteration of fresh weight (FW) in the whole plant (roots and shoots) depending on the HM concentrations. The reduction in fresh weight plants was more pronounced in As treatment than those treated with Al. Our data showed that all tested As concentrations declined FW significantly, both root and shoot biomass declined markedly at each As level (Figure 1B and D). This drop is above 50% in the different organs when doses 500 and 800 µM were incorporated in the medium leading to a decrease in the plants’ length without visible chlorosis altering leaves. It varied from 5962 to 1362 mg FW and from 2133 to 1377 mg FW in shoots and roots, respectively. Furthermore, the salt treatment alone led to a different morphological response in T. gallica . The biomass production significantly decreased in the shoots. Contrarily, the addition of NaCl to the medium did not have any significant impact on roots (Figure 1B and D).
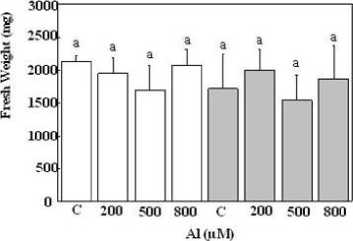
Similarly, plants treated with increasing Al concentrations showed a significant drop in FW (Figure 1A and C). This reduction is around 40% compared to control plants. However, in certain cases, there was no discernible variation in the root fresh FW of plants treated with increasing doses of aluminium. In salty medium, with the exception of plant treated with 200 mM NaCl which displayed a decline in FW, plants exposed to combined stress demonstrated no observable changes in fresh weight compared with those treated with Al only. As observed above, roots demonstrated a similar behaviour as shown with plants treated by Al and control plants.
-
3.2. Aluminium and Arsenic accumulation
-
3.3. MDA content
-
3.4. Enzyme activities
In Tamarix gallica , the results showed that the HM accumulation was influenced by the concentration of the metal in the irrigation solution. The content of As was around 52 and 50 μg.g-1 FW at 500 and 800 μM, respectively at the level of the aerial and root parts (Figure 2A and C). The addition of NaCl in the medium affected the concentration of As at the aerial parts, while in the roots, the addition of salt did not reduce its content with the exception of the 800 µM As, where a decline estimated at 35% was observed. The same effect was observed in the case of leaves from a concentration of 500 μM.
The endogenous aluminium rate was correlated with the increasing concentration of aluminium in the nutrient solution (Figure 2B and D). Thus, the content of this ion, in the root tissues, was only 2.16 mg / g FW for a dose of 200 μM AlCl 3 and reached 3.2 and 5.4 mg.g-1 at 500 and 800 Μm AlCl 3 , respectively. As opposed to the As, the addition of the salt in the medium enhanced metal uptake in roots and shoots (Figure 2 B and D).
The subjection of plants to different concentrations of arsenic for 90 days induced a significant augmentation in MDA levels in T. gallica leaves compared to control plants (two/three- fold). While this rise was observed only at high concentrations of the metal ion (500 and 800 μM), at low doses (200 μM) there was no significant difference between control and untreated plants (Figure 3 A).The values were about 10 and 14 mmol g-1 FW under As and NaCl exposure at 800 µM, respectively, compared with the controls (5 mmol g-1 FW).
The introduction of salt into the irrigation solution led to the rise in the MDA content for plants treated only with NaCl. It is important to highlight that that MDA production under combined stress (As added to NaCl) exceeded that observed under As alone (Figure 3A). Furthermore, plants subjected to combined stress (As, NaCl) showed an augmentation in membranous lipoperoxidation compared to those treated only with salt. The MDA content was notably amplified, reaching a factor of 2.82 when compared to control plants (Figure 3A).
After exposure to different concentrations of aluminium, an increase in MDA content was observed in T. gallica compared to control plants. This rise remained constant regardless of the concentration of applied Al. Thus, the production of MDA was increased twice compared to the control plants (Figure 3B). The irrigation of plants with Al in the presence of NaCl (200 mM) did not produce significant changes compared to control plants (Figure 3B).
The stressed individuals presented a variation in the enzyme activity. These differences were more certain in the GPOX and APX activity (peroxidase activity) (Figure 4 and 5).Our results revealed that the predominant antioxidant peroxidase in T. gallica turned out to be the APX in the shoots.
Under As stress, there was a slight promotion in APX activity. This augmentation was strong up to 57 % at 800 µM. Interestingly, the salt added to the medium did not alter the activity of the studied antioxidant as compared to the controls and a huge activity at a high level of As in combined stress was noted (Figure 4A).
APX exhibited enhanced activities in individuals suffering from Al stress (Figure 5A). We noted an increase of 86%, which remained constant regardless of the concentration of Al in the irrigation solution. However, although APX activity diminished with the addition of the salt, it recovered levels under higher concentrations of metals (Figure 5A).
There was a drop in GPOX activity in the leaves of T. gallica under As stress at 200 µM. Although this enzyme activity showed no significant changes when exposed to 500 µM As, at a higher concentration of 800 µM As, it decreased by half compared to 200 µM (Figure 4B). The reduced activities of GPOX were detected with NaCl addition under high-stress levels. Indeed, no difference was detected at the level of GPOX activity after the application of increasing doses of Al (Figure 5B). On the other hand, the salt added to the culture medium caused a significant drop in GPOX activity in plants subjected to 200 mM NaCl. Moreover, a substantial decline was observed with increasing doses of Al in the medium (Figure 5B).
Similarly, As stress was evident in the pronounced decrease in SOD activities, irrespective of As stress extent (Figure 4C). While SOD activities decreased by more than 50% at 200 mM NaCl, they exhibited a strong improvement, especially at 500 and 800 µM when combined with salt.
The variation of the SOD activity following the poisoning of plants by Al revealed that the latter had no obvious effect (Figure 5C). Indeed, the activity of this enzyme exhibited consistency across varying concentration in the medium except for a slight increase noted at the 500 μM dose. However, salt addition had an inhibitory effect on SOD activity in plants subjected to 200 mM NaCl, and this inhibition intensified with the increasing doses of Al in the nutrient solution. Elevations of 29%, 64% and 100% were recorded at 200, 500 and 800 μM Al, respectively, in the presence of salt (Figure 5C).
-
3.5. Proline and glycine betaine contents
When examining leaf proline content, T.gallica exhibited stable concentrations consistent concentrations across treatments, with only minor and statistically insignificant fluctuations, except for the control which had lower proline content. The rising in content was observed in control compared to other As concentrations (Figure 6A).The addition of the salt in the medium, supplemented with As, did not modify proline trend. A significant increase in proline content was observed as compared to the control, whereas among treatment plants, no variation in their contents was evident (Figure 6A).
In T. gallica , the exposure of plants to Al for 90 days induced an enhancement of the proline content. The effect was more pronounced for plants treated with a high concentration of aluminium. The proline level was increased twofold, compared to those of the control plants. Plants subjected to the combined stress (Al, NaCl) had a higher production of proline compared to those treated only by Al alone (Figure 6C). The concentration at 800 μM in the presence of salt was amplified threefold compared to untreated plants and 1.5 of the plants treated with 800 μM Al (Figure 6C).
Shoots
Figure
1: Biomass production in shoots (A, B) and roots (C, D) tissues of Tamarix gallica under different concentrations of As or Al (0, 200, 500, 800 µM) in the absence or presence of 200 mM NaCl in the nutrient solution (mean values ±SD, n=3). Different lower-case letters represent statistically significant differences (p<0.05)
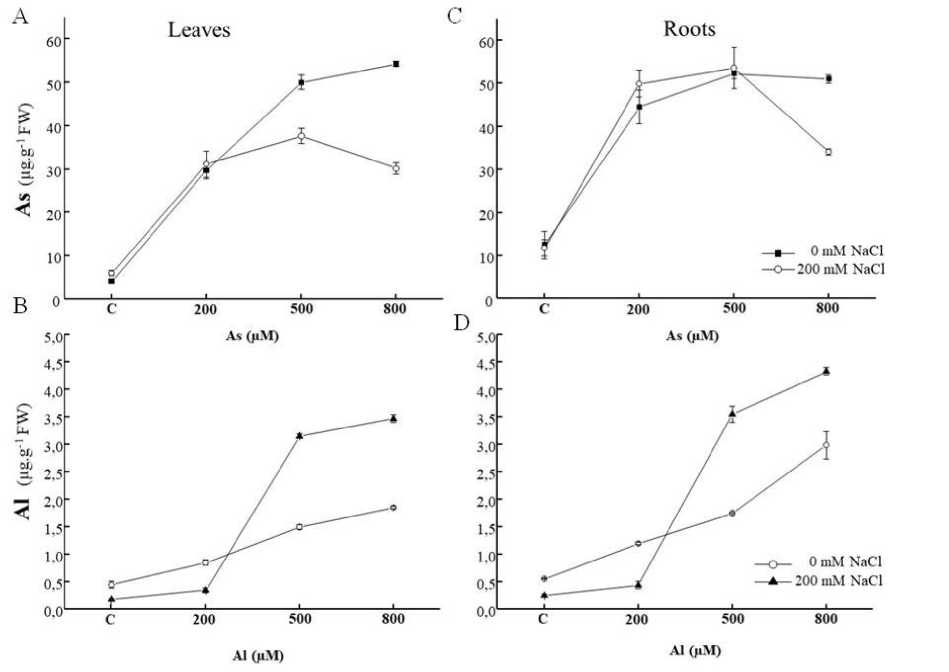
Figure 2: Arsenic (A, B) and Al (C, D) concentrations in shoots and roots tissues of Tamarix gallica (mean ± SD, n=3) exposed to different doses (0, 200, 500, 800 μM) of As or Al in the absence or presence of 200 mM NaCl in the nutrient solution
В 200 шМ NaCI
А О П1М NaCI
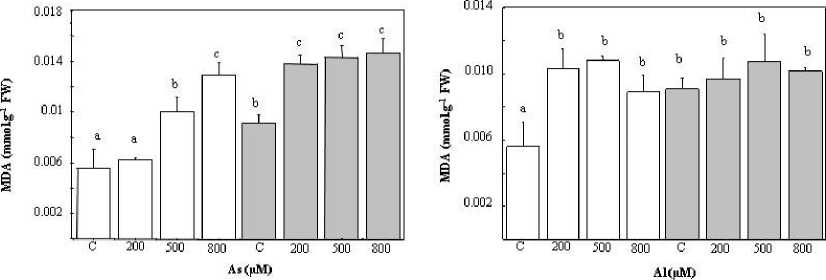
Figure 3: MDA content in the leaves of Tamarix gallica under different concentrations (0, 200, 500, 800 μM) of As (A) or Al (B) in the absence or presence of 200 mM NaCl in the nutrient solution (mean values ± SD, n=3). Different lower-case letters represent statistically significant differences (p<0.05)
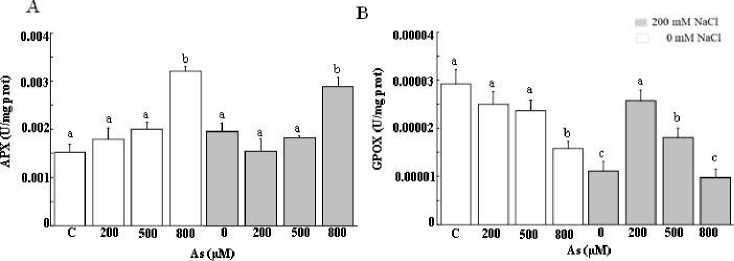
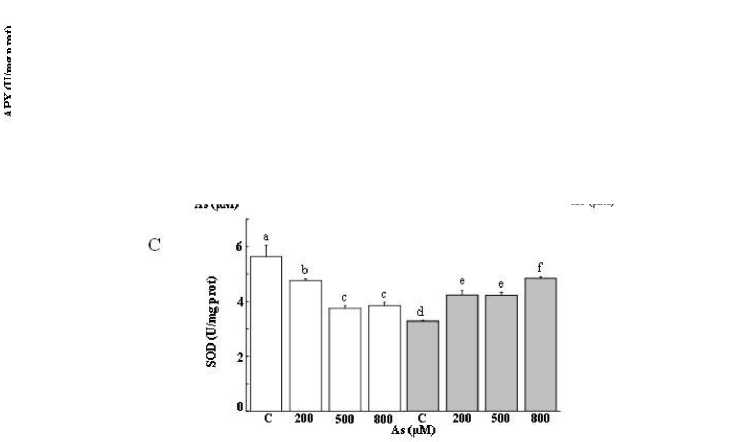
Figure 4: Enzymatic activity (APX (A), GPX (B) and SOD (C) in the leaves of Tamarix gallica (mean values ± SD) under different concentrations of As (0, 200, 500, 800 μM) in the absence or presence of 200 mM NaCl in the nutrient solution (mean values ± SD, n=3). Different lower-case letters represent statistically significant differences (p<0.05)
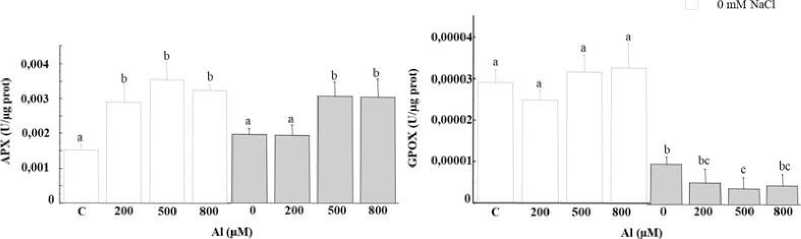
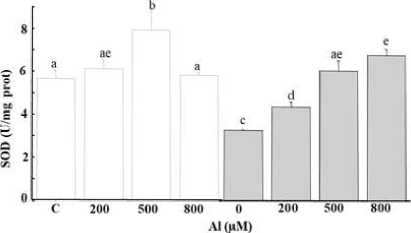
Figure 5: Enzymatic activity (APX (A), GPX (B) and SOD (C) in the leaves of Tamarix gallica under different concentrations of Al (0, 200, 500, 800 μM) in the absence or presence of 200 mM NaCl in the nutrient solution (mean values ± SD, n=3). Different lower-case letters represent statistically significant differences (p<0.05)
200 mM NaCl
А Во mM NaCI
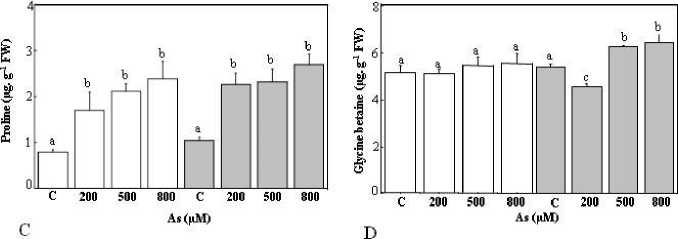
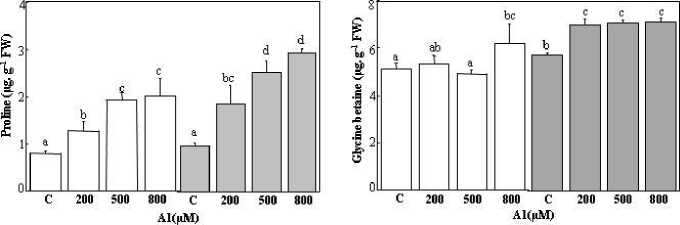
Figure 6: Proline and glycinebetaine content in the leaves of Tamarix gallica under different concentrations (0, 200, 500, 800 μM) of As (A, C) or Al (B, D) in the absence or presence of 200 mM NaCl in the nutrient solution (mean values ± SD, n=3). Different lower-case letters represent statistically significant differences (p<0.05)
obvious differences were detected among treatments for
Regarding the glycine betaine concentration, no plants treated with As (about 5 µg.g-1 FW), while T.
gallica demonstrated a significant rise in this amino acid concentration, concomitant with adding the salt to the medium (Figure 6B). In fact, the increase was stronger at 500 and 800 µM As, as compared to control plants. In contrast, plants treated with 200 mM NaCl did not exhibit any changes in GB content (Figure 6B).
The production of glycine betaine in plants subjected to aluminium seemed to be little affected by the presence of Al in the irrigation solution (Figure 6D). The GB content remained constant despite variation in the metal concentration in the culture medium, except for a decrease observed at 500 μM. Interestingly, plants exposed to the combined stress (Al, NaCl) showed a higher production of glycine betaine than those treated only by Al alone (Figure 6D).
DISCUSSION
In this study, our aim was to elucidate the biochemical response of T. gallica to different stressors, including arsenic, aluminium and combined stress (Al / As+ 200 mM NaCl).
While in our study (Sghaier et al., 2015; 2019), the accumulation of metal As or Al in T.gallica was previously described, in the present research work, the accumulation of metal in relation to fresh weight was discussed. As or Al accumulation in plant increased with the increase in metal concentration in the medium (Figure 2). This result suggested that T. gallica could absorb and accumulate high As or Al concentrations. All plants exposed to 800 µM As(V) remained viable and As or Al concentrations in roots and shoots continued to increase till the end of to increase throughout the entire treatment period of 90 days. This observation suggests that the plant was not saturated with toxic ions, indicating its capacity to accumulate even higher amounts of these elements (Figure 2). In contrast, aluminum (Al) exhibited an increased uptake in the presence of salt in the medium. However, while the addition of salt to the medium decreased As uptake by the plant at 800 µM, it enhanced Al uptake (Figure 2). The increase in Al in the salty medium might be attributed to the increased Al3+ uptake and its translocation in complexed form. In the case of T. gallica, which refers to salt cedars, the plant has the ability to eliminate harmful ions by utilizing specialized cells in its leaves known as salt bladders. This mechanism is responsible for reducing the uptake of arsenic. The presence of salinity can alter the accessibility of heavy metals in the soil by diminishing the sorption of metals in the soil and causing a shift of metals from the below-ground sections to the aboveground parts of the plants (Wahla and Kirkham, 2008). Most plants considered to be tolerant had mechanisms to keep much of their As load in the root (Leonardi et al., 2021). Salinity reduced Cd2+ absorption and translocation in Kosteletzkya virginica (Han et al., 2012), whereas Cd2+ translocation factor rose with the addition of NaCl in Sesuvium portulacastrum (Ghnaya et al., 2007).
Moreover, the growth of shoots and roots was dramatically affected by arsenic stress alone or in combined stress (Figure 1A and C). The decline in growth could be attributed to both water and nutrient deficiency, which is also the factor behind the depression in root development under As treatments. This condition led to an inhibited division of endodermal cells, induced by the rise in apoplastic As doses, which resulted in a decline in root length (Sarath et al., 2022).
However, when exposed to Al stress alone, roots appeared to be unaffected by high Al concentrations, while shoots exhibited alterations in response to the high metal extent (Figure 1B and D). This could potentially induce various toxic cellular changes related to cell division, localization and expression of the nucleolar proteins (Zhang et al., 2014). Aluminiumwas linked to phosphorus (P) in a reduced utilizable and insoluble form giving rise to P deficiency for plant development. The combined stress enhanced the growth of the shoots without any effect on roots growth (Figure 1B and D). Some salt cedars have been identified for their ability to thrive in polluted soils (Conesa et al., 2006) and contaminated soils without showing symptoms of intoxication (Manousaki et al., 2008). Our experiment aligns with these observations, as we found that no sign of chlorosis or necrosis was detected after 3 months of exposure to high concentrations of metals.
It was, indeed, reported that the decreased shoot and root development of Sueda maritima was observed under As exposure alone or combined treatments, which hypothesized that As stress did not set serious impacts on nutrient metabolism (Panda et al., 2017). Nevertheless, there exist genotypes where root development was unaffected even at very high Al doses, demonstrating that species vary in their Al/As stress reaction mechanisms at the cellular and tissue levels (Bojórquez-Quintal et al., 2017). Moreover, one of the possible reasons behind defunding the enhancement of plant growth provoked by Al was the promotion of nutrient absorption. In hyperaccumulator plants, Al could either stimulate or had no impact on nutrient metabolism (Bojórquez-Quintal et al., 2017). Indeed, it had been reported that Al could stimulate channels and Mg transporters in Al-resistant plants (Bojórquez-Quintal et al., 2017).
The obtained findings suggested that severe metals stress alone engendered oxidative stress determined by the increment MDA content. They clearly pointed out the installation of membrane lipoperoxidation in T. gallica after exposition to different doses of As or Al, which reflected the installation of a state of oxidative stress in the studied species (Figure 3). In addition, the salt stress, in turn, seems to induce a membrane lipoperoxidation (Reginato et al., 2014). The MDA content varied depending on the metals type and doses added to the soil. The MDA content was higher in As stress than Al (Figure 3).
In the case of arsenic stress, the overproduction of membrane lipids might be due to arsenic-induced ROS. These could instantly attack the hydrogen atom of a methylene group next to an unsaturated carbon atom. In tandem with our own findings, the application of arsenic treatment resulted in the lipid peroxidation of the membrane in rice seedlings (Gaikwad et al., 2020). The damage to the membrane is evident in both treatments, as reflected in the increased MDA content. Plants subjected to salt stress were altered via osmotic stress, nutritional deficiency, ion toxicity, etc., inducing the overproduction of ROS (Rahman et al., 2021). Despite the harmful effects of high ROS production, it played a key role not only in the expression of stress response genes, but also in the activation of the metal resistance approach (Sarath et al., 2022).
Furthermore, Al bound to membranes could instantly cause membrane stiffening, and therefore might facilitate the catalysis of membrane peroxidation (Wang et al., 2015). Likewise, Shamsi et al. (2008) had shown that Al treatment indirectly produced ROS that caused a severe imbalance, namely ROS production and antioxidant defence, resulting in increased lipid peroxidation via the stimulation of MDA production (Shahnez et al., 2011). Recent studies have demonstrated that some modifications in gene expression might participate in the alleviation of Al toxic effects. For instance, Wu et al. (2015) revealed that the overexpression of OsPIN2 would impair the Al-activated formation of ROS and reduce lipid peroxidation (LPO) in rice roots.
In contrast, the constant or attenuated level of the malondialdehydes appeared to be a characteristic of salinity-tolerant plants (Reginato et al., 2014). In agreement with our findings, several studies, namely about Atriplex atacamensis (Vromman et al., 2016), Suaeda maritima (Panda et al., 2017) and Kosteletzkya pentacarpos (Zhou et al., 2019), have reported that both treatments As and salt do not have any impact on H 2 O 2 levels. This supports the hypothesis that salinity helps plants to overcome HMs stress in case salt has no impact like (Zn and Pb) or increased HMs accumulation in the aerial part (Zhou et al., 2019).
Besides, our data corroborated that T. gallica used Pro and GB as its primary defense mechanisms against the lethal effect of As and to scavenge ROS and then activated the enzymatic antioxidant essentially SOD and APX . While under Al stress, Pro appeared as first line to defence toward emerged as the initial line of defense against ROS, followed by the activation of GPOX and SOD. However, the elevated doses of As or Al altered the antioxidant machinery (enzymatic and non-enzymatic). Martınez-Domınguez et al. (2010) reported that Spartina densiflora faced oxidative stress in its habitat and harmonized its antioxidative system depending on the level of metal pollution.
It is trusty to mention that As led to the inhibition of SOD activity in T. gallica notably upon As exposure. Besides, the level of decline was variable for As stress alone or in combined stress (Figure 4C). The SOD activity in the leaves remained constant at a lower Al concentration. Meanwhile, an augmentation in activity was noted at 500 µM, followed by a subsequent decline at higher Al levels, despite the activity remaining comparable to the control (Figure 5C). The presence of salt notably hindered SOD activity, however, an increase in SOD activity was recorded subsequently to the exposure to the combined stress Al/As and salt (Figure 4C and 5C).
According to the literature similar results were obtained, indicating that Salicornia brachiate exhibited an increase in SOD activity in response to heavy metals such as Cd, Ni and As. However, at higher concentrations, a decline in SOD activity was observed, suggesting a modification in oxygen scavenging function of SOD (Sharma et al., 2010). Vitoria et al. (2001) suggested that the decrease in SOD activity at elevated doses of heavy metals is attributed to the binding of metal ions to the active centre of the enzyme. The inactivation of enzymes under such conditions is often linked to interference with sulfhydryl groups, interaction with the enzyme-substrate complex, or protein active groups, and denaturation of the enzyme protein, as observed in the case of As exposure (Reboredo et al., 2021). Conversely, the observed increase in SOD activity may be associated with either an overproduction of ROS or the overexpression of genes encoding SOD (Israr et al., 2006).
Unchanged GPOX activity was noted as a response to Al stress (Figure 5B), demonstrating that the GPOX enzyme reached a high level for the trapping of H 2 O 2 generated by Al stress (Panda et al., 2017). GPOX activity was significantly impaired, dropping to nearly half of its original level under 800 µM As (Figure 4B). Meanwhile, a sharp reduction was observed in combined stress (Al+ salt). However, there was an increase in GPOX activity at low As concentrations when combined with the salt, followed by a decrease at higher levels of metal combined with salt. The addition of the salt in the medium altered dramatically the enzyme’s activity (Figure 4B and 5B). Under As stress, enhanced APX activities was noticed at 800 µM, either alone or combined with salt (Figure 4A). Additionally, APX activity was stimulated under Al stress (Figure 5A). APX was inhibited after subjection to the salt or 200 µM
Al +200mM NaCl followed by stimulation at 500, 800 µM combined with APX activity was stimulated under aluminum stress (Figure 5A). However, APX was inhibited after exposure to salt or 200 µM aluminum + 200mM NaCl, followed by a subsequent stimulation at 500 and 800 µM when combined with salt (Figure 5A).
It is noteworthy to note that APX and GPOX showed simultaneous enhancement and inhibition, indicating their collaborative role in conferring As or Al tolerance alone or combined with salt. Notably, APX serves a distinct physiological function compared to guaiacol peroxidase (GPOX). While GPOX had a crucial role in the biosynthesis of lignin and antioxidant defence by consuming H 2 O 2 , APX shows considerable variations in activity based on plant species and environmental conditions (Foyer and Noctor, 2003).
In fact, the hampered peroxidase activity might be due to blockade of essential functional groups, the substitution of essential metals with trace elements, modification in protein structure or integrity, and disruption of signal transduction of antioxidant enzyme. This enhanced peroxidase activity was associated with the presence of phytotoxic metal that were not linked to cell walls or stored in vacuoles(Hsu and Kao, 2007). Kumari et al. (2019) reported that plants were more tolerant to heavy metal stress through the enhancement of APX activity.
Other studies have shown different patterns in the behaviour of GPOX/APX activities in response to Al/As stress. An augmentation of GPOX around 31% was observed in Vigna trilobata (L.) after exposure to 6 mM of Al (Arundhathi et al., 2016). Furthermore, in a separate study on Cakile maritima , Ben Amor et al (2006) observed an increase in ascorbate peroxidase (APX) activity after 20 days of exposure to salt treatment. Nonetheless, as observed, in wheat seedlings, APX activity decreased under low concentrations of arsenic as well as at high arsenic doses in the wheat seedlings (Li et al., 2007). Besides, Shri et al. (2009) mentioned that arsenic treatment induced an increase in APX activity in rice plants.
The obtained results demonstrated that both treatments (As/Al+salt) and salt stress led to the generation of H 2 O 2 that was trapped either by APX or
GPOX, serving the purpose of maintaining appropriate levels H 2 O 2 necessary for stress responses (Panda et al., 2017). Moreover, the application of 200 μMAs +200 mM NaCl induced a 54% rise in POX activity compared to untreated plants, thus highlighting POX’s role in Sueada maritima , as antioxidative defence strategies (Demir et al., 2013). Indeed, APX was included in the ASH-GSH cycle through H 2 O 2 reduction. According to Hsu and Kao (2007), the pre-treatment of Oryza sativa with H 2 O 2 induced an enhancement in APX activity and protected rice plants when exposed to Cd. Nevertheless, the POD activities increased with rising doses of NaCl concentration, but decreased under moderate and severe Cd stresses, indicating the enhanced and inhibited scavenging processes of H 2 O 2 (Wali et al., 2017).
The obtained results highlighted the complexity of the synergy between the different antioxidant enzymatic activities in order to regulate the antioxidant metabolism of plants.
Taken all together, the activation or deactivation of an enzyme in the defence system depends on factors such as the type of metal/metalloid, dosage, duration of exposure and plant species. Under harsh stress conditions, a plant may be too vulnerable to initiate sufficient activity antioxidant enzymes for selfpreservation. However, the low activity of the antioxidant enzyme activity may indicate the weak stress, as proven by unchanged MDA values across treatments in combined stress (As/ Al+ salt) or under Al stress alone.
It is trusty to note that antioxidant enzymes are not the only way of neutralizing the most reactive ROS, (HO·). Hence, assaying antioxidant molecule levels, namely proline and glycine betaine were required to have a complete scenario of the scavenging capacity (Kofronová et al., 2020).
Hence, to sustain the ionic equilibrium into vacuoles, the cytoplasm cumulates compatible solutes (Parida and Das, 2005). Among these compatible solutes, proline (Pro) and glycine betaine (GB) were found (Moghaieb et al., 2004).
The intensity of stress directly correlates with elevated proline levels registered (Szabados and Savouré, 2010). Besides its function as osmoprotective, proline plays a crucial role in reinforcing the antioxidant system and fighting stress damage. It functions as a singlet oxygen deactivator (Lehmann et al., 2010) and hydroxyl radical scavenger (Lehmann et al., 2010). This implies that this molecule could be accumulated in cells towards Cd, Cu, and other heavy metals (Lefévre et al., 2009).
Many metal-tolerant species such as Armeria maritima, Deschampsia cespitosa , and Silene vulgaris , demonstrated high proline content (Sharma and Dietz, 2006). Our findings accord well with those obtained by Backor et al. (2004) who reported that proline accumulation was one strategy of heavy metal resistance in plants. In addition, Shackira and Puthur (2017) signalled the antioxidant and osmoregulatory roles of proline in Acanthus ilicifolius towards metal stress. Similarly, Pavlik et al. (2010) revealed that the rising in the proline extent of Spinacia oleracea was linked to arsenic stress.
Additionally, heavy metals have the potential to induce of quaternary ammonium compounds like glycine betaine. GB accumulation serves a protective function for thylakoids, maintaining membrane integrity and preserving other cellular structures. Moghaieb et al (2004) demonstrated that GB also plays a role in regulating the quaternary structures of complex proteins, contributing to the preservation of chloroplasts and photosystem II (Moghaieb et al. 2004). This protective mechanism is attributed to GB's involvement in osmotic adjustment, mitigating the hampered effects of stress and facilitating the accelerated restoration of damaged PSIIs (Siddiqui et al. , 2010).
In Atriplex halimus L. Cd exposure led to an increase in osmotic adjustment, resulting in the formation of compatible sugars as glycine betaine (Levèvre et al., 2009). T. gallica exhibited an increase in the proline content after treatment with Al and As (Figure 6A and C), whereas, there was no variation in GB content after exposure to different As and Al concentrations (Figure 6B and D). The unchanged GB content could be related to the lack of upregulationof BADH, a key gene for glycine betaine biosynthesis (Wali et al., 2016). Indeed, a decline in free amino acid like glycine, cysteine and proline could be caused by the generation of stress- sensitive proteins as metallothioneins, phytochelatins (Sarath et al., 2022).
Conversely, the exposure to 200 mM NaCl increased the synthesis of GB under Al stress and at high As concentrations. This aligns with the finding of Wali et al. (2016), who observed an increase of about 20% of glycine betaine leaf accumulation after exposure to 200 mM NaCl. Osmotic adjustment in Salicornia europaea and Suaeda maritima was achieved with a significant rise in glycine betaine (Moghaieb et al., 2004). Otherwise, low doses of salinity did not improve proline and GB levels in plants not subjected to heavy metals, and the water uptake remained unaffected, suggesting altered water content serves as a signal the signals for osmolyte synthesis (Sarath et al., 2022).
Moreover, the combined use of NaCl with As in Chenopodium quinoa, and salt with CdCl 2 improved the metal resistance of the halophyte Bruguiera cylindrica (Sruthi and Puthur, 2021). Obviously, both these examples boosted that the combined application of NaCl and metal stress in halophytes significantly enhanced the metal resistance of the halophytes compared to when each stressor was administered individually (Sarath et al. 2022).
CONCLUSIONS
The present research work investigates crosstolerance mechanisms of salt with arsenic/ aluminum in the halophyte Tamarix gallica , shedding light on its phytoremediation abilities. T. gallica demonstrated its potential to thrive under elevated concentrations of As/Al without displaying any visible symptoms of toxicity. Interestingly, this species showed a different behaviour to cope with as/ Al stress alone or combined with salt .
The study revealed that T. gallica had an enzymatic antioxidant system, complemented by non-enzymatic antioxidants, effectively defending itself against cell damage caused by arsenic and aluminum stress. Additionally, the plant's tolerance and growth at high concentrations of aluminum or arsenic, either individually or in combination with salt, suggest the existence of additional mechanisms necessitating further investigation.
Significantly, T. gallica exhibited enhanced tolerance to metal stress in the presence of salinity. The latter were characteristics of sebkha, which is the native habitat of this species. Sebkha is recognized as a saline area for dumping industrial waste, making T. gallica a suitable candidate for phytostabilization/ phytoremediation in these local zones. The findings underscore the potential of T. gallica as a resilient and effective species for addressing environmental challenges in saline areas contaminated with industrial pollutants.
CONFLICTS OF INTEREST
The author declare that he has no potential conflicts of interest.


